Abstract
This is an initial report of a cytogenetic study in two economically important species of Epinephelus, namely E. bleekeri and E. coeruleopunctatus, using the G-banding technique. Chromosome numbers of the two species are 2n = 48. The differences were found to be karyotype variations, with 48 telocentric chromosomes in E. bleekeri and two submetacentric and 46 telocentric chromosomes in E. coeruleopunctatus. Statistically, there were no karyotype variations between male and female individuals of each of the species. This basic knowledge is highly important for other technologies, such as those used in hybridization programming.
Introduction
Groupers are a category of fish with economic importance, used not only as resources for food but also for ornamentation and aquariums. They belong to the subfamily Epinephelinae of the family Serranidae. The subfamily was classified into 15 genera by Froese and Pauly (Citation2013). The genus Epinephelus is one of the most important genera. Each genus is identified mainly by morphological characteristics, such as body configuration, size and number of body parts, or color patterns. However, there are many overlapping morphological characteristics among grouper species. Compounding these are the many intraspecific variations, especially of color patterns. This problem makes it difficult to identify species and misidentification is a recurrent issue.
Reports on species diversity of groupers in Thailand have classified 20–25 species (Suvatti Citation1950; Duangsawasdi Citation1964; Froese and Pauly Citation2013). Due to their importance, wild species should be collected for the purposes of studying their germplasm. These studies can lead to more sustainable usage of groupers in a variety of ways, such as improving the breeding of cultivars, growth rate, hatchling collection for aquaculture, and identification. Cytogenetics has long been an essential tool to examine the transmission of genetic material from one generation of cells to the next. Changes in chromosome structure, which manifest directly on the transmitting genotype, are expressed as specific individual patterns, which also affect the growth and evolution of life. Therefore, the study of cytogenetics demonstrates how genetic variations occur in the chromosome of each species. When examining specific chromosome numbers and types for each species, some groups with close evolution appear to have many similar chromosome pairs, for example, orang-utans (Pongo pygmaeus), gorillas (Gorilla gorilla), chimpanzees (Pan troglodytes), and humans (Homo sapiens). Chromosome numbers and their karyotypes are often used as essential taxonomic data for those animals that are difficult to classify according to their morphological aspects. For example, the identification of the four genera of the family Hylobatidae is based on each having a different chromosome number (Geissmann Citation2002). It follows that there are some variations of Epinephelus species, including E. malabalicus (Zou et al. Citation2005), E. fuscoguttatus (Liao et al. Citation2006; Wei et al. Citation2009), E. moara (E. bruneus) (Guo et al. Citation2006), and E. coioides (Wang et al. Citation2010), showing a chromosome number of 48 with varied karyotypes. Cytogenetic studies provide important basic knowledge which can have applications for many other studies, such as for the detection of ploidy in fishes (Pradeep et al. Citation2011; Pradeep, Srijaya, Bahuleyan, et al. Citation2012).
This research is an initial study reporting the chromosome number and karyotypic characters of two species, E. bleekeri and E. coeruleopunctatus.
Materials and methods
Epinephelus bleekeri and E. coeruleopunctatus were collected from the Andaman Sea in Phuket Province, Thailand. Species identification was performed based on morphological characteristics according to Heemstra and Randall (Citation1993).
Chromosomes were directly prepared in vivo (Chen and Ebeling Citation1968; Nanda et al. Citation1995) as per the following process. Phytohemagglutinin (PHA) solution was injected into abdominal cavities of the fishes. Twenty-four hours later, colchicine was injected intramuscularly and/or into the abdominal cavities of the fishes, and was left there for 2–4 hours. The fishes were anesthetized in ice-cold water and dissected. The kidneys, spleens, and/or gills were cut into small pieces. The pieces were mixed and squashed with 0.075 M KCl. The large pieces of tissue were discarded, and then 15 ml samples of the cell sediments were transferred to centrifuge tubes and were incubated for 25–35 minutes. The fresh and cool fixative solution (3:1 methanol/glacial acetic acid) was added to stop the samples’ reaction with the KCl, then centrifuged at 1200–1500 rpm for 10 minutes. Afterwards, the supernatant was discarded, 7–8 ml of the fixative solution was again added, and the samples were centrifuged once again in order to wash the cell sediments. The cell sediments were resuspended in 1 ml of the fixative solution and kept at –20°C for further chromosomal study.
