Abstract
Pollen fertility in 21 germplasm collections of ginger was determined by glycero-carmine staining and in vitro germination. Pollen fertility based on staining ranged from 5.59% to 67.73%, while in vitro germination ranged from 2.35% to 60.31% in different collections analyzed. High pollen stainability was not always followed by high in vitro germination in collections analyzed. However, the in vitro germination percentage was always lower than the percentage of stainability in all the collections. Highest in vitro pollen germination was recorded in acc. no. 195 (60.31%) followed by acc. no. 821 (50.67%). Somatic chromosome number analysis of the collections revealed that the two collections with high pollen fertility (acc. nos. 195 and 821) were tetraploids with 2n = 44 while most of the other collections had 2n = 22, the normal chromosome number. One collection with aneuploid chromosome number of 2n = 24 had lower pollen germination (4.82%), similar to many diploid collections. Tetraploids are identified for the first time from germplasm collections of ginger. The role of polyploidy in improving pollen fertility in ginger is discussed.
Introduction
Ginger (Zingiber officinale Rosc.) is one of the most important and most widely used spices as well as a condiment vegetable used worldwide. It is cultivated widely in most tropical and subtropical countries. Essential oil and oleoresin extracted from the fleshy aromatic rhizomes are used for flavoring purposes and in medicine. Ginger was more valued for its medicinal properties in ancient India and China and played an important role in primary health care. In European medicine also ginger was among the most highly valued of all mild carminatives and it was a component of many pharmaceutical preparations (Purseglove et al. Citation1981; Ravindran et al. Citation2005; Parthasarathy et al. Citation2012).
Crop improvement work in ginger is largely restricted to clonal selection from germplasm collections and rare attempts of induced mutations and induced tetraploidy (Ramachandran and Nair Citation1992a; Sasikumar et al. Citation1994; Adaniya and Shirai Citation2001; Smith et al. Citation2004; Ravindran et al. Citation2005). Recombination breeding through hybridization and selection is not possible in ginger due to the absence of viable seed set. This has been attributed to high pollen sterility (Pillai et al. Citation1978; Jayachandran and Vijayagopal Citation1979; Ratnambal Citation1979; Das et al. Citation1999b; Dhamayanthi et al. Citation2003). Some investigators are of the opinion that self incompatibility mechanism also operates to prevent successful fertilization and seed set (Das et al. Citation1999b; Dhamayanthi et al. Citation2003). It has been suggested that cytological factors such as heterozygosity for interchanges and heterozygous paracentric inversions and subsequent meiotic abnormalities are the reasons for high pollen sterility in ginger (Ramachandran Citation1969, Citation1982; Ramachandran and Nair Citation1992a, Citation1992b; Adaniya and Shoda Citation1998b).
Variation in pollen fertility based on stainability among different genotypes (cultivars) of ginger has been indicated by a few workers (Ratnambal and Nair Citation1981; Adaniya and Shoda Citation1998a; Das et al. Citation1999b; Dhamayanthi et al. Citation2003; Subbarayudu et al. Citation2014). Earlier pollination experiments with cultivars having low pollen fertility have not resulted in fruit or seed set (Jayachandran and Vijayagopal Citation1979; Das et al. Citation1999b; Dhamayanthi et al. Citation2003). Valsala et al. (Citation1996) indicated the successful use of in vitro pollination methods for inducing seed set in ginger. However, they did not demonstrate the origin of “seed” as the actual product of fertilization occurred in vitro. It is evident from the above literature that any study on fruit and seed set in ginger requires genotypes having relatively higher pollen and ovule fertility. Pollen fertility can be assessed by staining and germination studies and ovule fertility can only be assessed by pollination with fertile pollen.
Considering the above, the present study was undertaken to identify the genotypes having high pollen fertility from the germplasm collections of ginger, suitable for pollination studies to induce fruit and seed set. The somatic chromosome number of these genotypes was also verified to ascertain the possible role of numerical variation in chromosomes on pollen fertility.
Materials and methods
Plant materials
Flowers for pollen fertility analysis were derived from plants maintained at the ginger germplasm repository of ICAR – Indian Institute of Spices Research, Calicut. Twenty-one germplasm collections of ginger cultivars, namely acc. nos. 7, 12, 19, 21, 23, 26, 31, 35, 38, 45, 49, 53, 78, 82, 92, 106, 125, 145, 147, 195 and 821 were used for pollen fertility analysis. All these accessions were collected from different locations in India. Rhizomes of these accessions were used for collecting root samples for chromosome number analysis.
Pollen stainability
Pollen stainability studies were performed following the acetocarmine-glycerine staining as described by Ratnambal and Nair (Citation1981) for ginger. The fresh pollen was collected from the anthers at the time of anthesis (1.30–3.30 p.m.) and mixed well on a drop of 1:1 mixture of 2% (w/v) aceto carmine and glycerine on a clean microscopic slide, and put the coverslip. The entire pollen collected from an anther was used for preparing one slide. The observation was taken after 2 h. The pollen grains deeply stained as red were scored as fertile while those unstained, partially stained and stained gray were considered as sterile. Ten microscopic fields each from six slides were counted and percentage of pollen fertility was calculated for the counts from each slide.
