Abstract
Cytological features and karyomorphology of two endemic giant pill-millipedes (Arthrosphaera fumosa and A. magna) of the Western Ghats were studied based on the meiotic cell divisions using Giemsa staining technique. Different meiotic stages of spermatogonial cell divisions were compared and karyotypes were constructed from the metaphase stages. The diploid chromosome number, 2n = 30, in A. fumosa as well as A. magna with XY sex determination system was seen. Most of the meiotic chromosome behaviors were similar between the millipede species. All meiotic chromosomes in A. fumosa belonged to acrocentric type, while in A. magna 27 were acrocentrics, two (3rd and 10th) were sub-metacentrics and one (5th) was metacentric. The relative lengths of autosomes in A. fumosa and A. magna were 21.9–109.3 and 38.8–90.6, respectively. The X chromosome was longer than the Y chromosome in both millipedes. The relative lengths of the X chromosomes of A. fumosa and A. magna were 130.1 and 103.6, while for the Y chromosome they were 113.6 and 92.6, respectively. Based on this study and earlier cytological and karyological studies on Arthrosphaera, the diploid chromosome number has been predicted to be higher than 30. Further assessment is needed of identified and unidentified species distributed in the Western Ghats of India and other geographic locations to follow the chromosomal evolutionary pattern.
Introduction
The millipedes (Class: Diplopoda), being major saprophagous fauna, are involved in mechanical fragmentation of organic matter in the soil, which enhances the rate of decomposition by the gut as well as by soil microorganisms (Hopkin and Read Citation1992). Although the diplopods consist of ~80,000 species, only up to 12,000 species have systematic description (Pitz and Sierwald Citation2010). The Order Sphaerotheriida is represented by ~325 species of giant pill-millipedes with patchy distribution in South Africa, Madagascar, the Oriental region, New Zealand and Australia (Pocock Citation1892, Citation1899; Wesener and van den Spiegel Citation2009; Wesener et al. Citation2010). The recent molecular studies revealed that the giant pill-millipede Arthrosphaera brandtii (Family: Arthrosphaeridae) in Madagascar belongs to paraphyletic group of the genus Spaheromimus, which is the sister taxon of the genus Arthrosphaera of the Indian Subcontinent (Wesener et al. Citation2010). Currently, distribution of giant pill-millipedes of the genus Arthrosphaera is confined to the regions of Southern India, Sri Lanka and Madagascar (Wesener and van den Spiegel Citation2009; Wesener et al. Citation2010).
So far, 0.1% of diplopods have been analyzed for chromosomes and most studies were confined to the giant pill-millipedes of the Indian Subcontinent (Fontanetti et al. Citation2002). Most cytological explorations of Diplopoda are from the Oriental Zoogeographic region (Indian species) and a few studies are available from outside the Asia Pacific (Achar Citation1987; Fontanetti et al. Citation2002; Kadamannaya et al. Citation2010). Cytological studies on Arthrosphaera have evolutionary significance because of their endemism and disjunct distribution. The low vagility of Arthrosphaera has attracted cytologists to study the chromosomal evolution of speciation (Achar Citation1987). The giant pill-millipede has been tagged for the highest number of chromosomes in the Class Diplopoda (Fontanetti Citation2000). The diploid chromosome number of Arthrosphaera varies between 26 and 30 with a male XY and female XX sex determination pattern (Bessiere Citation1948; Achar Citation1983b). Chowdaiah (Citation1966c) initiated cytological studies on Arthrosphaera using spermatogonial meiotic stages of Arthrosphaera zebraica sampled from the Sorab region of Karnataka, Southern India. Subsequently, Achar (Citation1983b, Citation1984a, Citation1986) explored the karyotypes of some more Arthrosphaera species of the Western Ghats using testicular epithelium and opined that chromosomal evolution occurred mainly due to Robertsonian rearrangement and pericentric inversions. Most recently, Kadamannaya et al. (Citation2010) compared the karyotypes of Arthrosphaera dalyi and A. zebraica. In order to extend the investigations on Arthrosphaera, the present study reports the cytology and karyotype of two Arthrosphaera species (A. fumosa and A. manga) distributed in the Western Ghats of India, based on spermatogonial meiosis.
