Abstract
In this study, somatic chromosome numbers of 27 Jurinea Cass. taxa (25 species and two subspecies), collected from different localities in Iran, were counted. Apart from Jurinea macrocephala DC. and J. pulchella DC. these are reported here for the first time. Except for J. eriobasis DC. with 2n = 36 and J. gedrosiaca Bornm. with 2n = 32 all the materials studied showed a somatic number of 2n = 34. Regarding the dysploidy observed in this genus two theories based on a decrease in aneuploidy and a polyploidy pillar complex are justified and discussed here.
Introduction
Plant taxonomy is initially based on morphological characteristics. Because these features vary, other characters such as chromosome number and chromosome morphology have been increasingly used in plant taxonomy. These data are also important to elucidate the origin, speciation and phylogenetic relationships of plants (Stebbins Citation1971; Cai et al. Citation2004; Pavlova and Tosheva Citation2005). In plants, mechanisms such as chromosomal rearrangements and changes in the basic chromosome number or in ploidy level can usually be accompanied by diversification and sometimes speciation (Levin Citation2002). Determining chromosome number in many populations throughout the distributional range of a given species represents a primary and essential step, in which events such as dysploidy, aneuploidy or polyploidy can be revealed (Sánchez-Jiménez et al. Citation2009).
The Asteraceae is the largest plant family and comprises more than 1600 genera and 23,000 species (Anderberg et al. Citation2007; Funk et al. Citation2009). Its many genera and species, its worldwide distribution and the fact that it comprises many useful plants have made it the subject of many karyological studies (Watanabe Citation2013).
For about 60% of the genera of Asteraceae chromosome counts have been reported (Akhtar Citation2005). Many genera surveyed are monobasic for x = 9 and x = 7, 25 genera appear to have more than one base number, and 18 of these have a base number of 8 or 9. The Old World genera have not been surveyed as extensively as New World genera; reports are available for 46% of genera, and x = 8 is predominant. Approximately 26% of the genera are monobasic for x = 9; a considerable number of the genera having more than one base number do not include x = 9 which was considered to be the ancestral number in Asteraceae (Stebbins Citation1971; Solbrig Citation1977; Stuessy Citation1977): however the most common is x = 8 which more likely is to be the ancestral base number for this family. Variation in chromosome number is probably through hybridization, euploidy and aneuploidy in Asteraceae (Häffner Citation2000; Funk et al. Citation2009). It is obvious by the data that the tribe Cardueae could be divided into two phyletic groups: a large group with x = 9 and 8, and a smaller group of genera with x = 6, 5 and 4. Nevertheless, the chromosome numbers range considerably among different genera of the tribe which can be correlated to relevant morphological variability; these characters are proved to be useful in the classification of this tribe. Chromosomal features are fundamental to have a better understanding of the relationships in Cardueae, particularly at the generic level (Akhtar Citation2005).
The monophyletic tribe Cardueae is constituted of five subtribes, namely Carduinae Cass., Centaureinae (Cass.) Dumort., Carlininae (Cass.) Dumort., Cardopatiinae Less., and Echinopinae (Cass.) Dumort (Susanna et al. Citation2006; Anderberg et al. Citation2007). Jurinea Cass. is a genus located within Carduinae. It was described based on two new species transferred from Serratula L. and Carduus L. (Cassini 1819). The genus comprises perennial herbs or rarely shrubby plants with purple, red, pink or whitish florets and involucres of linear to lanceolate phyllaries, the achenes are tetragonal with a distal corona around their apexes (Kožuharov Citation1976; Rechinger and Wagenitz Citation1979). Jurinea Cass. is a taxonomically complex genus comprising c.100 (Strid Citation1991) to 200 species (Susanna et al. Citation2006) or even 300 species (Iljin Citation1962). Native distribution of this genus covers Central Asia, Iran, Turkey and the Mediterranean region (Häffner Citation2000; Funk et al. Citation2009).
Jurinea is represented by 37 species in the Flora Iranica area, of which 25 species grow in Iran from which 22 are endemic (Rechinger and Wagenitz Citation1979). The number and position of taxa change every year, with new species being described, or some of the taxa recently displaced (Akhani Citation1996; Ghahreman and Mirtadzadini Citation2000; Mehregan and Assadi Citation2009; Mirtadzadini et al. Citation2011). So, from previous studies and our investigation, there are 26 species of Jurinea in the political boundary of Iran at present Rechinger & Wagenitz (Citation1979).