The cell suspensions were dropped onto a clean and cold slide by micropipette, which was followed by the air-dry technique. The slide was conventionally stained with 20% Giemsa solution for 30 minutes.
Chromosome counts were performed on the mitotic metaphase cells under a light microscope. Twenty clearly observable and well-spread cell chromosomes of both male and female individuals were selected and photographed. The lengths of the short arm of the chromosome (Ls) and the length of the long arm of the chromosome (Ll) were measured and were calculated to find the total arm length of the chromosome (LT = Ls + Ll). The relative length (RL), centromeric index (CI), and standard deviation (SD) of RL and CI were estimated. CI was also computed to classify the types of chromosomes according to Chaiyasut (Citation1989). All parameters were used in karyotyping.
For karyotyping, the chromosome pictures were used for homologous chromosome pairing. The initiation of chromosome pairing was used to determine each chromosome centromere. Then, the lengths of each chromosome’s long arm and short arm were measured and computed for the length of that chromosome. The karyotyping arrangement was ranged from the longest to the shortest, except for the sex chromosomes, which are always the last pair and are shown at the bottom left side of the range. The chromosome number is usually shown at the bottom of each picture. Regarding the arrangement, the short arm is normally shown on top, whereas the long arm is situated at the bottom of each karyotyping.
Results
The cytogenetic study of the two sample species E. bleekeri and E. coeruleopunctatus using G-banding technique are shown below.
Epinephelus bleekeri
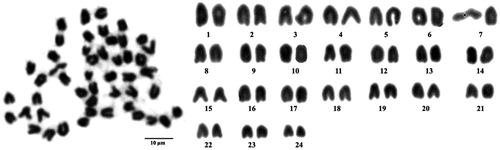
The chromosome number of this species is 2n = 48. Karyotypes are present at 48 telocentric chromosomes, shown in Figure .
Epinephelus coeruleopunctatus
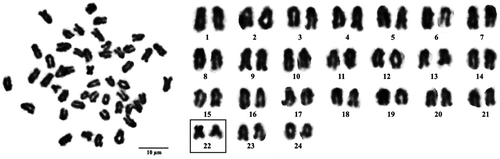
The chromosome number of this species is 2n = 48. Karyotypes comprise of two submetacentric and 46 telocentric chromosomes, shown in Figure .
Discussion
There have been many publications concerning cytogenetic studies on Epinephelus species, starting with a study of E. diacanthus in 1974 (Natarajan and Subrahmanyam Citation1974), up to a study of E. coioides in 2010 (Wang et al. Citation2010). All publications show the same chromosome counting of 2n = 48, also in accordance with the number given to E. bleekeri and E. coeruleopunctatus in this research. Variations between species have often occurred in previous karyotyping. For example, Wang et al. (Citation2010) reported that E. coioides were comprised of two submetacentric and 46 acrocentric chromosomes; Wei et al. (Citation2009) reported two subtelocentric and 46 telocentric chromosomes; and Guo et al. (Citation2006) reported 48 acrocentric chromosomes in E. moara.
This study is an initial report demonstrating a cytogenetic study of the two sample species E. bleekeri and E. coeruleopunctatus using the G-banding technique. The results show that the two species show an equal chromosome number of 2n = 48 with karyotype variations. Epinephelus bleekeri included 48 telocentric chromosomes, whereas E. coeruleopunctatus comprised of two submetacentric and 46 telocentric chromosomes. There were statistically no differences between male and female individuals of each of the species. That means no sex-linked chromosome was observed in the two species. We expected that the results would be the same for all species in the genus. Besides providing basic knowledge, cytogenetic studies have been successfully used for detection of ploidy in tilapias (genus Oreochromis), which were temperature shocked (Pradeep, Srijaya, Bahuleyan, et al. Citation2012; Pradeep, Srijaya, Papini, et al. Citation2012). This technique is very important in hybridization programming of fishes or any other animals, as it can detect and correctly identify the induced diploid or triploid offspring, which may be further selected as maternal and/or paternal material for further breeding.
