Abstract
Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. are commonly used in the traditional management of cancer in Nigeria. The genotoxic and anti-genotoxic effects of aqueous extracts of these plants was evaluated using the Allium cepa L. assay. Onion bulbs were exposed to 0.5–10 mg ml–1 concentrations (v/v) of each extract, prepared as practiced locally, for analyses of root growth inhibition and induction of cytogenetic damage, respectively. There was concentration-dependent, significant (p < 0.05) inhibition of root growth by the extracts when compared with the negative control. The EC50 for the decoctions of S. mombin, N. lotus and L. cylindrica were 1.3, 1.8 and 16.2 mg ml–1 respectively. All the extracts had mitodepressive effects on cell division, and induced chromosomal aberrations (p ˂ 0.05). They showed chemopreventive activity through reduction of cytological aberration induced by lead nitrate. These findings suggest inhibitory, anti-proliferative and anti-cytogenetic damage activities of aqueous extracts of the tested medicinal plants on A. cepa.
Introduction
The use of medicinal plant extracts for the treatment of human diseases is an ancient practice which has greatly increased in recent years. The World Health Organization estimated that up to 80% of the world’s population relies on traditional medicinal systems for some aspect of primary health care (Farnsworth et al. Citation1985). The secondary metabolism of higher plants has been shown to be an almost inexhaustible source of compounds with possible biological activity (Santos et al. Citation2006). Many potential carcinogens are present in the environment, and due to their great number and variety they cannot be completely avoided. The use of anti-mutagens and anti-carcinogens in everyday life has been suggested to be an effective procedure for preventing human cancer and genetic diseases (Ferguson Citation1994). Higher plants used extensively in traditional medicines are increasingly being screened for their role in modulating the activity of environmental genotoxicants (Sreeranjini and Siril Citation2011).
Medicinal plants contain bioactive compounds which can act to block or reverse carcinogenesis at early stages (Lippman et al. Citation1994). In Nigeria, several plant-based remedies are used in the management of cancer with varying degrees of success. However, the efficacy of most of these plants is yet to be scientifically confirmed due to lack of or inadequate in vivo and in vitro studies which could outline the mechanism of the anticancer activity of these plant based products.
Spondias mombin (Linn), Nymphea lotus (Linn) and Luffa cylindrica (Linn) (syn Luffa aegyptiaca Mill) are commonly used in the traditional management of cancer in Nigeria. They have been reported to have antibacterial, antifungal, antioxidant and hepatoprotective effects (Corthout et al. Citation1994; Esimone et al. Citation2006; Qizhen et al. Citation2006; Hassan et al. Citation2009; Yisa Citation2009; Akinjogunla et al. Citation2010; Hamenoo Citation2010; Ismail et al. Citation2010; Pal and Manoj Citation2011; Shete et al. Citation2011).
In view of the widespread use of these plants in traditional medicine, and due to the lack of information on their genotoxic and anti-genotoxic potential, it is important to evaluate the probable effect of these plant extracts on the genetic material. And because of a strong correlation between genomic damage and carcinogenesis (Hoejimakers Citation2001), understanding of the anti-genotoxic potentials of the plant extracts may be useful in cancer chemoprevention. As parts of an ongoing study in our laboratory, we evaluated the potential of aqueous extracts of S. mombin, N. lotus and L. cylindrica to induce cytogenotoxicity and anti-cytogenotoxicity in root tip cells in Allium cepa.
Materials and methods
Collection of medicinal plants
The leaves of S. mombin, whole plants of N. lotus and fruits of L. cylindrica were collected at different locations within the premises of the University of Ibadan, Nigeria. These were taken to the University of Ibadan Herbarium for authentication and voucher specimens (S. mombin UIH-22350, N. lotus UIH-22349, L. cylindrica UIH-22348) were deposited. The leaves and whole plants were washed with tap water, dried in shade, ground and stored in the dark while the fruits were washed with tap water and used fresh.
Preparation of extracts
Extractions were carried out as done locally: 50 g samples of S. mombin and N. lotus and 100 g of L. cylindrica were boiled separately in 1 l of tap water. The decoctions were filtered with Whatman® (Maidstone, UK) no.1 (11 μm) filter to remove the suspended particles and stored at 4°C until use. They were designated aqueous S. mombin (ASM), aqueous N. lotus (ANL) and aqueous L. cylindrica (ALC) respectively.