Materials and methods
Pill-millipedes and sampling
Arthrosphaera fumosa Pocock is known from the high altitudes, while Arthrosphaera magna Attems is mainly confined to the foothill regions of the Western Ghats in India (Ambarish and Sridhar Citation2013). Adult individuals of A. fumosa and A. magna were randomly collected from Karike (12°45′ N, 75°38′ E) and Adyanadka (12°41′ N, 75°6′ E), respectively during the rainy season (July–September 2012). They were identified based on the keys by Pocock (Citation1899) and Attems (Citation1936). Representative specimens were deposited in the Zoological Museum, Department of Applied Zoology, Mangalore University (Arthrosphaera fumosa: MUAZPMAf-01; Arthrosphaera magna: MUAZPMAm-02). Animals were maintained in glass tanks in the laboratory by offering decomposing mixed moist leaf litter. After acclimatization, active adult males were chosen for the cytological studies.
Cytological preparations
Testes were dissected out in normal saline from millipedes after 4 h of colchicine treatment (0.5%, 0.5 ml). The whole testis pre-treatment was carried out for 1 h at 37°C in different molarities of KCl (0.16–0.21 M). According to the methodology outlined by Achar (Citation1983b), pre-treated testes were minced thoroughly in normal saline and centrifuged at 1100 rpm for 5 min. The cell button obtained was re-suspended in hypotonic solution and the supernatant was discarded. After two changes in hypotonic solution, the cell button was centrifuged and fixed in a fresh fixative (acetic acid-methanol, 1:3) for 30 min. After two changes in fixative, the material was re-suspended in a small quantity of fresh fixative. Cell suspension was dropped on ice cold slides, air-dried and preserved. The slides were treated with Giemsa (2.5%) stain and observed for chromosomes under the 100× objective of a microscope (Olympus-CX41RF, Center Valley, PA, USA).
Karyotype
Different meiotic stages were screened for chromosomal behavior and the spermatogonial metaphase chromosomes were used for construction of karyotype according to the method of Levan et al. (Citation1964). Measurement of individual chromosome length from the well-spread metaphase plate was taken in pixels and converted into centimeters to calculate the relative length (RL = [length of the chromosome/total length of haploid set] × 1000) and centromeric index (CI = [length of the short arm/length of the chromosome] × 100). The average of all the chromosomes (including the sex pair) was considered for calculation of the RL.
Based on the size, 1–14 autosomal pairs were arranged in descending order followed by the largest pair of sex chromosomes X and Y.
Results
Arthrosphaera fumosa
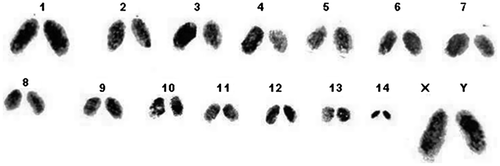
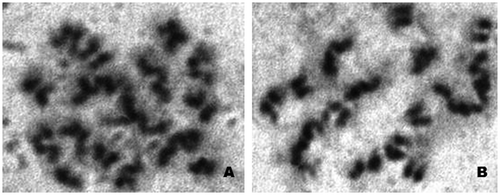
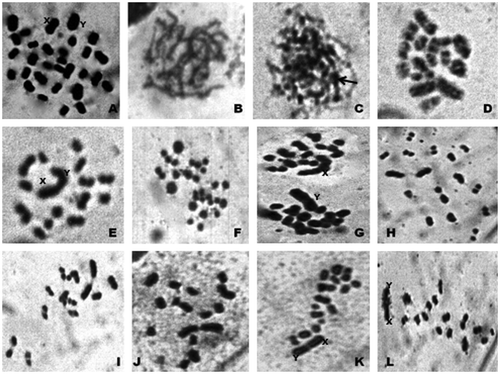
The diploid chromosomal complex of A. fumosa consists of 30 acrocentric chromosomes (Figure ). The sex chromosome pair was conspicuous, heteromorphic, the chromosome X was slightly larger than the Y and relatively darkly stained. The sex chromosomes were disposed at the periphery, while the seriation of autosomes was gradual. Different stages of chromosomes in early meiotic prophase were clearly visible. The pachytene stage was recognized by pairing of homologous chromosomes along the length. Characteristic fuzziness was seen in diplotene stages. In diakinesis, the chromosomes were more condensed and chiasmata were situated at their terminal part. The late diakinesis shows terminalization of chiasmata, which was incomplete in sex chromosomes. Meiotic metaphase-I exhibited 15 dumbbell-shaped bivalent chromosomes. The sex chromosomes showed lagging during the first meiotic division, but they showed normal movement along with autosomes in anaphase-II. Figure reveals a typical karyotype of 28 acrocentric autosomes with two sex chromosomes (X and Y). The relative length of autosomes of A. fumosa ranged between 21.9 and 109.3, while for sex chromosomes the range was 113.6–130.1 (Table ). During cytological analysis of whole testis, a few plates of mitotic metaphase from the testicular epithelium were also seen (Figure ). In the mitotic metaphase, it is difficult to distinguish the sex chromosomes as clearly as in spermatogonial metaphase.
