Abstract
Detailed cytological characteristics of both sporophytic as well as gametophytic generation of two bulbaceous species, Ledebouria revoluta and Drimiopsis botryoides, were studied by investigating together pollen mitosis and root tip mitosis along with meiosis. A comparative study showed there was no significant difference between chromosome characteristics of haploid and diploid plants. The chromosome number of L. revoluta was 2n = 30 (in root tip) and n = 15 (in pollen) and that of D. botryoides was 2n = 66 (in root tip) and n = 33 (in pollen). Both genera have a monomodal chromosome with gradual decreases in size from the longest, 6.79 μm, to the shortest, 1.50 μm, in L. revoluta, and 9.65 to 1.55 μm in D. botryoides. The karyotypic formula of L. revoluta was 20m + 10sm and D. botryoides was 30m + 14sm + 16 st + 6t. All the meiotic events were found to be normal in both genera and a clear 15 and 33 bivalents were observed in L. revoluta and D. botryoides accordingly. Very few meiotic abnormalities were observed in L. revoluta (about 2.4%); a comparatively higher percentage (nearly 7.9%) of abnormalities were observed in D. botryoides in the form of laggard, bridge, chromosome stickiness, early or late anaphasic movement, etc. However, the ultimate product of meiosis, i.e. microspores/pollens, are normal with an expected haploid chromosome number (n = 15 and 33 accordingly). Overall, L. revoluta is karyotypically less asymmetrical with a lower chromosome number than D. botryoides, indicating that D. botryoides is evolutionarily more advanced than L. revoluta.
Introduction
Ledebouria revoluta (L.f.) Jessop [Syn: Scilla indica (Wight) Baker. or Scilla hyacinthina (Roth) J.F.Macbr.] and Drimiopsis botryoides Baker [Syn: Drimiopsis kirkii Baker] are bulbaceous species of the family Asparagaceae (The Plant List 2013). Traditionally used medicinal plants have recently attracted the attention of pharmaceutical and scientific communities. Both species have long been widely used in the ethno-medicinal systems of India and South Africa. Scaly bulbs of these plants have cardio-protective potentialities along with antioxidant and antimicrobial properties (Tripathi et al. Citation2001; du Toit et al. Citation2007; Sakthivel et al. Citation2013; Muleya et al. Citation2014). Many important cardiac glycosides and homoisoflavanones had been isolated from Ledebouria and Drimiopsis (Jha and Sen Citation1980; Moodley et al. Citation2006; du Toit et al. Citation2007). Beside its medicinal importance, D. botryoides is also used as an ornamental plant for its attractive dark green mosaic leaves with a leathery texture (Haque and Ghosh Citation2014).
The chromosome number is the most frequently documented cytological character. For nearly a century, botanists have been interested in the resolution and documentation of chromosome numbers for taxa (Goldblatt and Lowry Citation2011). These data have been documented in journal articles, books and more recently in the form of online databases (Bennett and Leitch Citation2011; Rice et al. Citation2015). Chromosome counts exist for only around 25–30% of angiosperms (Bennett Citation1998; Guerra Citation2008). Among flowering plants, the chromosome number ranges from 2n = 4, found in four monocotyledons, Zingeria biebersteiniana, Colpodium versicolor, Ornithogalum tenuifolium and Rhynchospora tenuis, and two dicotyledons, Haplopappus gracilis and Brachyscome dichromosomatica (Castiglione and Cremonini Citation2012) to 2n = 640, found in the dicotyledonous stonecrop Sedum suaveolens (Uhl Citation1978). The effectiveness and reliability of cytological techniques for studying the evolution of angiosperms have been recognized for a long time (Sharma and Sharma Citation2012). Chromosome numbers have been extensively utilized as an important phylogenetic character in the context of cytotaxonomy (Guerra Citation2012; Glick and Mayrose Citation2014). The karyotype concept provides basic information about chromosome number, morphology of an individual chromosome, chromosomal aberrations, chromosomal homology and ploidy level in plants (Peruzzi and Eroğlu Citation2013). The karyotype features that are most commonly documented in comparative evolutionary studies, and that can be used to determine the correct systematic position of plant species, are based on morphological characteristics of chromosomes such as size, arm ratio and secondary constructions. Expensive techniques including chromosome banding and fluorescence in situ hybridization (FISH) may be necessary to establish a relationship between taxa with uniform (i.e. symmetric) karyotypes; however, taxa with asymmetric karyotypes can be studied with simple squashing and solid staining with orcein/carmine. Chromosome numbers of angiosperms are usually reported as sporophytic diploid numbers 2n and also as gametophytic haploid numbers n. This haploid number n not always as monoploid or basic chromosome (x) number. So, to confirm the basic chromosome number of a species, karyotypic studies of both diploid somatic cells (2n) and the haploid pollen cell (n) are essential along with corresponding meiotic chromosomal behavior study.