Phytochemical analysis
The extracts were screened for the presence of tannins, alkaloids, saponins, resin, steroids, reducing sugar and glycosides according to standard procedures (Sofowora Citation1993; Trease and Evans 1989; Parekh and Chanda Citation2007).
Allium cepa assay
Onions (Allium cepa L., 2n = 16, Family Amaryllidaceae), obtained commercially at the Bodija market, Ibadan, Nigeria, were sun-dried for 2 weeks. The dry bulbs were used in the modified A. cepa assay (Fiskesjö Citation1997; Bakare and Wale-Adeyemo Citation2004) to evaluate the genotoxic and anti-genotoxic effects of the plant extracts. The treatment (incubation) of roots was carried out in two different applications under the same conditions. For each application 12 onion bulbs were used per concentration of each of the test samples. Five concentrations (v/v): 0.5, 1.25, 2.5, 5 and 10 mg ml–1 for ASM and ANL and 1, 2.5, 5, 10 and 20 mg ml–1 for ALC were considered. Tap water and lead nitrate (10 ppm) were used as the negative and positive control respectively.
Application 1
A series of 12 cleaned onion bulbs were placed on top of 100 ml beakers filled with different concentrations of aqueous plant extracts and incubated in the dark at room temperature for 72 hours with the test samples being changed at 24-hour intervals. At 48 hours, the meristematic cell region from the cut root tips of two onion bulbs was processed for the preparation of microscope slides.
Application 2
In this application, the effect of aqueous plant extracts on lead nitrate pretreated roots was investigated. For this purpose, a series of 12 cleaned onion bulbs were placed on top of beakers filled with lead nitrate (10 ppm) for 24 hours. After the lead nitrate treatment onion bulbs were treated with five different concentrations of plant extract for 48 hours and incubated in the dark at room temperature. At 24 hours of treatment with the extract, the meristematic cell region from the cut root tips of two onion bulbs was processed for the preparation of microscope slides.
Root growth and cytogenetic analysis
In each application, on the third day (72 hours), the length of the roots of the remaining 10 onion bulbs at each concentration were measured (in cm) and used as an index of general toxicity (root growth inhibition). From the weighted averages for each concentration and the control, the percentage root growth inhibition in relation to the negative control and the EC50 for each extract were determined (Fiskesjö Citation1985). The effect of each sample on the morphology of growing roots was also examined.
For the slide preparation, the cut root tips (at 48 hours) were fixed in ethanol:glacial acetic acid (3:1 v/v), after which the roots were hydrolyzed in 1 N HCl at 60°C for 5 minutes and then washed three times in distilled water. Two to three root tips were squashed on each slide and stained with acetocarmine for 10 minutes. Six slides were prepared for each concentration, of which four were used for microscopic observation at 1000× magnification. In total 4000 cells were observed for each concentration. Chromosomal aberrations were characterized and classified. The mitotic index was calculated as the number of dividing cells per total cells scored at each concentration. The frequency of aberrant cells (%) was calculated based on the number of aberrant cells per total cells scored per slide for each concentration of the extract (Bakare et al. Citation2000).
Statistical analysis
Statistical analyses were performed using the SPSS 17.0® software package. Data on root length, mitotic index and chromosomal aberrations were compared using analysis of variance (ANOVA) followed by Tukey test (p < 0.05). The EC50 from the root length data was determined using Probit regression analysis. Correlation between root length and mitotic index was determined using Pearson correlation coefficient.
Results
Preliminary phytochemical analysis showed the presence of tannins, saponins, sterol, glycosides and resins in ASM; tannins, saponins and sterols in ANL; alkaloids, saponins, sterol and resins in ALC. Data on the root lengths and growth inhibition are presented in Table . Restricted root growth was observed with all tested samples. The EC50 for ASM, ANL and ALC were 1.4, 1.8 and 16.2 mg ml–1 respectively. The results of the cytological effects are summarized in Tables –. There was a concentration-dependent reduction in mitotic index in all the extracts compared to the negative control. The mitotic index was positively correlated (ASM, r = 0.893; ANL, r = 0.769; ALC, r = 0.748) to the root lengths. Chromosomal aberration induced by ANL and ASM were not significantly different from those of the negative control except at concentration of 5 mg ml–1 of both extracts (Tables and ). ALC significantly (p < 0.05) induced chromosomal aberration except at the highest tested concentration (Table ). ASM and ALC were able to reduce the frequency of chromosomal aberration induced by lead nitrate. ANL only showed a significant ameliorative effect at concentrations 2.5, 5 and 10 mg ml–1.