Figure 1. Photomicrographs of chromosomes in male meiosis of Arthrosphaera fumosa (2n = 30): A, spermatogonial metaphase; B, pachytene; C, diplotene; D, diakinesis; E, metaphase-I (side view); F, early anaphase; G, anaphase; H, anaphase (two sets) (arrow, lagging of sex chromosomes); I, dia-metaphase-I; J, metaphase-II; K, metaphase-I (polar view); L, early anaphase-II.
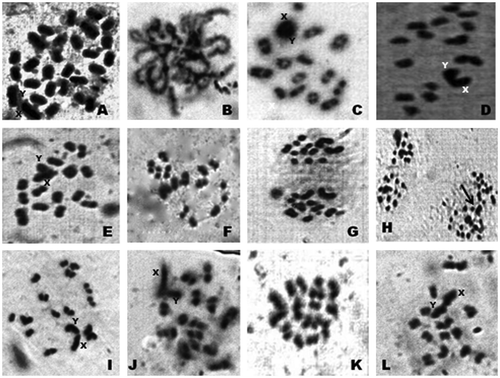
Table 1. Relative length (RL) (n = 5 ± SD), centromeric index and nature of chromosomes of Arthrosphaera fumosa and Arthrosphaera magna based on karyotype analysis (2n = 30).
Arthrosphaera magna
The diploid chromosome number in spermatogonial metaphase was 30, encompassing acrocentrics, metacentric and sub-metacentrics (Figure ). The X and Y constituted the largest pair of acrocentric heteromorphic chromosomes. The X chromosome was slightly larger compared to the Y chromosome. Among the autosomes, all belonged to acrocentrics except for the 3rd, 5th and 10th pairs. The 5th pair was metacentric, while the 3rd and 10th pairs were sub-metacentrics. Observation of different stages of meiotic prophase revealed the pairing of homologous chromosomes all along the length in pachytene stage. In zygotene, the chromosomes appeared as threads with several darkly stained parts. The diakinesis stage was characterized by highly condensed conspicuous bivalents and the sex dyad was very conspicuous due to its characteristic configuration. Terminalization of chiasmata of sex chromosome pair was incomplete even during late diakinesis. Metaphase-I consists of highly condensed clearly visible chromosomes. In anaphase-I, sex chromosomes exhibited lagging because of their large size, however, they showed normal segregation in anaphase-II and separation of sister chromatids were clearly visible. Figure represents the karyotype of A. magna showing acrocentric, metacentric and sub-metacentric chromosomes along with sex pair. The relative length of autosomes ranged from 38.8 to 90.6, while for the sex chromosomes the range was 92.6–103.6 (Table ).
Figure 4. Photomicrographs of chromosomes in male meiosis of Arthrosphaera magna (2n = 30): A, spermatogonial metaphase; B, pachytene; C, zygotene (arrow, darkly stained region); D, diplotene; E, diakinesis; F, early anaphase; G, anaphase-I; H and I, metaphase-II; J, metaphase-I (polar view); K, metaphase-I (side view); L, early anaphase-II.