The structure and behavior of plant chromosomes during reduction division is important because genetic variation of both gametes and the future sporophyte depends on it. In particular, meiotic abnormalities have received considerable attention in cytogenetic investigations. Polyploidy is very common among plant species and this raises special issues for the analysis of meiosis in many plants, quite apart from the fundamental issues of meiosis. Cytologically, both Ledebouria revoluta and Drimiopsis botryoides are complex with various chromosome counts. The chromosome number of L. revoluta have been reported as 2n = 20, 22, 28, 30, 44, 45, 46, 58, 60, 64, 68 and that of D. botryoides as 2n = 30, 44, 55, 60, 66, 68, 80 (Table ). So in addition to mitotic karyotype study, the meiotic behavior, particularly the bivalent state is very important to determine the right ploidy status of these species.
Table 1. Previous chromosome counts of Ledebouria revoluta (L.f.) Jessop and Drimiopsis botryoides Baker.
The effects of climate change during the last two decades have led to concern about the life cycles and diversification of the world’s vegetation, including wild medicinal and aromatic plants (Cavaliere Citation2009). All of the previously published cytological reports on L. revoluta and D. botryoides are at least 20–50 years old. A significant climatic change has occurred during this time period and plant genetic composition may change in response to the selection pressure of climate change. Furthermore, in most of the previous cytological reports, both the genera had been considered under the family Liliaceae; but in recent times, both belong to the family Asparagaceae (according to The Plant List 2013). Therefore, a detailed re-evaluation of the karyotype and meiotic behavior is needed. There have been no karyotypic studies of the gametophytic generation of these species. In this paper we study the detailed karyotypes of gametophytic and sporophytic generation along with meiotic behavior; this will enrich the cytological database of both L. revoluta and D. botryoides and help us to understand the evolutionary advancement of these species.
Material and methods
For the somatic chromosome study, young and healthy root tips (0.5 cm long) were pre-treated either with a saturated solution of para-dichlorobenzene (\xFEDB) at 16–18°C for 4 h or with 2.5 mM 8-hydroxyquinoline at 10–12°C for 3.5 h. Then pre-treated root tips were fixed, stained with 2.0% aceto-orcein and squashed following the methods described by Haque and Ghosh (Citation2013). Karyotype analysis was based on at least 10 high-quality metaphase plates. The chromosomes were sorted into different categories on the basis of the arm ratio [m = 1.0–1.69, sm = 1.7–2.99, st = 3.0–6.99, t = 7.0–∞] following Levan et al. (Citation1964). According to Huziwara (Citation1962), the total form percentage (TF %) was calculated to evaluate karyotype symmetry/asymmetry. During preparation of the karyotype, the homologous chromosomes were tentatively paired on the basis of their morphology and size and were arranged in order of decreasing length. An ideogram was constructed by arranging the chromosomes in homologous pairs by order of their length. Mean length was calculated for each chromosome pair and these values were then used to calculate total chromatin length of each diploid karyotype. Since a single set of chromosomes is present in haploid cells, so there is no need for calculating the mean length of chromosome pairs during haploid karyotype preparation. All chromosome plates were observed in Leica DM750 microscope [© Leica Microsystems (Switzerland) Ltd.], and individual chromosomes are measured with the help of “Leica application suite” (version 4.0) software and photographed by the Leica DFC295 camera.
For meiotic and pollen mitotic studies, flower buds of the appropriate size were fixed in freshly prepared Carnoy’s solution (absolute ethanol, chloroform and acetic acid at 6:3:1 ratio) for 24 h at 4°C. After overnight fixation, buds were stored in 70% ethanol at 4°C until further study. Anthers were smeared in 2% aceto-carmine following Sharma and Sharma’s (Citation1980) methods and photographed by the Leica DFC295 camera. Gametophytic karyotype analysis was based on at least 10 well-scattered metaphase plates of haploid pollen grains.
Results
Karyomorphological details of L. revoluta and D. botryoides are summarized in Table . According to Stebbins’s classification (Stebbins Citation1971), both species (L. revoluta and D. botryoides) belonged to class 3C karyotype asymmetry category.
Table 2. Karyomorphological characteristics of sporophytic plants of Ledebouria revoluta and Drimiopsis botryoides.
Mitotic chromosome analysis of Ledebouria revoluta
The chromosome number of gametophytic plant cells (i.e. pollen) was n = 15 and that of sporophytic plant cells (i.e. root tip cells) was 2n = 30 (Figure a,d). The karyotype was asymmetrical and monomodal with gradual decreases in chromosome size from 6.73 μm to 1.46 μm in the gametophyte (Figure b, c; Table ) and 6.79 μm to 1.50 μm in the sporophyte (Figure e, f; Table ). The ratio of the largest and smallest chromosome lengths was 4.61 for the gametophyte and 4.53 for the sporophyte. The centromeric position varies from median to submedian region with centromeric index (Ic) 47.85–29.34. The karyotypic formula was 20m + 10sm. The total chromatin length was 36.73 ± 1.97 μm (mean of gametophytic and sporophytic TCL) and the total forma percentage was 39.64% (mean of gametophytic and sporophytic TF %).