Table 4. Cytological effects of aqueous extracts of Luffa cylindrica on Allium cepa cells.
Table 3. Cytological effects of aqueous extracts of Nymphea lotus on Allium cepa cells.
Table 2. Cytological effects of aqueous extracts of Spondias mombin on Allium cepa cells.
Table 1. Effects of aqueous extracts of Spondias mombin, Nymphea lotus and Luffa cylindrica on Allium cepa root growth.
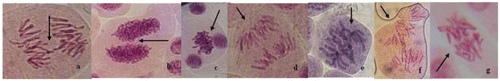
Figure show the types of aberrations observed upon exposure of root tips of A. cepa to different concentrations of the plant extracts alone or after lead nitrate treatment. The most frequent aberrations induced by the extracts were associated with spindle dysfunction (disturbed spindle, disoriented and vagrant chromosomes) rather than clastogenicity (fragments, anaphase bridge). The most frequent aberration induced by lead nitrate is sticky chromosomes, followed by disturbed spindles. The extracts at most concentrations were able to reduce the frequency of sticky chromosomes close to the frequency in the negative control.
Figure 1. (Color online) Chromosomal aberrations (arrowed) induced in Allium cepa root tips by aqueous extracts of Spondias mombin, Nymphea lotus and Luffa cylindrica. (a) Delayed chromatid; (b) binucleated cell at prophase; (c) sticky chromosome; (d) bipolar anaphase; (e) vagrant chromosome; (f) disoriented chromosomes; (g) spindle disturbance at anaphase. Magnification 1000 ×.
Discussion
Mutations are the cause of innate metabolic defects in cellular systems, triggering morbidity and mortality in living organisms. A large number of synthetic and natural substances, apart from various genotoxic physical and biological agents, are known to act as mutagenic, co-carcinogenic and/or carcinogenic agents (Mitscher et al. Citation1986). Many natural compounds in plants have been reported to have anti-mutagenic and anti-carcinogenic effects (Steinmetz and Potter Citation1996), acting through many complex mechanisms. In this study, we investigated the genotoxic and anti-genotoxic properties of aqueous extracts of three plants, namely S. mombin, N. lotus and L. cylindrica, which are widely used herbs in Nigeria for traditional management of cancer with claims of efficacy. The Allium test has been extremely useful in biological monitoring, investigation of environmental pollution, determination of toxicity as well as evaluation of cytotoxicity and anti-proliferative potentials of medicinal plants (Bakare et al. Citation2000, Citation2012, Citation2013; Majewska et al. Citation2003; Teixeira et al. Citation2003; Akinboro and Bakare Citation2007; Oyedare et al. Citation2009; Sultan and Celik Citation2009; Frescura et al. Citation2012; Pastori et al. Citation2013).
Our data showed that all the extracts caused a decline in root growth and mitotic index (MI); and is similar to previous studies wherein extracts of different medicinal plants caused decreased mitotic cell division in A. cepa root tips (Akinboro and Bakare Citation2007; Oyedare et al. Citation2009; Sultan and Celik Citation2009; Pastori et al. Citation2013), Eruca sativa (Frescura et al. Citation2013) and in mice (Nabeel et al. 2008). The decline in MI shows interference in the cell cycle. Toxic influence, expressed in repression of root growth, can lead to a decrease in proliferative activity of cells and hence the positive correlation between root growth inhibition and mitotic inhibition observed. A decline of MI below 22% in comparison to negative control can have a lethal impact on the organism (Antonsiewicz Citation1990), while a decrease below 50% usually has sublethal effects (Panda and Sahu Citation1985) and is called the cytotoxic limit value (Sharma Citation1983). The decrease in the MI explains the potential cytotoxicity and suggests the inhibitory, mitodepressive and anti-proliferative effects of the tested extracts on A. cepa root tip meristem cells.