Based on chromosomal information found in the literature (Baksay Citation1956; Kuzmanov and Kožuharov Citation1967; Borhidi Citation1968; Chouksanova et al. Citation1968; Küpfer Citation1969a, Citation1969b; Holub et al. Citation1971; Kuzmanov and Ancev Citation1973; Lungeanu Citation1973; Kuzmanov and Georgieva Citation1977; Humphries et al. Citation1978; Kieft and Loon Citation1978; Chichiricco et al. Citation1979; Sokolovskaia and Probatova Citation1980; Vir and Kachroo Citation1985; Kuzmanov et al. Citation1986; Magulaev Citation1976, Citation1982, Citation1986; Jee et al. Citation1989; Kochjarova Citation1990; Blanca and Cueto Citation1992; Kuzmanov et al. Citation1990, Citation1993; Vogt and Oberprieler Citation1993; Diaz De La Guadia Citation1995; Gagnidze and Gviniashvili Citation1997; Dogan et al. Citation2009, Citation2010), it is evident that Jurinea is poorly known cytologically. No cytotaxonomic record of this genus has been reported from Iran so far.
The main objective of this study is a first step to reveal cytological information about the described Jurinea taxa from Iran and enlarge the chromosomal data in the genus. We believe this study will play a positive role in resolving the morphologically unsolved problems of this taxonomically complex genus.
Materials and methods
The examined plant materials are presented in Table . Plant materials were collected from different localities in Iran, in 2008–2012. Voucher specimens are deposited in the herbarium of Department of Biology at the University of Isfahan (HUI).
Table 1. List of Jurinea taxa studies (all samples were collected by Parishani & Mirtadzadini).
Chromosome counts were made on somatic metaphases using standard squash techniques. Mitotic chromosome preparations were obtained from the root-tip of germinating seeds collected in the wild. Root-tip meristems were obtained by germinating seeds on wet filter paper in Petri dishes at approximately 18°C. Actively growing roots were excised in the morning, when they were about 0.5–1 cm long. Times from 8 am to 4 pm were tested and the best results were obtained at 9.30 am.
Root tips of 1–2 cm were cut off and pretreated with saturated solution of α-bromo-naphthalene at 4°C for 16 h, fixed in fresh Carnoy’s solution (3:1 ethanol:glacial acetic acid) for 24 h and stored at 4°C until use. Root tips were washed in distilled water to remove the fixative, hydrolyzed in 1N HCl for 20–25 min at room temperature and stained in 1% aceto-orcein at least for 8 h (Sharma and Sharma Citation2001; Singh Citation2002; Martin et al. Citation2006; Dogan et al. Citation2009). Squashing was done in a drop of acetic acid 45%. The best metaphase plates were photographed using a BX40 microscope (Olympus, Tokyo, Japan) with digital camera attachment. To assess the existence of previously published chromosome counts in the studied species, we used the chromosome number databases of plant chromosome numbers cited by Dogan et al. (Citation2009), as well as the online chromosome number databases Index to Plant Chromosome Numbers (Missouri Botanical Garden, http://www.tropicos.org/Project/IPCN) and Index to Chromosome Numbers in Asteraceae (Watanabe Citation2013).
Results and discussion
Many taxonomic and phylogenetic problems concerning the genus Jurinea have not been resolved till now. However, more extensive karyologic and genomic data are needed in addition to taxonomic and phylogenetic criteria to throw some light on these problems.
Our observations of all the Iranian Jurinea species revealed 2n = 34 to be the most common diploid number, and only two species had different numbers (2n = 32 and 2n = 36). The results are given below based on the sectional position of the species and the endemism reports are substantially according to Flora Iranica (Rechinger and Wagenitz Citation1979).
Sect. Corymbosae Benth. in Benth. et Hook. F., Gen. Plant. 2: 473, 1873
1. J. pulchella DC.
This species is a diploid with 2n = 34 (Table , Figure ). Our record of 2n = 34 for this species confirms the previous determination on specimens from Turkey reported by Dogan et al. (Citation2009).
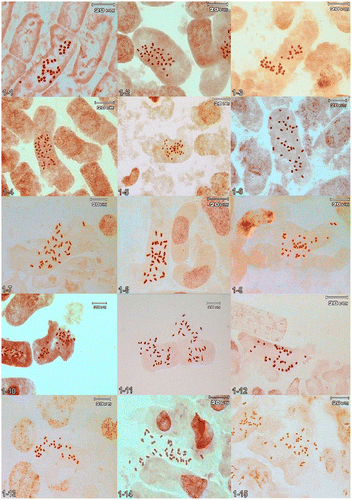
Figure 1. (Colour online) Somatic metaphases: 1-1, Jurinea pulchella 2n = 34; 1-2, J. leptoloba 2n = 34; 1-3, J. heterophylla 2n = 34; 1-4, J. giviensis 2n = 34; 1-5, J. gedrosiaca 2n = 32; 1-6, J. macrocephala 2n = 34; 1-7, J. macrocephala subsp. elbursensis 2n = 34; 1-8, J. multicaulis 2n = 34; 1-9, J. proteoides 2n = 34; 1-10, J. cordata 2n = 34; 1-11, J. inuloides 2n = 34; 1-12, J. viciosoi 2n = 34; 1-13, J. meda 2n = 34; 1-14, J. prasinophylla 2n = 34; 1-15, J. sharifiana 2n = 34.