References
- Chaiyasut K. 1989. Cytogenetics and cytotaxonomy of the genus Zephyranthus. Bangkok: Department of Botany: Faculty of Sci-ence, Chulalongkorn University.
- Chen TR, Ebeling AW. 1968. Karyological evidence of female heterogamety in the mosquito fish, Gambusia affinis. Copeia. 1968(1):70–75.10.2307/1441552
- Duangsawasdi M. 1964. A study on the groupers (Epinephelidae) in Thai waters [bachelor thesis in fisheries]. Faculty of Fisheries: Kasetsart University, Bangkok, Thailand.
- Froese, R, D.Pauly 2013. FishBase [World Wide Web electronic publication]. www.fishbase.org, version (10/2013).
- Geissmann T. 2002. Taxonomy and evolution of gibbons. Evolutionary Anthropology: Issues, News, and Reviews. 11(S1):28–31.
- Guo F, Wang J, Su YQ, Wang DX, Xu LN. 2006. Study on the karyotype of Epinephelus moara. Marine Sciences. 2006(8):1–3.
- Heemstra PC, JE.Randall 1993. Groupers of the world (family Serranidae, subfamily Epinephelinae). FAO Fisheries Synopsis No. 125 Vol. 16. Available from ftp://ftp.fao.org/docrep/fao/009/t0540e/t0540e00.pdf.
- Liao JQ, Yin SW, Chen GH, Huang H, Lei CG, Lou TT. 2006. The karyotype of grouper Epinephelus fuscoguttatus. Fisheries Science. 2006(11):567–569.
- Nanda I, Schsrtl M, Fiechtinger W, Schlupp I, Parzefall J, Schmid M. 1995. Chromosomal evidence for laboratory synthesis of triploid hybrid between the gynogenetic teleost Poecilia formosa and its host species. Journal of Fish Biology. 47(4):619–623.
- Natarajan R, Subrahmanyam K. 1974. A karyotype study of some teleosts from Portonovo waters. Proceedings of the Indian Academy of Sciences – Section B. 79(5):173–196.
- Pradeep PJ, Srijaya TC, Bahuleyan A, Renjithkumar CR, Jose D, Papini A, Chatterji AK. 2012. Triploidy induction by heat-shock treatment in red tilapia. Caryologia. 65(2):152–156.10.1080/00087114.2012.711678
- Pradeep PJ, Srijaya TC, Jose D, Papini A, Hassan A, Chatterji AK. 2011. Identification of diploid and triploid red tilapia by using erythrocyte indices. Caryologia. 64(4):485–492.
- Pradeep PJ, Srijaya TC, Papini A, Chatterji AK. 2012. Effects of triploidy induction on growth and masculinization of red tilapia [Oreochromis mossambicus (Peters, 1852) × Oreochromis niloticus (Linnaeus, 1758)]. Aquaculture. 344–349:181–187.10.1016/j.aquaculture.2012.03.006
- Suvatti C. 1950. Fauna of Thailand. Bangkok: Department of Fisheries.
- Wang SF, Su YQ, Ding SX, Cai Y, Wang J. 2010. Cytogenetic analysis of orange-spotted grouper, Epinephelus coioides, using chromosome banding and fluorescence in situ hybridization. Hydrobiologia. 638(1):1–10.10.1007/s10750-009-9980-9
- Wei Y, Fan T, Jiang G, Sun A, Xu X, Wang J. 2009. Establishment of a novel fin cell line from Brown-marbled grouper, Epinephelus fuscoguttatus (Forsskål), and evaluation of its viral susceptibility. Aquaculture Research. 40(13):1523–1531.10.1111/are.2009.40.issue-13
- Zou JX, Yu QX, Zhou F. 2005. The karyotypes C-bands patterns and Ag-NORs of Epinephelus malabaricus. Shuichan Xuebao. 29(1):33–37.