Figure 1. Mitotic chromosome study of gametophytic and sporophytic generation of Ledebouria revoluta: (a) metaphase plate of pollen grain (gametophyte) showing n = 15 chromosomes; (b) karyogram of pollen grain; (c) idiogram of haploid gametophytic plants; (d) metaphase plate of root-tip cell (sporophyte) showing 2n = 30 chromosomes; (e) karyogram of diploid root-tip cell; (f) idiogram of diploid sporophytic plants.
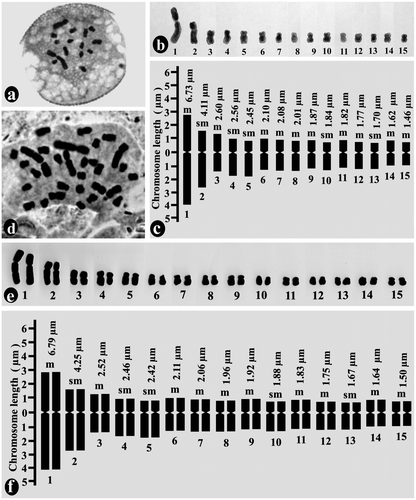
Table 3. Chromosome parameters of haploid pollen grains of Ledebouria revoluta.
Table 4. Chromosome parameters of diploid root-tip cells of Ledebouria revoluta.
Mitotic chromosome analysis of Drimiopsis botryoides
The chromosome number of pollen grains of D. botryoides was n = 33 and that of root-tip cells was 2n = 66 (Figures a, a). Here also the karyotype was asymmetrical, and monomodal with gradual decreases in chromosome size from 9.45 μm to 1.42 μm in the gametophyte (Figure b,c; Table ) and 9.65 μm to1.55 μm in the sporophyte (Figure b,c; Table ). The ratio of the largest and the smallest chromosome lengths was 6.65 for the gametophyte and 6.23 for the sporophyte. The centromeric position varies from the median to the terminal region with Ic ranging from 49.61 to 11.11. The karyotypic formula was 30m + 14sm + 16st + 6t. The total chromatin length was 159.73 ± 2.54 μm (mean of gametophytic and sporophytic TCL) and the TF % was 26.83% (mean of gametophytic and sporophytic TF %).
Figure 2. Mitotic chromosome study of pollen grain (gametophytic generation) of Drimiopsis botryoides: (a) metaphase plate of pollen grain showing n = 33 chromosomes; (b) karyogram of pollen grain; (c) idiogram of haploid gametophytic plants.
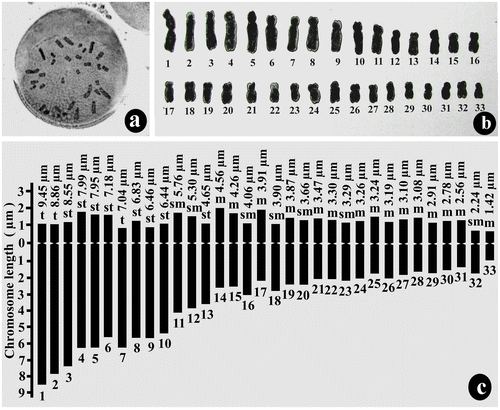
Figure 3. Mitotic chromosome study of root-tip cell (sporophytic generation) of Drimiopsis botryoides. (a) metaphase plate of root-tip cell showing 2n = 66 chromosomes; (b) karyogram of root-tip cell; (c) idiogram of diploid sporophytic plants.
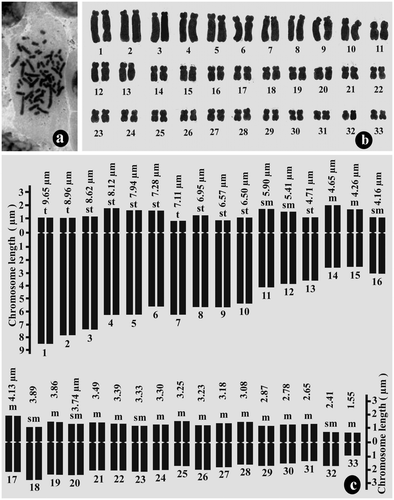
Table 5. Chromosome parameters of haploid pollen grains of Drimiopsis botryoides.
Table 6. Chromosome parameters of diploid root-tip cells of Drimiopsis botryoides.