2. J. leptoloba DC.
This species is endemic to Iran (Rechinger and Wagenitz Citation1979). The somatic chromosome number is 2n = 34 (Table , Figure ). This is the first chromosome count for this species.
3. J. heterophylla Jaub. et Spach
The chromosome number of this species is determined as 2n = 34 (Table , Figure ). To our knowledge, this is the first report for this taxon. This taxon is endemic to Iran (Rechinger and Wagenitz Citation1979).
4. J. giviensis Mirtadz.
This taxon is endemic to Iran and is found in a limited area of Ardebil province (Mirtadzadini et al. Citation2011). The somatic chromosome number is 2n = 34 (Table , Figure ). According to our data, this is the first chromosome count for this species.
5. J. gedrosiaca Bornm.
This taxon is endemic to Iran (Rechinger and Wagenitz Citation1979). The chromosome number in this species showed 2n = 32 (Table , Figure ). According to our data, this is the first chromosome count for this species.
Sect. Derderia (Jaub. et Spach) Boiss. in Boiss. Fl. Or. 3: 568, 1875
6. J. macrocephala DC.
This taxon exhibited 2n = 34 (Table , Figure ). Our record of 2n = 34 for this species confirms the previous determination on specimens from Turkey reported by Dogan et al. (Citation2009).
7. J. macrocephala DC. subsp. elbursensis Wagenitz
This taxon is endemic to Iran and is found in a limited area of Karaj and Qazvin (Rechinger and Wagenitz Citation1979). It exhibited 2n = 34 (Table , Figure ). The chromosome number of the taxon is given here for the first time.
8. J. multicaulis DC.
This taxon is endemic to Iran (Rechinger and Wagenitz Citation1979). The chromosome number in this species showed 2n = 34 (Table , Figure ). According to our data, this is the first chromosome count for this species.
9. J. proteoides Boiss. et Hausskn. ex Boiss.
This taxon exhibited 2n = 34 (Table , Figure ). This species is endemic to Iran and is found in a limited area of Chaharmahal-Bakhtiari province (Rechinger and Wagenitz Citation1979). This is the first chromosome count for this species.
10. J. cordata Boiss. et Hausskn. ex Boiss.
The somatic chromosome number of this taxon is 2n = 34 (Table , Figure ). This taxon is endemic to Iran (Rechinger and Wagenitz Citation1979). The chromosome number of the taxon is reported here for the first time.
11. J. inuloides Boiss. et Hausskn. ex Boiss.
This taxon is endemic to Iran (Rechinger and Wagenitz Citation1979). It exhibited 2n = 34 (Table , Figure ). This is the first chromosome count for this species.
Sect. Bellae Iljin, Fl. URSS. 27:720 (1962)
12. J. viciosoi Pau
This is an Iranian endemic and perennial species that grows on rocky slopes, road sides and stony pastures of Bazoft’s altitudes (Rechinger and Wagenitz Citation1979). It is determined that the somatic chromosome number is 2n = 34 (Table , Figure ). According to our data, this is the first chromosome count for this species.
13. J. meda Bornm.
This taxon is endemic to Iran (Rechinger and Wagenitz Citation1979). The chromosome number in this species is 2n = 34 (Table , Figure ). The chromosome number of the taxon is given here for the first time.
14. J. prasinophylla Rech. f.
This taxon possessed 2n = 34 chromosomes (Table , Figure ). This species is endemic to Iran (Rechinger and Wagenitz Citation1979). This is the first chromosome count for this species.
15. J. sharifiana Rech. f. et Esfand.
This taxon is endemic to a limited area in north-east Semnan Province (Rechinger and Wagenitz Citation1979). The chromosome number in this species was 2n = 34 (Table , Figure ). According to our data, this is the first chromosome count for this species.
Sect. Penduliflorae Iljin, Fl. URSS. 27:721 (1962)
16. J. kopetensis Rech. f.
The chromosome number of this species was determined to be 2n = 34 (Table , Figure ). This is the first report on the chromosome number of the species. It is endemic to Iran (Rechinger and Wagenitz Citation1979).