Discussion
Chromosome features
Application of air-drying method using Giemsa staining serves as one of the most efficient techniques for cytological investigations of diplopods (Achar and Chowdaiah Citation1979). However, cytological studies in Arthrosphaera are hampered due to difficulties in obtaining female metaphase chromosomes. Moreover, meiotic metaphase chromosomal analysis is less efficient compared to that of mitotic metaphase for construction of karyotype. The behavior of meiotic chromosomes of A. fumosa and A. magna in the present study was almost similar in exhibiting a characteristic diffused state of chromosomes in prophase as seen in many diplopods (Chowdaiah and Kanaka Citation1979; Achar Citation1987; Fontanetti et al. Citation2002). The pachytene stages were represented by chromosome pairing along the length, while in zygotene they appear like threads with darkly stained regions. No bouquet formation was noticed in the zygotene stage, as reported in A. craspedota and A. disticta (Chowdaiah and Kanaka Citation1974; Ambarish and Sridhar, unpublished observations). In metaphase-I, chromosomes were highly conspicuous and attained a dumbbell shape due to high condensation. The sex chromosomes showed lagging in anaphase-I followed by normal segregation in the second meiotic division. Diplotene was represented by highly condensed as well as less condensed regions of chromosomes. Pycnotic condensation was a common phenomenon in Arthrosphaera as suggested by Achar (Citation1984a), and the number of pycnotic knobs precisely denotes the chromosome number of a specific species. Arthrosphaera zebraica had a characteristic single chromatid bridge in anaphase-I (Chowdaiah Citation1966a), while such a feature was not evident in our study.
Arthrosphaera has the highest number of chromosomes among the diplopods, with a majority of acrocentrics (Chowdaiah and Kanaka Citation1974). Chromosomes of Arthrosphaera were mainly grouped into large (allosomes) and small (autosomes) categories (Achar Citation1984a). A few large and many small chromosomes are the striking feature of giant pill-millipedes, which has been considered as a mechanism of spindle adaptation (Chowdaiah and Kanaka Citation1974). Lewis and John (Citation1963) opined that more chromosomes can be accommodated on the spindle due to their small size. Our study supports an XY type of sex determination in A. fumosa and A. magna, which is of a primitive type as they were less clearly differentiable from autosomes. Similarly, the allosomes were less distinguishable from autosomes in many species of diplopods in Brazil (Fontanetti et al. Citation2002) as well as in India (Achar Citation1983b). They exhibited lagging during the first meiotic division and behaved normally during the second equational division. According to Chowdaiah (Citation1966b), pre-reduction in first meiosis followed by successions are the two important features of sex chromosomes in giant pill-millipedes. Although the majority of species in Diplopoda showed an XY type of sex determination mechanism, including the genus Arthrosphaera, in some instances XO/XXO sex determination mechanisms were reported from India (Achar and Chowdaiah Citation1980; Achar Citation1984a, Citation1986). Natarajan (Citation1959) showed XO sex determination in the millipede Phyllogonostreptus nigrolabiatus. This study was reconfirmed by the report by Sharma and Handa (Citation1974) indicating XO and XXO types of sex determination in Gonoplectus malayus and P. nigrolabiatus, respectively.
Studies on the temperate giant pill-millipedes (genus Glomeris) have shown a few important characteristic chromosomal behaviors (Warchałowska-Śliwa et al. Citation2004). Glomeris hexasticha and G. connexa possess 2n = 16 with an XY sex determination pattern. Occurrence of paracentromeric C-bands was an important observation in Glomeris based on C-heterochromatin analysis. The C-band thickness as well numbers varied between allosomes and autosomes in Glomeris spp. The 36 G-bands of Carlogonus acifer and identical bands of Glomeris were the two major banding studies applied on diplopods (Achar and Chowdaiah Citation1980; Achar Citation1983a; Warchałowska-Śliwa et al. Citation2004). About 60% of heterochromatin content in the genome of diplopods of unrelated orders (e.g. Acanthopetalum sicanum and Enologus oxypygum) was reported from Spain (Vitturi et al. Citation1997). In two species of diplopods of the genus Pseudonannolene (P. silvestris and P. tocaiensis), a high content of constitutive heterochromatin has been reported (Da Silva et al. Citation2005). In P. tocaiensis, the constitutive heterochromatin region is highly condensed and exhibits bouquet formation (De Freitas et al. Citation2003). However, so far none of the Arthrosphaera spp. were subjected to heterochromatin and chromosome banding studies.