Meiotic chromosome analysis of Ledebouria revoluta and Drimiopsis botryoides
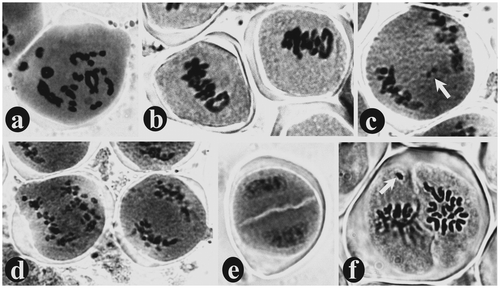
Bivalents with several chiasmata was observed at diplotene stage (Figure a). During diakinesis, a total of 15 bivalents were observed in L. revoluta and 33 bivalents in D. botryoides, which confirms their diploid numbers 2n = 30 and 2n = 66 respectively (Figures a, b). All the bivalents are moved to the equatorial region during metaphase-I (Figures b, c). At anaphase-I, one chromosome of each homolog migrates toward the opposite pole through anaphasic movements driven by the spindle apparatus (Figure d) and ultimately reaches two opposite poles during telophase-I (Figure j). A perfect diad with two distinct cells covered by a common wall was observed. These are the first haploid cells produced from diploid pollen mother cell (PMC) through meiosis-I (Figure e). At metaphase-II, all the chromosomes of each haploid cell of the diad are arranged in the equatorial region (Figure k). In case of L. revoluta a haploid number of chromosomes (i.e. 15 chromosomes) are clearly countable in each cell of the diad (Figure f). Ultimately, four haploid microspores are formed at the end of meiosis-II, which are typically arranged in a tetragonal configuration covered by a common wall to form microspore tetrads (Figure m). Sometimes meiotic division is asynchronous between the anthers of the same bud or even the PMCs of the same anther. Asynchrony was even observed between the two cells of the diad, i.e. within the same PMC during the second meiotic division (Figure l). Only 2.4% meiotic abnormalities occurred in L. revoluta, in the form of early/late anaphasic movements of chromosome, bivalent/univalent laggards (Figure c), and chromosome unable to reach the equatorial plane during metaphase-II (Figure f); whereas total 7.9% meiotic abnormalities were observed in PMCs of D. botryoides, in the form of univalent/bivalent laggards (Figure c), stickiness in metaphasic chromosomes (Figure e), one or two chromosomes unable to reach the equatorial plane during metaphase-I (Figure d, e), laggards, dicentric bridge and acentric fragments (Figure h), early/late anaphasic movements of chromosomes (Figure f, g), and unequal separation, i.e. more than the haploid number of chromosomes are moved toward one pole and less than the haploid number moved towards the opposite pole (Figure i).
Figure 4. Study of meiotic chromosomes in Drimiopsis botryoides stained with aceto-carmine: (a) diplotene of prophase-I showing thread like bivalents with chiasmata; (b) diakinesis of prophase-I showing 33 bivalents; (c) metaphase-I showing all chromosomes arranged in the equatorial plane of PMC; (d) abnormal metaphase-I where one chromosome has failed to reach the equatorial plane (arrow); (e) abnormal metaphase-I where two chromosomes have failed to reach the equatorial plane (arrow) and the rest of the chromosomes are sticky in nature; (f) abnormal anaphase-I with late anaphasic movements of one chromosome pair (arrow); (g) abnormal anaphase-I with early anaphasic movements of one chromosome (arrow); (h) abnormal anaphase-I with dicentric bridge and acentric fragment (arrows); (i) abnormal anaphase-I with unequal separation; (j) telophase-I showing two sets of chromosomes at two opposite poles; (k) metaphase-II showing all chromosomes of each haploid cell of the diad at the equatorial plane; (l) a diad with asynchronous second division; one cell in metaphase and another in telophase; (m) a perfect tetrad with four tetragonally arranged cells (i.e. microspores) covered by a common wall.
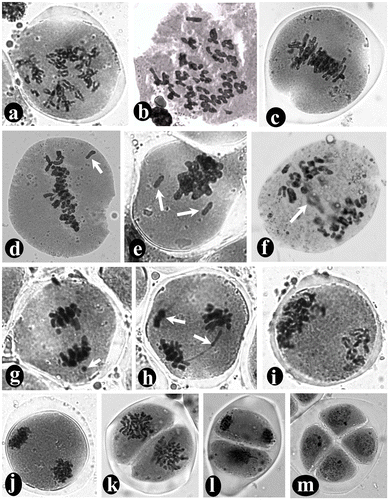
Figure 5. Study of meiotic chromosomes in Ledebouria revoluta stained with aceto-carmine: (a) diakinesis of prophase-I showing 33 bivalents; (b) metaphase-I; (c) anaphase-I with one bivalent laggard (arrow); (d) anaphase-I; (e) a perfect diad with two distinct cells covered by a common wall; (f) metaphase-II showing each haploid cell of the diad containing 15 chromosomes arranged in equatorial plane. In one cell of the diad, a chromosome failed to reach the equatorial plane (arrow).