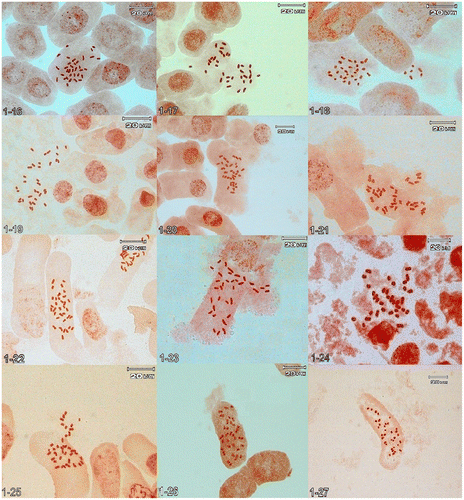
Figure 1. (Continued) 1-16, Jurinea kopetensis 2n = 34; 1-17, J. radians 2n = 34; 1-18, J. monocephala subsp. sintenisii 2n = 34; 1-19, J. dumulosa 2n = 34; 1-20, J. antunowi 2n = 34; 1-21, J. catharinae 2n = 34; 1-22, J. ramosissima 2n = 34; 1-23, J. gabrieliae 2n = 34; 1-24, J. stenocalathia 2n = 34; 1-25, J. eriobasis 2n = 36; 1-26, J. mobayenii 2n = 34; 1-27, J. bungei 2n = 34.
17. J. radians Boiss.
This taxon is endemic to eastern Iran (Rechinger and Wagenitz Citation1979). It possessed 2n = 34 chromosomes (Table , Figure ). The chromosome number of the taxon is given for the first time.
Sect. Suffrutices Iljin: Fl. URSS. 27: 721, 1962
18 J. monocephala Aitch. et Hemsl. subsp. sintenisii (Bornm.) Wagenitz
This taxon is distributed in Iran and Turkmenistan (Rechinger and Wagenitz Citation1979). The chromosome number of this species was determined to be 2n = 34 (Table , Figure ). This is the first report on the chromosome number of the species.
Sect. Stechmannia (DC.) Boiss., Fl. Or. 3: 568, 1875.
19. J. dumulosa Boiss.
This taxon is a common species endemic to Iran and Afghanistan (Rechinger and Wagenitz Citation1979). It possessed 2n = 34 chromosomes (Table , Figure ). According to our data, this is the first chromosome count for this species.
20. J. antunowi C. Winkl.
This taxon is distributed in Kopet Dagh region of Turkmenistan and limited area of north-east Iran (Rechinger and Wagenitz Citation1979; Akhani Citation1996).The chromosome number of this species was determined to be 2n = 34 (Table , Figure ). This is the first report on the chromosome number of the species.
21. J. catharinae Iljin
This taxon is endemic to north-east Iran (Rechinger and Wagenitz Citation1979). The chromosome number in this species showed 2n = 34 (Table , Figure ). According to our data, this is the first chromosome count for this species.
22. J. ramosissima DC.
This species is an endemic taxon to central and eastern Iran and a limited area of south-west Afghanistan (Rechinger and Wagenitz Citation1979). The chromosome number of this species was determined to be 2n = 34 (Table , Figure ). This is the first report on the chromosome number of the species.
23. J. gabrieliae Bornm.
This species is endemic to Iran (Rechinger and Wagenitz Citation1979). It was determined that the somatic chromosome number is 2n = 34 (Table , Figure ). This is the first chromosome count for this species.
24. J. stenocalathia Rech. f.
The chromosome number of this species was determined to be 2n = 34 (Table , Figure ). This is the first report on the chromosome number of the species. It is endemic to Iran (Rechinger and Wagenitz Citation1979).
25. J. eriobasis DC.
This species is endemic to Iran (Rechinger and Wagenitz Citation1979). Its somatic chromosome number was determined as 2n = 36 (Table , Figure ). This is the first count for this species.
Indefinite section
26. J. mobayenii Ghahr. & Mirtadz.
The chromosome number of this species was determined to be 2n = 34 (Table , Figure ). This is the first report on the chromosome number of the species. This species is distributed in a limited region of south-west Kerman province (Ghahreman and Mirtadzadini Citation2000).
27. J. bungei Boiss.
This species is endemic and it is distributed only in the central regions of Iran (Rechinger and Wagenitz Citation1979). The chromosome number in this species is 2n = 34 (Table , Figure ). According to our data, this is the first chromosome count for this species.
In spite of our intensive exploration around the country, we could not find Jurinea ancyrensis Bornm. [belonging to Sect. Derderia (Jaub. et Spach) Boiss.] that Rechinger and Wagenitz (Citation1979) said was in Iran. We explored the mentioned places and found taxa similar to J. multicaulis DC. and J .macrocephala DC.