MI measures the proportion of cells in the M-phase of the cell cycle and its inhibition could be interpreted as cellular death or a delay in the cell proliferation kinetics (Rojas et al. Citation1993). Reduction in the mitotic activity could be due to inhibition of DNA synthesis or a blocking in the G2 phase of the cell cycle, preventing the cell from entering mitosis (Sudhakar et al. Citation2001). Mitodepressive effects of some plant extracts, e.g. the ability to block the synthesis of DNA and nucleoproteins, was reported earlier (Mercykutty and Stephen Citation1980; Schulze and Krischer Citation1996). They probably inhibit the initiation of their biosynthesis; and such action occurring in the interphase nucleus, apart from influencing the ultimate structure of the chromosome during cell division, could also cause reduction of number of other stages (Akinboro and Bakare Citation2007). Reduction in mitotic activity can also be due to impaired nucleoprotein synthesis and reduced level of ATP to provide energy for spindle elongation, microtubule dynamics and chromosomal movement (Majewska et al. Citation2003).
Chromosomal aberrations are changes in chromosome structure resulting from a break or exchange of chromosomal material. The aberrations induced by ASM and ANL were not significantly different from those of the negative control except at a concentration of 5 mg ml–1 of both extracts, while ALC was found to induce chromosomal aberrations that are significantly different from the negative control. This shows weak genotoxicity of ASM, ANL and ALC. The observed chromosomal aberrations may be due to the presence of certain phytochemical components of the extracts. Some naturally occurring compounds in plants such as polyphenols, alkaloids and tannins, have been implicated in causing chromosomal damage at certain concentrations (Ene and Osuala Citation1990; Yamanaka et al. Citation1997; Hayakawa et al. Citation1999). The aberrations observed herein might be due to the presence of alkaloids and tannins in these extracts as the preliminary phytochemical analysis of these extracts showed the presence of alkaloids, tannins, saponins, steroids, terpene, flavonoids, phenolics, anthraquinones and cardiac glycoside. This is in line with previous reports on the phytochemical analysis of the tested plant extracts (Akinjogunla et al. Citation2010; Balakrishnan and Huria Citation2011; Muthumani et al. Citation2011; Omotayo and Borokinni 2012).
Lead is naturally found in small amounts in the earth crust and is largely used in the production of containers of foods, stills, batteries, paints, and leathers. Human activities such as burning of fossil fuels, mining, and manufacturing are sources of lead (Truta et al. Citation2011). Dash et al. (Citation1988) reported that lead has diverse effects on the cell, some of which are enzyme inhibition, chromosome aberration, mutation and clastogenic effects leading to spindle impairment and malfunction. Lead nitrate has been reported to be mutagenic in A. cepa (Liu et al. Citation1994), wheat (Truta et al. Citation2011), mice (Madhavi et al. Citation2007) and human cultured cells (Yedjou and Tchounwou Citation2007).
In this study, lead nitrate induced aberrations such as sticky chromosomes, disturbed spindles and fragments. ASM and ANL reduced lead nitrate induced chromosomal aberration in A. cepa meristematic cells. Although ALC induced chromosomal aberrations, it also reversed lead nitrate induced aberrations at concentrations 5, 10 and 20 mg ml–1, showing it to be anti-genotoxic at these concentrations. According to Ferguson (Citation2001), there is evidence that certain compounds can both induce and prevent DNA damage. Crude extracts are known to be composed of complex mixtures of phytochemicals which may work synergistically, additively or antagonistically. The decreased frequency of aberrations in the lead nitrate + ALC group may be as a result of decreased levels of the component responsible for the observed aberrations in the group exposed to ALC alone.
Most of the observed abnormalities were due to spindle failure (disturbed spindles at metaphase and anaphase, vagrant, disoriented chromosomes or chromatin dysfunction such as stickiness). This shows these extracts to be spindle inhibitors. Drugs that inhibit the function of the mitotic spindle have been proven to be exceptionally successful in the clinic as chemotherapeutic anti-cancer drugs (Schmidt and Bastians Citation2007). This may be responsible for the success acclaimed with these plants in the traditional management of cancer; however they may be genotoxic to normal cells if they are not selective in their mode of action.
To the best of our knowledge, this is the first report on the genotoxicity and anti-genotoxicity of L. cylindrica and the first report on the genotoxicity and anti-genotoxicity of S. mombin and N. lotus in a plant system. Spondias mombin and N. lotus appear to act as biomutagens, acting on the repair and replication of lead induced DNA damage. However, inhibition of mutagenesis is often complex, acting through multiple mechanisms (Musarrat et al. Citation2006). Luffa cylindrica induced genotoxic and anti-genotoxic effects in A. cepa probably because the components have the ability to interact synergistically and antagonistically on different occasions on the genetic material in the test system. Further study is needed to isolate the bioactive components in the tested plants. We are currently assessing the mutagenic and anti-mutagenic effect as well as oxidative damage of aqueous and methanolic extracts of these plants in vivo.