Discussion
Both L. revoluta and D. botryoides are complex with diverse chromosome counts within the species. The diploid chromosome number of L. revoluta has been reported as 2n = 20, 22, 28, 30, 44, 45, 46, 58, 60, 64 and 68 (Table ). Our present findings show that normal in vivo grown plants of L. revoluta are diploid with 2n = 30 chromosomes, which agreed with the previous studies (Sen Citation1973a; Jha and Sen Citation1980; Dixit et al. Citation1989; Nair Citation1989; Chakravarty and Sen Citation1992). However, some mixoploid plants had also been induced either by γ-irradiation or generate spontaneously during in vitro culture (Chakravarty and Sen Citation1987, Citation1989, Citation1992, Citation2001). The chromosome count (2n = 30) was documented in these previous reports, but the karyotype was not described except for in Chakravarty and Sen (Citation1992). Although the diploid number found here is the same, the karyotype was very different from that described by Chakravarty and Sen (Citation1992). They found one pair of acrocentric, three pair of metacentric and 11 pair of sub-metacentric chromosomes, whereas our findings confirm 10 pairs of metacentric and five pairs of sub-metacentric chromosomes, i.e. the acrocentric chromosomes are absent. To defend our findings, which differ from earlier reports, we have prepared the karyotype from both haploid (i.e. pollen) as well as diploid (i.e. root-tip) plants, which makes our results more authentic. Like L. revoluta, different chromosome counts have been reported in D. botryoides as 2n = 30 (Vijayavalli and Mathew Citation1988); 2n = 44, 55 (Stedje and Nordal Citation1987); 2n = 44, 55, 66 (Stedje Citation1994); 2n = 60 (Vij et al. Citation1982); 2n = 66 (Sharma Citation1970); 2n = 66, 68, 126 (Sen Citation1973b); 2n = 68 (Mahalakshmi and Sheriff Citation1970); and 2n = 80 (Darlington and Wylie Citation1955). However, all of these reports are old and only emphasized chromosome counts; detailed karyological characters are poorly known. The present study of the detailed karyotype from both gametophytic (i.e. pollen) and sporophytic (i.e. root-tip) plants not only enriches the karyotypic database but also help us to understand the cytotaxonomy of D. botryoides.
The main advantage of preparing karyotypes from haploid pollen is they contain only a single set of chromosomes, so there is no confusion about accurate pairing of homologous chromosomes through orcein staining technique. According to Sapre (Citation1975), pollen mitotic studies reveal chromosome morphology so well that there is no need for root-tip squashes. During our study on pollen mitotic metaphase, the centromeric positions are clearly observed in all the chromosomes present in the haploid set of L. revoluta and D. botryoides (Figure a, a). Detailed karyomorphology from pollen mitosis was also studied in Aloe (Sapre Citation1975; Das et al. Citation2010).
Study of chromosome structure and behavior in meiosis is important, as genetic recombination occurs during pachytene of meiosis-I, causing variation both in gametes and in future sporophytes. During our study we observed a perfect bivalent structure as a result of proper pairing of homologous chromosomes (Figures a, b). This observation confirms the true ploidy level (i.e. diploid) of our observed plants. Putative alignment of chromosomes and their behavior during reduction division has been used in several cases for determining the base number, ploidy level, type of ploidy and also for detecting structural chromosomal changes that a taxon might have undergone during evolution (Sharma and Sharma Citation2012). The events of meiosis are controlled by a large number of genes, and mutations in these genes cause several anomalies that may affect plant fertility or cause total sterility (Pagliarini Citation2000). The phases of meiosis are generally regular, but a few abnormalities are also noted in L. revoluta (2.4%) and D. botryoides (7.9%). Similar types of abnormalities have also been noted in other monocots, e.g. Bellevalia of the family Hyacinthaceae (Dane Citation2006), Aloe vera of the family Aloaceae (Haque and Ghosh Citation2013), Allium and Fritillaria of the family Liliaceae (Zhang et al. Citation2012; Kartal Citation2015). Both genetic and environmental factors are responsible for chromosome stickiness (Pagliarini Citation2000). According to Gaulden (Citation1987), the sticky chromosomes can arise from the defective functioning of specific non-histone proteins involved in chromosome organization, which are desirable for chromatid separation and segregation. Chromosome stickiness may arise from incorrect folding of chromatin fibers into a single chromatid, as a result of which chromatin fibers intermingle and chromosomes become attached to each other by means of sub-chromatid bridges (Klasterskii et al. Citation1976). Chromosomal bridges may form due to stickiness, unequal exchange or dicentic chromosomes (Chaudhari and Chaudhary Citation2012). A typical dicentric bridge and acentric fragment have been observed in D. botryoides (Figure h), formed by a single crossing over occurring within the loop formed through paracentric inversion. Abnormality in meiotic behavior is common and spontaneous in both species, but a higher percentage occurred in D. botryoides. These outcomes are corroborated by previous studies which found meiotic abnormalities occurring spontaneously in Chlorophytum comosum (Gudadhe et al. Citation2012) or in other species of the studied genus, e.g. Drimiopsis barteri (Oyewole Citation1984). Although different meiotic abnormalities are observed, unpredictably at tetrad stage microspores appeared normal in both L. revoluta and D. botryoides. Similar findings were also noted in Aloe and Fritillaria (Sapre Citation1975; Haque and Ghosh Citation2013; Kartal Citation2015). The synchrony in meiotic cell division can increase the efficiency of sexual reproduction through ensuring sufficient fertility of the organism (Magnard et al. Citation2001); however, asynchronous meiotic division seems more common in vegetatively reproducing plants. Both L. revoluta and D. botryoides reproduce vegetatively, and therefore the asynchrony during meiotic division is quite usual. Cytokinesis of meiosis in both L. revoluta and D. botryoides is successive, and the tetrads show tetragonal (or isobilateral) arrangement similar to most monocotyledons (Haque and Ghosh Citation2013; Kartal Citation2015).