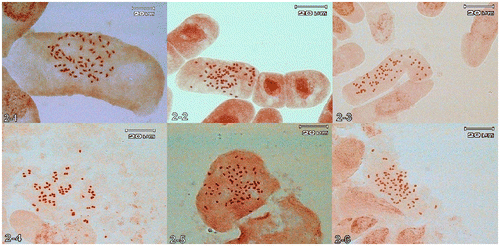
In the present study, all the Jurinea species studied were determined to be comprised of a basic chromosome number of x = 17 with the exception of two species (J. gedrosiaca with x = 16 and J. eriobasis with x = 18). Although it was rare, during cytogenetic analyses a few cells revealed polyploidy in some of the preparations. However, in 11 of the species tetraploid cells (2n = 4x = 68) were determined in the same preparations where diploid chromosomes (2n = 2x = 34) were counted (mixoploidy). They were: J. bungei, J. inuloides, J. leptoloba, J. macrocephala DC. subsp. elbursensis, J. monocephala, J. pulchella, J. radians, J. sharifiana, J. eribasis, J. viciosoi and J. giviensis (Figure to ).
Figure 2. (Colour online) Polyploidy in somatic metaphases: 2-1, Jurinea monocephala subsp. sintenisii 2n = 68; 2-2, J. radians 2n = 68; 2-3, J. viciosoi 2n = 68; 2-4, J. eriobasis 2n = 72; 2-5, J. bungei 2n = 68; 2-6, J. macrocephala subsp. elbursensis 2n = 68.
Since the level of chromosome number variation of Jurinea is low (mostly 2n = 34), it indicates that chromosomal counting is not a good parameter to use in distinction of the Jurinea species. Some species of Jurinea are dysploids, with 2n = 24, 30, 32, 35, 36 and 58 (such as J. gedrisiaca with 2n = 32 and J. eriobasis with 2n = 36 in the present study; Missouri Botanical Garden, http://www.tropicos.org/Project/IPCN; Watanabe Citation2013). Due to the morphological characters of J. gedrosiaca (it is very similar to Tricholepis species) and its chromosome number, it is suggested that this taxon belongs to the genus Tricholepis rather than Jurinea. Based on the absence of spiny phyllary in involucre and white hair cover and also diploid chromosome number in J. eriobasis, it can separated from section Stechmannia (DC.) Boiss.
Although Dogan et al. (Citation2011) provided, as well as the karyotypes, haploid idiograms of each chromosome count for the Turkish Jurinea species, the somatic chromosomes of most Jurinea species examined here were very small and it was difficult to determine the exact positions of the centromere. Consequently it was not possible to determine the precise chromosomal morphologies. Based on the chromosome counts made in this study we found x = 17 to be the most frequent number among the Iranian Jurinea species, which is in accordance with Dogan et al. (Citation2009) who reported 2n = 34 for Turkish taxa. In addition, on the basis of the reports indexed in Watanabe (Citation2013), 2n = 34 is the most common number in this genus (Table ). Due to the predominance of dysploidy in Astreaceae (Anderberg et al. Citation2007; Funk et al. Citation2009) the taxonomic boundary of the genus Jurinea and its chromosomal formula should be interpreted based on a kind of palaeo eupolyploidy or aneupolyploidy. Since no diploid with x = 8 or 9 is known in the Jurinea–Sassurea group in sensu Susanna and Garcia-Jacas (Anderberg et al. Citation2007; Funk et al. Citation2009), 2n = 34 can be justified based on two theories.
Table 2. A summary of the number of chromosome count reports (2n) for the Jurinea–Saussurea group in sensu Susanna and Garcia-Jacas (Anderberg et al. Citation2007) based on Watanabe (Citation2013) and Wang et al. (Citation2013).
First is the suggestion of Wang et al. (Citation2013) who believe that the diploid number of 2n = 36 observed in Dolomiaea is a derivation from an ancestral diploid with the same number, and 2n = 34 counted in a generic complex including Jurinea and Himalaiella is generated via descending aneuploidy. They also mentioned that no polyploids based on x = 17 and 18 were found in the harsh habitats in the areas under their study. On the basis of the above interpretation and also taking into account the diploid numbers reported for the monophyletic Jurinea–Saussurea group (Funk et al. Citation2009) (Table ), in order to form this complex a serial of descending and ascending aneuploidy would be required. In addition, the presence of repeated similar diploid numbers in different taxa requires us to accept several homoplasic events.
The second suggestion can be hypothesized on the basis of a kind of polyploidy pillar complex (Ehrendofer Citation1959) in which regardless the Jurinea’s sister groups this theory can not be explained. In this theory it is necessary to assume the presence of a complex of ancestral palaeodiploid species carrying x = 6, 7, 8, and 9 (Figure ). Hybridization among the latter diploids, combined with a chromosomal doubling strategy to circumvent the unbalanced meioses, generated the numbers 26, 30, and 34. The diploids of 24, 28, 32 and 36 are direct consequences of auto-polyploidization. This suggestion justifies the presence of 2n = 26 in the closely related genus i. e., Saussurea without any need to a serial of aneuploidies from 36 to 26; this number is the most common in the latter taxon (Table ).