Karyotype evolution
The range of relative length of autosomes (21.9–109.3) and sex chromosomes (XY, 130.1 and 113.6) in A. fumosa are not comparable with other Arthrosphaera [A. dalyi (39.4–99.9) (XY, 194.7 and 161), A. disticta (30.1–91) (XY, 139.1 and 117.1) and A. zebraica (35.80–90.7) (XY, 177.8 and 126.1)] (Kadamannaya et al. Citation2010). The range of relative length of autosomes (38.8–90.6) of A. magna is partially comparable with that of A. zebraica. In our study, A. fumosa and A. magna consist of 30 and 27 acrocentric chromosomes, respectively, supporting the earlier studies in the family Sphaerotheriidae (Achar Citation1983b). As some species of Arthrosphaera consist of metacentric and sub-metacentric autosomes (Table ) (e.g. A. dalyi, 2n = 30; A. davisoni, 2n = 26; A. magna, 2n = 30; A. nitida, 2n = 30), it is predicted that the genus Arthrosphaera is represented by chromosomes more than 2n = 30.
Table 2. Chromosome number and nature of chromosomes in Arthrosphaera.
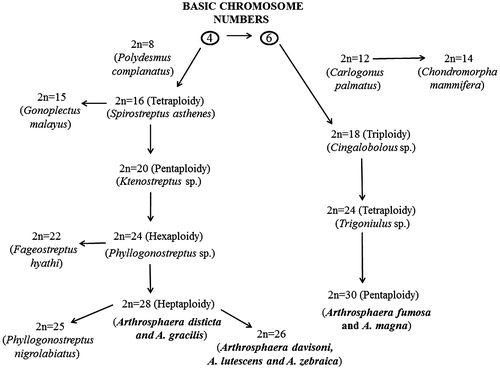
The diploid chromosome number in Diplopoda ranges from 8 to 30 and it was proposed by Achar (Citation1987) that the diplopod chromosome number had evolved from the basic chromosome array of 4 and 6. Robertsonian rearrangement and pericentric inversions were the two major events resulting in altered chromosome number in opposite directions during the evolution of karyotype in Diplopoda (Achar Citation1984a). Centric fusion resulted in association, leading to a decreased number of chromosomes, while centric fission resulted in dissociation of chromosomes. Two arrays of chromosome number can be predicted from the Diplopoda including the giant pill-millipedes investigated so far, and the directionality of karyotype evolution has been given with specific examples in Figure . The first array with 4 as a basic chromosome number shows four polyploidy levels: tetraploidy (2n = 16) (e.g. Spirostreptus asthenes), pentaploidy (2n = 20) (e.g. Ktenostreptus sp.), hexaploidy (2n = 24) (e.g. Phyllogonostreptus sp.) and heptaploidy (2n = 28) (e.g. Arthrosphaera disticta and A. gracilis). The second array with 6 as a basic chromosome number shows three ploidy levels: triplody (2n = 18) (e.g. Cingalobolous sp.), tetraploidy (2n = 24) (e.g. Trigoniulus sp.) and pentaploidy (2n = 30) (e.g. Arthrosphaera magna and A. fumosa). Both 4 and 6 chromosome series consist of the genus Arthrosphaera (A. disticta, 2n = 28; A. fumosa and A. magna, 2n = 30), occupying the last group in the direction of evolution with the highest chromosome number. Some diplopods showed diploid chromosome numbers of 14 (e.g. Chondromorpha mammifera), 15 (e.g. Gonoplectus malayus), 22 (e.g. Fageostreptus hyathi), 25 (e.g. Phyllogonostreptus nigrolabiatus) and 26 (e.g. Arthrosphaera davisoni, A. lutescens and A. zebraica. The diploid chromosome number 26 is the lowest chromosome number in the genus Arthrosphaera so far reported, which stands as an outlier of the group like other aberrant groups of Diplopoda, indicating the possibility of duplication of the basic complement due to evolutionary forces like polysomy or Robertsonian changes. Most of the chromosome numbers are multiples of the basic numbers 4 or 6 and an extensive ploidy seen in Polydesmus gracilis (Achar Citation1984b) is major evidence for polyploidy being one of the most important factors in karyotype evolution in diplopods.