Chromosome studies are vital to unraveling the process of evolution (Vosa Citation2005). The karyotype is the highest level of structural and functional organization of the nuclear genome. Karyotype asymmetry could be explained by the shifting of the centromere from median to subterminal and terminal positions as well as increasing the ratio of largest and smallest chromosomes size (Sharma Citation1956). By comparing the chromosomes of different taxa much can be learned about patterns and mechanisms of karyotype evolution and its significance for diversification and speciation (Weiss-Schneeweiss and Schneeweiss Citation2013). Based on a theoretic approach, the minimum-interaction hypothesis states that long-term chromosome evolution usually tends to evolve as a whole towards increasing number and acrocentric chromosomes through centric fission (Imai et al. Citation1986); this trend is beneficial in diminishing genetic threats due to harmful reciprocal translocations and in increasing the potential of genetic divergence (Imai et al. Citation2002). Asymmetric karyotypes in higher plants are considered to occur in more evolutionarily advanced taxa (Vosa Citation2005). The presence of acrocentric chromosomes in D. botryoides denote it is karyotypically more asymmetric than L. revoluta where acrocentric chromosomes was totally absent and ratio value between largest and smallest chromosomes was relatively lower (4.65) than D botryoides (6.23). The trend for chromosome number to increase with evolutionary advancement has been observed in monophyletic slipper orchids Phragmipedium and Paphiopedilum (Cox et al. Citation1998). With a higher chromosome number (2n = 66), greater TCL (159.73 ± 2.54 μm) and lower TF % (26.83), the genus D. botryoides is evolutionarily more advanced than L. revoluta, having a comparatively lower chromosome number (2n = 30), smaller TCL (36.73 ± 1.97 μm) and higher TF % (39.64). Similarly, CitationCox et al. (Citation1997) observed a proportional relationship between the degree of evolutionary advancement and an increase in chromosome number in slipper orchids. According to Sharma and Sharma (Citation2012), to determine the relationship between various taxa with confidence, chromosomes need to be viewed in combination with other aspects of an organism, such as breeding system, habit, morphology, distribution and biochemistry; because the phenotypic aspects of the chromosomes cannot be more informative than the expression of genes as seen in the morphology of a plant. So, on the basis of our cytological findings, we can assume that Drimiopsis is evolutionarily more advanced than Ledebouria. However, the present cytological study demands additional supportive studies on other aspects to establish evolutionary progression confidently; research is ongoing on morphology, sexual and asexual reproduction system, biochemistry, and the molecular marker system to address these demands.
In conclusion, our combined study on mitosis, meiosis and pollen mitosis enriches the cytological database of both Ledebouria and Drimiopsis. In addition, this study throws light on the evolutionary advancement between them on the basis of cytological characteristics.
Acknowledgments
SMH acknowledges the Ministry of Minority Affairs and University Grant Commission for providing the Maulana Azad National Fellowship. Both the authors are thankful to Swami Kamalasthananda, Principal, Ramakrishna Mission Vivekananda Centenary College, Rahara, Kolkata (India), for the facilities provided for the present study. They also acknowledge the Department of Science & Technology (DST) “Fund for Improvement of Science & Technology infrastructure in universities & higher educational institutions” (FIST) program for infrastructural facilities.
Funding
This work was supported by University Grants Commission (INDIA) [grant number F1-17.1/2010/MANF-MUS-WES-5180].
References
- Bennett MD. 1998. Plant genome values: how much do we know? Proc Natl Acad Sci USA. 95:2011–2016.
- Bennett MD, Leitch IJ. 2011. Nuclear DNA amounts in angiosperms: targets, trends and tomorrow. Ann Bot. 107(3):467–590.
- Castiglione MR, Cremonini R. 2012. A fascinating island: 2n=4. Plant Biosyst. 146(3):711–726.
- Cavaliere C. 2009. The effects of climate change on medicinal and aromatic plants. HerbalGram. 81:44–57.
- Chakravarty B, Sen S. 1987. In vitro regeneration from callus cultures of Scilla indica (Roxb.) Baker. Curr Sci. 56(9):960–962.
- Chakravarty B, Sen S. 1989. Regeneration through somatic embryogenesis from anther explants of Scilla indica (Roxb.) Baker. Plant Cell Tiss Org. 19(1):71–75.
- Chakravarty B, Sen S. 1992. Chromosomes and nuclear DNA in regenerants of Scilla indica (Roxb.) Baker derived from two explant sources. Cytologia. 57(1):41–46.
- Chakravarty B, Sen S. 2001. Enhancement of regeneration potential and variability by γ-irradiation. Biol Plantarum. 44(2):189–193.
- Chaudhari AK, Chaudhary BR. 2012. Meiotic chromosome behaviour and karyomorphology of Aloe vera (L.) Burm. f. Chromosome Bot. 7(1):23–29.
- Cox AV, Abdelnour GJ, Bennett MD, Leitch IJ. 1998. Genome size and karyotype evolution in the slipper orchids (Cypripedioideae: Orchidaceae). Am J Bot. 85(5):681–687.