Figure 3. Scheme illustrating a hypothetical chromosomal relationship of the Jurinea–Saussurea group in sensu Susanna and Garcia-Jacas (Anderberg et al. Citation2007; Funk et al. Citation2009). A putative ancestral palaeodiploid series is considered on the base. Through successive hybridizations, back crossings and chromosome doublings a web of reticulate evolution is generated; this implies a polyploidy pillar complex scheme (Ehrendofer Citation1959). In order to retain some simplicity the back crossings are not illustrated in this scheme.
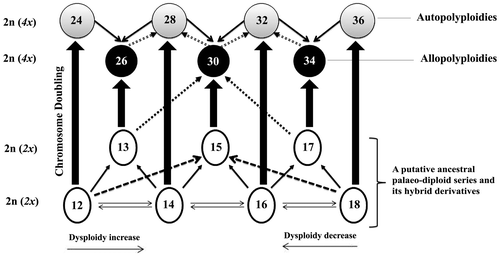
This justification seems to be more rational when accepting a putative ancestral diploid core of one or a pair of basic numbers (x = 7/8) from which, via aneuploidy, other ancestral diploids were generated. Very close relationships and chromosomal affinities caused a web of reticulate evolution at tetraploid level (Figure ), which is explicitly reflected by the reported chromosome numbers (see Table ). This situation is marked in the relevant taxonomic treatments in which 3–19 genera (Anderberg et al. Citation2007; Wang et al. Citation2007; Funk et al. Citation2009) were recognized in this group. Funk et al. (Citation2009) believed that all 16 generic segregates described within the group can be reduced into three genera: Saussurea, Jurinea and Dolomiaea.
However, as Table shows, in Jurinea 34 is the most frequent diploid number in Iran and the only number reported in Turkey (Dogan et al. Citation2009), in the centre of its distributional range. This number can be proposed as an allotetraploid which forms the core genome in this genus. Nevertheless, there are also some other lower and higher chromosome counts, which in the frame of a reticulate evolution can be attributed to a serial of introgressive hybridizations and back crossing, at tetraploid and the lower ploidy levels. This situation has brought about a complex in which taxonomic confusions are clearly seen among the taxonomic treatments applied to this group. Based on the results of this study we accept the lumping idea of Jurinea–Saussurea group (Funk et al. Citation2009).
Acknowledgements
This research was supported by the Office of Graduate Studies of the University of Isfahan.
References
- Akhani H.1996. Studies on the flora and vegetation of the Golestan national park, NE Iran, I: A new species and some new plant records. Annales Naturhistorisches Museum Wien. 95(B):97–105.
- Akhtar T. 2005. Taxonomic study of the tribe Cardueae from Pakistan with emphasis on conservation status and ethnobotanical [PhD thesis]. Quaid-I-Azam University, Islamabad, Pakistan.
- Anderberg AA, Baldwin BG, Bayer RG, Breiwier J, Jeffrey C, Dillon MO, Eldenäs P, Funk V, Garcia-Jacas N, Hind DJN, Karos PO, Lack HW, Nesom G, Nordenstam B, Oberprieler C, Panero JL, Puttock C, Robinson H, Stuessy TF, Susanna A, Urtubey E, Vogt R, Ward J, Watson LE. 2007. Compositae. In: Kadereit JW, Jeffrey C, editors. The families and genera of vascular plants. Berlin, Heidelberg: Springer Verlag; p. 61–573.
- Baksay L. 1956. Cytotaxonomical studies on the flora of Hungary. Annales Historico-Naturales Musei Nationalis Hungarici. Ser. Nova. 7:321–334.
- Blanca G, Cueto M. 1992. In numeros cromosomaticos de planta occidentales. Anales del Jardin Botanico de Madrid. 50:83.
- Borhidi A. 1968. Karyological studies on south east European plant species. I. Acta Bot. Acad. Sci. Hung. 14:253–260.
- Cai J, Wang H, Gu ZJ, Mill RT, Li DZ. 2004. Karyotypes of thirteen species of Pedicularis (Orobanchaceae) from the Hengduan Mountains region, NW Yunnan. China. Caryologia. 57(4):337–347.
- Cassini H. 1821. Proposition d’un nouveau genre de plantes (Jurinea). Bulletin des Sciences par la Société philomathique de Paris. 8:140–144.