Acknowledgments
This study was partly funded by the Alexander Von Humboldt Return Fellowship to AAB and the University of Ibadan Postgraduate School scholarship to ITO. We thank Dr. I.O. Oladosu of the Department of Chemistry for his assistance on the phytochemical analysis of the extracts and Prof. A. E. Ayodele of Department of Botany for his assistance in plant selection.
References
- Akinboro A, Bakare AA. 2007. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa (Linn.). J Ethnopharmacol. 112(3):470–475.
- Akinjogunla JO, Yah CS, Eghafona NO, Ogbemudia FO. 2010. Antibacterial activity of Nymphaea lotus (Nymphaeaceae) leaf extract against methicillin resistant Staphylococcus aureus (MRSA) and vancomycin resistant Staphylococcus aureus (VRSA) from clinical samples. Elect J Environ Agric Food Chem. 9(6):1064–1073.
- Antonsiewicz D. 1990. Analysis of the cell cycle in the root meristem of Allium cepa under the influence of ledakrin. Folia Histochem Cytobiol. 26:79–96.
- Bakare AA, Adeyemi AO, Adeyemi A, Alabi OA, Osibanjo O. 2012. Cytogenotoxic effects of electronic waste leachate in Allium cepa. Caryologia. 65(2):94–100.
- Bakare AA, Alabi OA, Gbadebo AM, Ogunsuyi OI, Alimba CG. 2013. In vivo cytogenotoxicity and oxidative stress induced by electronic waste leachate and contaminated well water. Challenges. 4(2):169–187.
- Bakare AA, Mosuro AA, Osibanjo O. 2000. Effect of simulated leachate on chromosomes and mitosis in roots of Allium cepa (L). J Environ Biol. 21(3):263–271.
- Bakare AA, Wale-Adeyemo AR. 2004. The mutagenic and cytotoxic effects of leachates from domestic solid wastes and Aba-Eku landfill, Nigeria on Allium cepa. Nat Environ Poll Technol. 3:455–462.
- Balakrishnan N, Huria T. 2011. Protective effect of Luffa cylindrica L. fruit in paracetamol induced hepatotoxicity in rats. Intern J Pharmac Biol Arch. 2(6):1761–1764.
- Corthout J, Pieters LA, Claeys M, Vanden Berghe DA, Viletinck AJ. 1994. Antibacterial and molluscicidal phenolic acid from Spondias mombin. Planta Medica. 60:460–463.
- Dash S, Panda KK, Pada BB. 1988. Biomonitoring of low levels of mercurial derivatives in water and soil by Allium micronucleus assay. Mutat Res. 203:11–21.
- Ene EE, Osuala CL. 1990. The mutagenic potentials of water extracts of Borreria filiformis (Hiern) Hatch and Dalz. and Vince rosea Linn. Nig J Bot. 3:35–40.
- Esimone CK, Omobuwajo RL, Sowemimo AA, Proksch P. 2006. Single-cycle vector based antiviral screening assays for high throughput evaluation of potential anti-HIV medicinal plants: a pilot study on some Nigerian herbs. Rec Progr Med Plant Res, Phytopharmacol Therap Values I. 19:50–60.
- Farnsworth NR, Akelere O, Bingel AS, Soejarto DD, Guo Z. 1985. Medicinal plants in therapy. WHO Bull. 63:965–981.
- Ferguson LR. 1994. Antimutagens as cancer chemopreventive agents in the diet. Mutat Res. 307:395–410.
- Ferguson LR. 2001. Role of plant polyphenols in genomic stability. Mutat Res. 475:89–111.
- Fiskesjö G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 102:99–112.
- Fiskesjö G. 1997. Allium test for screening chemicals; evaluation of cytologic parameters. In: Wang W, Gorsuch JW, Hughes JS, eds. Plants for Environmental Studies. Boca Raton, New York: CRC Lewis Publishers, 308–333.