- Cox AV, Pridgeon AM, Albert VA, Chase MW. 1997. Phylogenetics of the slipper orchids (Cypripedioideae: Orchidaceae): nuclear rDNA ITS sequences. Plant Syst Evol. 208(3):197–223.
- Dane F. 2006. Cytological and histological studies on reproductive system of hexaploid Bellevalia edirnensis Özhatay & Mathew (Hyacinthaceae). Acta Biol Hun. 57(3):339–354.
- Darlington CD, Wylie AP. 1955. Chromosome atlas of flowering plants. London: George Allen and Unwin.
- Das A, Mukherjee P, Ghorai A, Jha TB. 2010. Comparative karyomorphological analyses of in vitro and in vivo grown plants of Aloe vera L. BURM. f. Nucleus. 53(3):89–94.
- Dixit GB, Yadav SR, Salunkhe CB. 1989. Cytomorphological studies in Scilla hyacinthiana (Roth.) Macbr. complex from Maharashtra. Glimpses Cytogenet India. 2:124–134.
- du Toit K, Elgorashi EE, Malan SF, Mulholland DA, Drewes SE, van Staden J. 2007. Antibacterial activity and QSAR of homoisoflavanones isolated from six Hyacinthaceae species. S Afr J Bot. 73(2):236–241.
- Gaulden ME. 1987. Hypothesis: some mutagens directly alter specific chromosomal proteins (DNA topoisomerase II and peripheral proteins) to produce chromosomal stickiness, which causes chromosome aberrations. Mutagenesis. 2(5):357–365.
- Glick L, Mayrose I. 2014. ChromEvol: assessing the pattern of chromosome number evolution and the inference of polyploidy along a phylogeny. Mol Biol Evol. 31(7):1914–1922.
- Goldblatt P, Lowry II PP. 2011. The Index to Plant Chromosome Numbers (IPCN): three decades of publication by the Missouri Botanical Garden come to an end. Ann Missouri Bot Gard. 98(2):226–227.
- Gudadhe SP, Nathar VN, Dhoran VS. 2012. Meiotic abnormalities in Chlorophytum comosum (Thunb) Jacq. Int J Res Plant Sci. 2(2):29–34.
- Guerra M. 2008. Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenet Genome Res. 120(3-4):339–350.
- Guerra M. 2012. Cytotaxonomy: the end of childhood. Plant Biosyst. 146(3):703–710.
- Haque SM, Ghosh B. 2013. High frequency microcloning of Aloe vera and their true-to-type conformity by molecular cytogenetic assessment of two years old field growing regenerated plants. Bot Stud. 54:46. http://www.as-botanicalstudies.com/content/54/1/46
- Haque SM, Ghosh B. 2014. Somatic embryogenesis and synthetic seed production – a biotechnological approach for true-to-type propagation and in vitro conservation of an ornamental bulbaceous plant Drimiopsis kirkii Baker. Appl Biochem Biotechnol. 172(8):4013–4024.
- Huziwara Y. 1962. Karyotype analysis in some genera of Compositae. VIII. Further studies on the chromosome of Aster. Amer J Bot. 49(2):116–119.
- Imai HT, Maruyama T, Gojobori T, Inoue Y, Crozier RH. 1986. Theoretical bases for karyotype evolution. 1. The minimum-interaction hypothesis. Am Nat. 128(6):900–920.
- Imai HT, Satta Y, Wada M, Takahata N. 2002. Estimation of the highest chromosome number of eukaryote based on the minimum interaction theory. J Theor Biol. 217(1):61–74.
- Jha S, Sen S. 1980. A search for scilladienolides in Scilla indica Roxb. Curr Sci. 49(7):273–274.
- Johnson MAT, Brandham PE. 1997. New chromosome numbers in petaloid monocotyledons and in other miscellaneous angiosperms. Kew Bull. 52(1):121–138.
- Kartal C. 2015. Microsporogenesis, microgametogenesis and in vitro pollen germination in the endangered species Fritillaria stribrnyi (Liliaceae). Caryologia. 68(1):36–43.
- Klasterskii I, Natarajan AT, Ramel C. 1976. An interpretation of the origin of subchromatid aberrations and chromosome stickiness as a categories of chromatid aberrations. Hereditas. 83(2):153–162.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52(2):201–220.
- Magnard J-L, Yang M, Chen YCS, Leary M, McCormick S. 2001. The Arabidopsis gene tardy asynchronous meiosis is required for the normal pace and synchrony of cell division during male meiosis. Plant Physiol. 127(3):1157–1166.
- Mahalakshmi N, Sheriff A. 1970. Karyomorphological studies in Drimiopsis kirkii Baker. Proc Indian Acad Sci B. 72(6):270–276.
- Moodley N, Crouch NR, Mulholland DA, Slade D, Ferreira D. 2006. 3-Benzyl-4-chromanones (homoisoflavanones) from bulbs of the ethnomedicinal geophyte Scilla indica (Hyacinthaceae). S Afr J Bot. 72(4):517–520.
- Muleya E, Ahmed AS, Sipamla AM, Mtunzi FM. 2014. Free radical scavenging and antibacterial activity of crude extracts from selected plants of medicinal value used in Zululand. Pak J Nutr. 13(1):38–42.