Conclusions and outlook
So far, no cytological investigations have been carried out on A. fumosa, while only the karyotype was constructed for A. magna (Achar Citation1986). The present study added information pertaining to cytology and karyotype of these two tropical endemic giant pill-millipedes. There is a wide gap in chromosomal studies of female Arthrosphaera, preventing more precise understanding of the X chromosomes. It is likely that cultured tissues of female Arthrosphaera will provide clearer picture of autosomes as well as allosomes. In addition, banding techniques (e.g. NOR silver staining and fluorescence tagging) will help to study the Arthrosphaera chromosomes more precisely. Based on the present and earlier investigations, it is possible to predict that the diploid chromosome number of Arthrosphaera is higher than 30, as sub-metacentrics and metacentrics exist in some species (e.g. A. dalyi, A. magna and A. nitida: 2n = 30). Further investigations on the cytology and karyotype of identified, unidentified and allied species of Arthrosphaera existing in India, Sri Lanka and Madagascar may provide a clear perspective on the evolutionary pattern.
Acknowledgments
The authors are grateful to Mangalore University for permission to carry out this study in the Department of Biosciences. One of us (CNA) acknowledges the award of Fellowship, Department of Science and Technology, New Delhi, Government of India (DST/INSPIRE Fellowship/2011/(294): Award # IF110540). We are thankful to Drs. Jayaprakash and Nandini, N., Bangalore University, Bangalore for help in retrieving relevant literature.
References
- Achar KP. 1983a. The use of G-banding technique in the chromosome studies of a millipede species – Spirostreptus asthenes. Current Science. 52(11):540–543.
- Achar KP. 1983b. Karyological studies in nine species of Indian Diplopoda (Myriapoda). Nucleus. 26(3):191–197.
- Achar KP. 1984a. Analysis of male meiosis in five species of Indian Diplopoda (Myriapoda). Caryologia. 37(4):373–386.
- Achar KP. 1984b. Chromosome studies in Indian Diplopoda (Myriapoda) I. A note on the occurrence of polyploidy in Polydesmusgracilis. Current Science. 53(14):764–767.
- Achar KP. 1986. Analysis of male meiosis in seven species of Indian pill-millipede (Diplopoda: Myriapoda). Caryologia. 39(1):89–101.
- Achar KP. 1987. Chromosomal evolution in Diplopoda (Myriapoda: Arthropoda). Caryologia. 40(1–2):145–155.
- Achar KP, Chowdaiah BN. 1979. The use of air drying in the study of diplopod chromosomes. In: Camatini M, editor. Myriapod Biology. London: Academic Press. p. 21–23.
- Achar KP, Chowdaiah BN. 1980. The use of C-banding technique in the chromosome studies of a millipede species – Carlogonus acifer. Caryologia. 33(2):185–191.
- Ambarish CN, Sridhar KR. 2013. Observation on pill-millipedes of the Western Ghats (India). Journal of Agricultural Technology. 9(1):61–79.
- Attems C. 1936. Diplopods of India. Memoirs of the Indian Museum. 11:133–167.
- Bessiere C. 1948. La spermatogenese de quelques Myriapods diplopods. Archives de Zoologie Expérimentaleet Générale. 85(3):149–236.
- Chowdaiah BN. 1966a. Chromosome studies in two species of pill-millipedes (Diplopoda -Myriapoda). Caryologia. 19(2):135–141.