- Chichiricco G, Frizzi G, Tammaro F. 1979. In numeri cromosomici per la Flora Italiana. Information Botanique Italy. 11:659–660.
- Chouksanova NA, Sveshnikova LT, Alexandrova TV. 1968. Data on karyology of the family Compositae Giseke. Cytologia. 10:198–206.
- Diaz De La Guardia C. 1995. In numeros cromosomicospara la flora Espanola (769–773). Lagascalia. 18(1):119–122.
- Dogan B, Duran A, Martin E, Erdogan E, Hakki EE. 2010. Jurinea turcica (Asteraceae), a new species from North-West Anatolia, Turkey. Biologia, Section Botany. 65(1):28–32.
- Dogan B, Duran A, Martin E, Hakki EE. 2009. Chromosome numbers in Turkish species of Jurinea Cass. (Asteraceae). Caryologia. 62(1):16–23.
- Dogan B, Duran A, Martin E, Hakki EE. 2011. Karyotype analyses of the species of the genus Jurinea Cass. (Compositae) in Turkey. African Journal of Biotechnology. 10:722–729.
- Ehrendofer F. 1959. Differentiation-hybridization cycles and polyploidy in Achillea, Cold Spring Harbor Symposium. Quantative Biology. 24:141–152.
- Funk VA, Susanna A, Stuessy T, Bayer R, editors. 2009. Systematics, evolution and biogeography of the Compositae. Washington, DC: International Association for Plant Taxonomy.
- Gagnidze RI, Gviniashvili T. 1997. In IOPB chromosome data 11. Newslett. Int. Organ. Plant Biosyst. 26–27:20–21.
- Ghahreman A, Mirtadzadini SM. 2000. Two new species of the genera Jurinea and Scrophularia from southeast Iran. Iranian Journal of Botany. 8(2):245–250.
- Häffner E. 2000. On the phylogeny of the subtribe Carduinae (tribe Cardueae, Compositae). Botanischer Garten und Botanisches Museum, Berlin-Dahlem. 3–208. http://www.bgbm.fu-berlin.de/bgbm/library/publikat/englera.
- Holub J, Mesicek J, Javurkova V. 1971. A notated chromosome counts of Czechoslovak plants (16–30) Materials for Flora (SSR - 2). Folia Geobot. Phytotaxonomy (Praha). 6:179–214.
- Humphries C, Murray J, Bocquet G, Vasudevan K. 1978. Chromosome numbers of phanerogams from Morocco and Algeria. Botaniska Notiser. 131:391–406.
- Iljin MM. 1962. Jurinea. In: Shishkin BK, Bobrov EG, editors. Flora of the USSR 27. Akademiya Nauk SSSR Publishers, Moscow-Leningrad; p. 533–722.
- Jee KR, Vir U, Wafai BA, Kachroo P. 1989. Cytology of Senecio jacquemontianus (Decne.) Benth. ex Hook. f. (Asteraceae) from Kashmir Himalaya meiotic studies. Chromosomal Informational Service. 46:4–6.
- Kieft B, Loon V. 1978. In IOPB chromosome number reports LXII. Taxon. 27:519–535.
- Kochjarova J. 1990. Karyological study of the Slovak Flora. Acta Facultatis Rerum Naturalim Universitatis Botanica. 38:89–99.
- Kožuharov S. 1976. Jurinea. In: Tutin TG, Heywood VH. editors. Flora Europaea. Vol. 4. Cambridge: Cambridge University Press, p. 218–220
- Küpfer P. 1969a. In IOPB chromosome number reports XXII. Taxon. 18:433–442.
- Küpfer P. 1969b. Recherches cytotaxinomiquessur la flora des Montagnes de la Peninsule Iberique. Bulletin de la Societe Neuchateloise des Sciences Naturelles. 92:31–48.
- Kuzmanov BA, Ancev ME. 1973. In IOPB chromosome number reports XLI. Taxon. 22:459–464.
- Kuzmanov BA, Georgieva S. 1977. In IOPB chromosome number reports LVIII. Taxon. 26:557–565.
- Kuzmanov BA, Georgieva SB, Nikolova VA. 1986. Chromosome numbers of Bulgarian flowering plants. I. Fam. Asteraceae. Fitologiya (Sofia). 31:71–74.
- Kuzmanov BA, Jurukova-Grancarova PD, Georgieva SB. 1990. Chromosome numbers of Bulgarian angiosperms. Fitologiya (Sofia). 38:92.
- Kuzmanov BA, Jurukova-Grancarova PD, Georgieva SB. 1993. Karyological study of Bulgarian Asteraceae. IV. Fitologiya (Sofia). 44:3–15.
- Kuzmanov BA, Kožuharov SI. 1967. Karyotypes of four Bulgarian Compositae species. Acta Botanica Academiae Scientiarum Hungaricae. 20:469–472.