- Nair AS. 1989. Micropropagation of Scilla hyasynthiana (Roth) Macbride. Proc Indian Natn Sci Acad B. 55(2):121–124.
- Oyewole SO. 1984. Microsporogenesis and sexual sterility in Drimiopsis barteri. Cytologia. 49(1):87–93.
- Pagliarini MS. 2000. Meiotic behavior of economically important plant species: the relationship between fertility and male sterility. Genet Mol Biol. 23(4):997–1002.
- Peruzzi L, Eroğlu HE. 2013. Karyotype asymmetry: again, how to measure and what to measure? Comp Cytogenet. 7(1):1–9.
- Raghavan TS, Venkatasubban KR. 1939. Studies in the Indian Scilleae. II. The cytology of Scilla indica Baker. Cytologia. 10(1-2):189–204.
- Rice A, Glick L, Abadi S, Einhorn M, Kopelman NM, Salman-Minkov A, Mayzel J, Chay O, Mayrose I. 2015. The chromosome counts database (CCDB) – a community resource of plant chromosome numbers. New Phytol. 206(1):19–26.
- Sakthivel K, Palani S, Selvaraj R, Venkadesan D, Sivasankari H, Senthil BK. 2013. Cardioprotective and antioxidant potential of Scilla hyacinthine. J Biol Sci. 13(5):313–322.
- Sapre AB. 1975. Meiosis and pollen mitosis in Aloe barbadensis Mill. (A. perfoliata var. vera L., A. vera Auth. non Mill.). Cytologia. 40(3-4):525–533.
- Sarkar AK, Datta N, Chatterjee U. 1980. In: Chromosome number reports LXVII. Taxon. 29:360–361.
- Sen S. 1973a. Structural hybridity intra-and interspecific level in Liliales. Folia Biol. (Cracow). 21(2):83–197.
- Sen S. 1973b. Polysomaty and its significance in Liliales. Cytologia. 38(4):737–751.
- Sharma AK. 1956. A new concept of a means of speciation in plants. Caryologia. 9(1):93–130.
- Sharma AK. 1970. Annual Report, 1967–1968. Research Bulletin, 2. University of Calcutta (Cytogenetic Laboratory), pp. 1–50.
- Sharma AK, Sharma A. 1980. Chromosome techniques – theory and practice. 3rd ed. London: Butterworths.
- Sharma G, Sharma N. 2012. Cytology as an important tool for solving evolutionary problems in Angiosperms. Proc Natl Acad Sci India Sect B Biol Sci. 84(1):1–7.
- Sheeba MJ, Vijayavalli B. 1998. Cytological studies of Scilla indica. J Cytol Genet. 33:189–193.
- Sheriff A, Rao UG. 1981. Cytogeographical studies on Scilla indica in India – triploid. Cytologia. 46(1-2):69–74.
- Stebbins GL. 1971. Chromosomal evolution in higher plants. London: Addison-Wesley.
- Stedje B. 1994. A revision of the genus Drimiopsis (Hyacinthaceae) in East Africa. Nordic J Bot. 14(1):45–50.
- Stedje B. 1996. Karyotypes of some species of Hyacinthaceae from Ethiopia and Kenya. Nordic J Bot. 16(2):121–126.
- Stedje B, Nordal I. 1987. Cytogeographical studies of Hyacinthaceae in Africa south of the Sahara. Nordic J Bot. 7(1):53–65.
- Subramanian D. 1981. Cytopolymorphism in Scilla indica Baker. Proc Indian Sci Congr Assoc. 68 (Sect. VI):93.
- The Plant List [Internet]. 2013. Version 1.1. Available from: http://www.theplantlist.org/
- Tripathi YB, Singh AV, Dubey GP. 2001. Antioxidant property of the bulb of Scilla indica. Curr Sci. 80(10):1267–1269.
- Uhl CH. 1978. Chromosomes of Mexican Sedum II. Section Pachysedum. Rhodora. 80(824):491–512.
- Vij SP, Sharma M, Chaudhary JD. 1982. Cytogenetical investigations into some garden ornamentals III. Chromosomes of some monocot taxa. Cytologia. 47(3-4):649–663.
- Vijayavalli B, Mathew PM. 1988. Studies of south Indian Liliaceae: II. Cytology of species of four genera of the tribe Scilleae. New Bot. 15(2-3):61–68.
- Vosa CG. 2005. On chromosome uniformity, bimodality and evolution in the tribe Aloineae (Asphodelaceae). Caryologia. 58(1):83–85.
- Weiss-Schneeweiss H, Schneeweiss GM. 2013. Karyotype diversity and evolutionary trends in Angiosperms. In: Greilhuber J, Dolezel J, Wendel JF, editors. Plant genome diversity. Vol. 2. Vienna: Springer. p. 209–230. DOI: 10.1007/978-3-7091-1160-4_13
- Zhang J, Liu XR, Zhang FX, Liu JX. 2012. Microsporogenesis and development of the male gametophyte in Allium senescens L. (Liliaceae) in China. Plant Syst Evol. 298(9):1619–1624.