- Chowdaiah BN. 1966b. Cytological investigations on some Indian Diplopoda (Myriapoda). Nature. 210(5038):847.
- Chowdaiah BN. 1966c. Cytological studies of some Indian Diplopoda – I (Myriapoda). Cytologia. 31(3):294–301.
- Chowdaiah BN, Kanaka R. 1974. Cytological studies in six species of pill-millipedes (Diplopoda-Myriapoda). Caryologia. 27(1):55–64.
- Chowdaiah BN, Kanaka R. 1979. Chromosome cytology of seven species of Indian Diplopoda (Myriapoda). In: Camatini M, editor. Myriapod Biology. London: Academic Press; p. 9–20.
- Da Silva ST, Prado RA, Fontanetti CS. 2005. High content of constitutive heterochromatin in two species of Pseudonannolene (Diplopoda). Caryologia. 58(1):47–51.
- De Freitas VC, Taboga SR, Fontanetti CS. 2003. RNA reallocation during initial spermatic meiosis of Pseudonannolene tocaiensis Fontanetti, 1996. Caryologia. 56(3):495–499.
- Fontanetti CS. 2000. Description and chromosome number of a species of Pseudonannolene silvestri (Arthropoda, Diplopoda, Pseudonannolenidae). Revista Brasileira de Zoologia. 17(1):187–191.
- Fontanetti CS, Campos KA, Prado RA, Souza TS. 2002. Cytogenetic studies in Diplopoda. Cytologia. 67(3):253–260.
- Hopkin SP, Read HJ. 1992. The biology of millipedes. Oxford: Oxford University Press.
- Kadamannaya BS, Sreepada R, Sridhar KR. 2010. Chromosomal features of the endemic pill millipedes (Arthrosphaera: Sphaerotheriidae, Diplopoda) from Western Ghats, India. Cytologia. 75(4):467–475.
- Levan A, Fredga K, Sandburg AA. 1964. Nomenclature for centromeric position on chromosomes. Heriditas. 52(2):201–220.
- Lewis KR, John B. 1963. Spontaneous interchange in Chorthippusrunneus. Chromosoma. 14(6):618–637.
- Natarajan R. 1959. Cytological studies in Diplopoda (Myriapoda). Journal of Zoological Society of India. 11(1):91–101.
- Pitz KM, Sierwald P. 2010. Phylogeny of the millipede order Spirobolida (Arthropoda: Diplopoda: Helminthomorpha). Cladistics. 26(5):497–525.
- Pocock RI. 1892. Report upon two collections Myriyapoda sent from Ceylon by Mr. E. E. Green and from various parts of southern India by Mr. Edger Thurston of the govt. central museum, Madras. Bombay Natural History Society. 7:131–174.
- Pocock RI. 1899. A monograph of the pill-millipedes (Zephroniidae) inhabiting India, Cylon and Burma. Journal of Bombay Natural History Society. 12:269–285.
- Sharma GP, Handa SM. 1974. Comparative cytological studies on Gonoplectus malayus and phyllogonoplectus nigrolabiatus (Diplopoda: Myriapoda). Cytologia. 39(4):673–680.
- Vitturi R, Colomba MS, Caputo V, Sparacio I, Barbieri R. 1997. High heterochromatin content in somatic chromosomes of two unrelated species of Diplopoda (Myriapoda). Chromosome Research. 5(6):407–412.
- Warchałowska-Śliwa E, Maryańska-Nadachowska A, Kania G. 2004. Cytogenetic studies of Glomeris (Diplopoda, Glomeridae). Folia Biologica (Kraków). 52(1–2):67–71.
- Wesener T, Raupach MJ, Sierwald P. 2010. The origins of the giant pill-millipedes from Madagascar (Diplopoda: Sphaerotheriida: Arthrosphaeridae). Molecular Phylogeny and Evolution. 57(3):1184–1193.
- Wesener T, van den Spiegel D. 2009. A first phylogenetic analysis of giant pill-millipedes (Diplopoda: Sphaerotheriida), a new model Gondwanan taxon, with special emphasis on island gigantism. Cladistics. 25(6):1–29.