- Levin DA. 2002. The role of chromosomal changes in plant evolution. Oxford: Oxford University Press.
- Lungeanu I. 1973. In IOPB chromosome number reports XLII. Taxon. 22:647–654.
- Magulaev AJ. 1976. The chromosome numbers of flowering plants of the northern Caucasus (Part 2). The Flora of the Northern Caucasus. 2:51–62.
- Magulaev AJ. 1982. The number of chromosomes of the species of Asteraceae, Caryophyllaceae and Plantaginaceae of the North Caucasus. Biologicheskie Nauki. 11(227):74–79.
- Magulaev AJ. 1986. Chromosome numbers in some species of flowering plants of the Crimea and Caucasus floras. Botanicheskii Zhurnal. 71:1575–1578.
- Martin E, Dinc M, Duran A, Ozturk M. 2006. Karyotype of Lotus strictus Fisher & C. A. Mey. (Leguminosae), Centaurea amanicola Hub.-Mor. (Compositae) and Teucrium lamiifolium d’Urv. subsp. lamiifolium (Labiatae) on taxa. America-Eurasian. Journal of Scientific Research. 1(1):12–17.
- Mehregan I, Assadi M. 2009. Cousinia sect. Argenteae (Asteraceae, Cardueae), a new section including a new species from NE Iran. Willdenowia. 39(2):265–271.
- Mirtadzadini M, Rahiminejad MR, Vitek E. 2011. Jurinea giviensis (Asteraceae), a new species from Iran. Nordic Journal of Botany. 29(2):159–162.
- Pavlova D, Tosheva A. 2005. Notes on karyomorphology of Melilotus officinalis populations in Bulgaria. Caryologia. 57:151–158.
- Rechinger KH, Wagenitz G. 1979. Compositae-Cynareae III. Jurinea. In: Rechinger KH, editor. Flora Iranica. Vol. 139a. Graz: Akademische Druck, p. 180–209.
- Sánchez-Jiménez I, Pellicer J, Hidalgo O, Garcia S, Garnatje T, Vallès J. 2009. Chromosome numbers in three Asteraceae tribes from Inner Mongolia (China), with genome size data for Cardueae. Folia Geobotanica. 44:307–322.
- Sharma AK, Sharma A. 2001. Chromosome painting: Principal, strategies and scope. Calcultta: Kailash Baloni.
- Singh RJ. 2002. Plant cytogenetics. 2nd ed. New York: CRC Press.
- Sokolovskaia AP, Probatova NS. 1980. Chromosome numbers in some species from the sands of Sarykum (Daghestan ASSR). Botanicheskii Zhurnal. 65:1169–1172.
- Solbrig OT. 1977. Chromosomal cytology and evolution in the family Compositae. In: Heywood VH, Harborne JB, Turner BL, editors. The biology and chemistry of the Compositae. vol I. London: Academic Press; p. 269–281.
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Edward Arnold.
- Strid A. 1991. Jurinea Cass. In: Strid A, Kit T, editors. Mountain Flora of Greece. vol II. Edinburgh: Edinburgh University Press; p. 476–478.
- Stuessy TF. 1977. Heliantheae – systematic review. In: Heywood VH, Harborne JB, Turner BL, editors. The biology and chemistry of the Compositae. vol II. London: Academic Press; p. 621–671.
- Susanna A, Garcia-Jacas N, Hidalgo O, Vilatersana R, Garnatje T. 2006. The Cardueae (Compositae) revisited: Insights from its, trnL-trnF, and matK nuclear and chloroplast DNA analysis. Annals of the Missouri Botanical Garden. 93(1):150–171.
- Vir JD, Kachroo P. 1985. Chromosomal conspectus of some alpine-subalpine taxa of Kashmir Himalaya. Chromosomal Informational Service. 39:33–35.
- Vogt R, Oberprieler C. 1993. Chromosome numbers of North African phanerogams. I. Florae Mediterranean. 3:187–210.
- Wang YJ, Liu JQ, Miehe G. 2007. Phylogenetic origins of the Himalayan endemic Dolomiaea, Diplazoptilon and Xanthopappus (Asteraceae: Cardueae) based on three DNA regions. Annals of Botany. 99:311–322.
- Wang X, Liu BB, Ma YZ, Xie PH, He XY, Shang BL, Wang YJ. 2013. Chromosomal study on the Alpine genus Dolomiaea (Astraceae: Cardueae) from the Qinghai-Tibet Plateau and adjacent regions. Caryologia. 66:186–193.
- Watanabe K. 2013. Index to chromosome numbers in Asteraceae. http://www.lib.kobe-u.ac.jp/products/asteraceae/index.html.