- Frescura VD, Kuhn AW, Laughinghouse HD IV, Nicoloso FT, Lopes SJ, Tedesco SB. 2013. Evaluation of the allelopathic, genotoxic, and antiproliferative effect of the medicinal species Psychotria brachypoda and Psychotria birotula (Rubiaceae) on the germination and cell division of Eruca sativa (Brassicaceae). Caryologia. 66(2):138–144.
- Frescura VD, Laughinghouse HD, Tedesco SB. 2012. Antiproliferative effect of the tree and medicinal species Luehea divaricata on the Allium cepa cell cycle. Caryologia. 65(1):27–33.
- Hamenoo NA. 2010. Hepatoprotective and toxicological assessment of Spondias mombin l. (Anacardiaceae) in rodents. [M. Phil project]. [Kumasi]: Department of Pharmacology, Kwame Nkrumah University of Science & Technology.
- Hassan A, Rahman S, Deeba F, Mahmud S. 2009. Antimicrobial activity of some plant extracts having hepatoprotective effects. J Med Plants Res. 3(1):20–23.
- Hayakawa F, Kimura T, Hoshimo N, Ando T. 1999. DNA cleavage activities of (–)-epigallocatechin, (–)-epicatechin, (+)-catechin, and (–)-epigallocatechin gallate with various kind of metal ions. Biosci Biotechnol Biochem. 63:1654–1656.
- Hoejimakers JH. 2001. Genome maintenance mechanisms for preventing cancer. Nature. 411:366–374.
- Ismail M, Hussain MM, Dastagir MG, Billah M, Quader A. 2010. Phytochemical and antimicrobial investigation of Luffa cylindrica. BLACPMA. 9(4):327–332.
- Lippman SM, Benner SE, Hong WK. 1994. Cancer chemoprevention. J Clin Oncol. 12:851–873.
- Liu D, Jiang W, Wang W, Zhao F, Lu C. 1994. Effect of lead on root growth, cell division and nucleolus of Allium cepa. Environ Poll. 86:1–4.
- Madhavi D, Devi KR, Rao KK, Reddy PP. 2007. Modulating effect of Phyllanthus fruit extract against lead genotoxicity in germ cells of mice. J Environ Biol. 28(1):115–117.
- Majewska A, Wolska E, Śliwińska E, Furmanowa M, Urbańska N, Pietrosiuk A, Zobel A, Kuraś M. 2003. Antimitotic effect, G2/M accumulation, chromosomal and ultrastructure changes in meristematic cells of Allium cepa L. root tips treated with the extract from Rhadiola rosea roots. Caryologia. 56:337–351.
- Mercykutty VC, Stephen J. 1980. Adriamycin induced genetic toxicity as demonstrated by Allium cepa test. Cytologia. 45:769–777.
- Mitscher LA, Drake S, Gollapuri SR, Harris JA, Shankel DM. 1986. Antimutagenesis and anticarcinogenesis mechanisms. In: Shankel DM, Hartmen PE, Kada T, Hollaender A, editors. New York: Plenum Press
- Musarrat J, Aqil F, Ahmad I. 2006. Mutagenicity and antimutagenicity of medicinal plants. In: Ahmad I, Aqil F, Owais M, editors. Modern phytomedicine. Weinheim: Wiley-VCH, 271.
- Muthumani P, Meera R, Mary S, Jeena M, Devi P, Kameswari B, Priya BE. 2011. Phytochemical screening and anti inflammatory, bronchodilator and antimicrobial activities of the Seeds of Luffa cylindrica. Res J Pharmac Biol Chem Sci. 1(4):11–22.
- Nabeel M, Abderrahman S, Papini A. 2008. Cytogenetic effect of Arum maculatum extract on the bone marrow cells of mice. Caryologia. 61 (4): 383–387.
- Omotayo FE, Borokini TI. 2012. Comparative phytochemical and ethnomedicinal survey of selected medicinal plants in Nigeria. Sci Res Essays. 7(9):S989–999.
- Oyedare BM, Bakare AA, Akinboro A. 2009. Genotoxicity assessment of water extracts of Ocimum gratissimum, Morinda lucida and Citrus medica using the Allium cepa assay. BLACPMA. 8(2):97–103.
- Pal RK, Manoj J. 2011. Hepatoprotective activity of alcoholic and aqueous extracts of fruits of Luffa cylindrica Linn in rats. Annal Biol Res. 2(1):132–141.
- Panda BB, Sahu UK. 1985. Induction of abnormal spindle function and cytokinesis inhibition in mitotic cells of Allium cepa by the organophosphorus insecticide fensulfothion. Cytobios. 42(167–168):147–155.
- Parekh J, Chanda S. 2007. Antibacterial and phytochemical studies on twelve species of Indian medicinal plants. Afri J Biomed Res. 10:175–181.
- Pastori T, Flores FC, Boligon AA, Athayde ML, da Silva Cde B, do Canto-Dorow TS, Tedesco SB. 2013. Genotoxic effects of Campomanesia xanthocarpa extracts on Allium cepa vegetal system. Pharmac Biol. 51(10):1249–1255.
- Qizhen D, Yuanjin X, Lei L, Yang Z, Gerold J, Peter W. 2006. Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. J Agric Food Chem. 54:4186–4190.
- Rojas E, Herrera LA, Sordo M, Gonsebatt ME, Montero R, Rodriguez R, Ostrosky-wegman P. 1993. Mitotic index and cell proliferation kinetics for identification of antineoplastic activity. Anti-Cancer Drugs. 46):637–640.
- Santos FV, Mesquita SFP, Faria MJSS, Poersh A, Maciel MAM, Pinto AC, Morimoto HK, Cólus IMS. 2006. Absence of mutagenicity in somatic and germ cells of mice submitted to subchronic treatment with an extract of Croton cajucara Benth. (Euphorbiaceae). GenetMol. Biol. 29(1):159–165.
- Schmidt M, Bastians H. 2007. Mitotic drug targets and the development of novel anti-mitotic anticancer drugs. Drug Resist Updates. 10:162–181.
- Schulze E, Krischer S. 1996. Microtubule dynamics in interphase. Cell J Cell Biol. 102:1020–1021.
- Sharma CBSR. 1983. Plant meristems as monitors of genetic toxicity of environmental chemicals. Curr Sci. 52:1000–1002.
- Shete RV, Pawashe PM, Kore KJ, Otari KV. 2011. Protective role of Luffa cylindrica Linn against erythromycin estolate induced hepatotoxicity. Curr Pharm Res. 1(4):315–319.
- Sofowora A. 1993. Medicinal plants and traditional medicine in Africa. Ibadan (Nigeria): Spectrum Books, 191–289.
- Sudhakar R, Ninge Gowda KN, Venu G. 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 66(3):235–239.
- Sreeranjini S, Siril EA. 2011. Evaluation of anti-genotoxicity of the leaf Morinda citrifolia Linn. Plant Soil Environ. 57(5):222–227.
- Steinmetz KA, Potter JD. 1996. Vegetables, fruits and cancer prevention: a review of the epidemiological evidence. Nutrit Cancer. 18:1–29.
- Sultan AO, Celik TA. 2009. Genotoxic and antimutagenic effects of Capparis spinosa L. on the Allium cepa L. root tip meristem cells. Caryologia. 62(2):114–123.
- Teixeira RO, Camparoto ML, Mantovani MS, Vicentini VEP. 2003. Assessment of two medicinal plants, Psidium guajava L. and Achillea millefolium L., in in vitro and in vivo assays. Genet Mol Biol. 26(4):551–555.
- Trease GE, Evans WC. 1989. Pharmacognosy. 11th ed. London: Bailliere Tindall, 45–50.
- Truta E, Rosu CM, Bara IC. 2011. Lead-induced genotoxicity in wheat. Analele stiintifice ale Universitatii “Alexandru Ioan Cuza”. Sectiunea Genetica si Biologie Moleculara TOM XII. 51–58.
- Yamanaka N, Oda O, Nagao S. 1997. Green tea catechins such as (–)-epicatechin and (–)-epigallocatechin accelerate Cu2+ induced low density lipoprotein oxidation in propagation phase. FEBS Letters. 401:230–234.
- Yedjou CG, Tchounwou PB. 2007. N-acetyl-L-cysteine affords protection against lead-induced cytotoxicity and oxidative stress in human liver carcinoma (HEPG2) cells. Intern J Environ Res Pub Health. 4(2):132–137.
- Yisa J. 2009. Phytochemical analysis and antimicrobial activity of Scoparia dulcis and Nymphaea lotus. Austr J Bas Appl Sci. 3(4):3975–3979.