In vitro pollen germination
Pollen germination was tested by the sitting drop experiment (Shivanna and Rangaswamy Citation1993) using the culture medium standardized for pollen germination in ginger by Pillai et al. (Citation1978) constituting 8% sucrose, 3% gelatine and 60 ppm boric acid in double distilled water. Pollen was collected from freshly opened flowers, dusted on a drop of culture medium placed on micro-slides kept on humid chambers layered with moistened filter paper, incubated inside a BOD incubator at 26 ± 1°C for 20 h in the dark, and observed. Five microscopic fields each from six slides were counted and percentage of germinated pollen was calculated for each slide.
In vivo germination of pollen
The genotypes showing in vitro pollen germination above 10% were tested for in vivo pollen germination by pollinating freshly opened flowers. Six flowers each were randomly selected from each genotype and self pollinated by hand at the time of anthesis. The style with stigma was detached after 4 h of pollination from three flowers. These were stained by heating with cotton blue in lactophenol and examined under a microscope. The other three flowers were left undisturbed and observed daily for symptoms of fruit set.
Chromosome number analysis
The rhizomes were planted in seed pans filled with clean river sand and regularly watered. On initiation of sprouting and root emergence, root tips were collected for analysis. Actively growing root tips from the freshly emerging roots of 5–10 mm length were collected between 11.00 and 11.30 a.m. and pretreated with a 1:1 mixture of saturated paradichlorobenzene solution and 2 mM 8-hydroxyquinoline at 4–5°C for 4 h. After washing thoroughly in double distilled water, the root tips were fixed in a mixture of ethyl alcohol and acetic acid (3:1) for 24 h.
The fixed root tips were subjected to hydrolysis with 5 M HCl at 0°C for 4 min and stained in 2% aceto orcein for 4 h, and then squashed in 45% acetic acid. Mitotic metaphase stages were observed and chromosomes numbers were counted from temporary slides using a Leica DMRB (Leica, Germany) microscope under 100× objective. Six mitotic metaphase plates with good chromosome spread from 2–3 slides were used for counting the chromosome number in each genotype. The tetraploids identified were further verified by counting many metaphase plates from different root tips.
Morphological characters
Morphological characters of plants were visually observed and described.
Photography and photomicrography
Photographs of plants and rhizomes were taken using an Olympus SP 350 (Olympus, Japan) digital camera and reduced to appropriate size having required resolution. Photomicrographs were taken using a Leica DMRB microscope fitted with Moticam-2300 (Motic, China) digital photomicrographic system, and images were saved using Motic Images Plus-2.0 software. The digital photographs were reduced to appropriate size for presentation.
Statistical analysis
Data on percentage of pollen stainability and in vitro pollen germination were subjected to analysis of variance (ANOVA) using MSTATC software package and means were separated using Duncan’s multiple range test at p = 0.05. The data were subjected to angular transformation before analysis and for presentation original data was retained.
Results
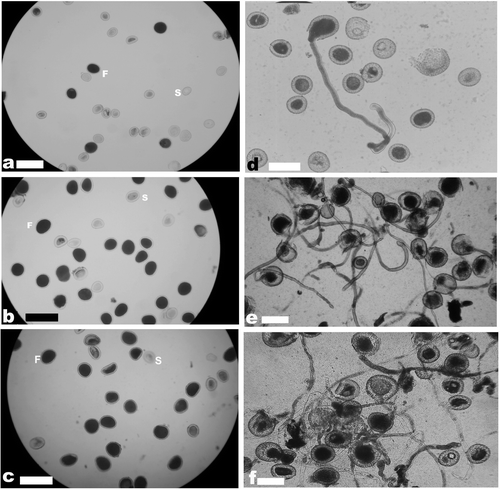
The germplasm collections analyzed showed wide variation in pollen stainability as well as in vitro pollen germination (Table ). Highest pollen stainability and in vitro germination was recorded in acc. no. 195 followed by acc. no. 821. Acc. no. 195 showed 67.73% pollen stainability and 60.31% in vitro germination, while acc. no. 821 showed 58.16% pollen stainability and 50.67% in vitro germination. Among the remaining genotypes, pollen stainability did not exceed 27.5%. Of those exhibiting stainability > 15% (acc. nos. 12, 19, 23, 45, 53, 78, 82, 145 and 147), only two (acc. nos. 12 and 82) showed in vitro germination > 10%. As a general trend, in vitro germination was always lower than the percentage of stainability. However, it was evident that higher stainability need not result in higher in vitro germination always as in the case of acc. nos. 45, 78 and 147. Pollen stainability and in vitro pollen germination in acc. nos. 7, 195 and 821 are presented in Figure .
Table 1. Chromosome number, pollen stainability and in vitro pollen germination in germplasm collections of ginger.
Figure 1. Pollen stainability and in vitro germination in diploid and tetraploid collections of ginger. (a) Pollen stainability in acc. no. 7; (b) pollen stainability in acc. no. 195; (c) pollen stainability in acc. no. 821; (d) in vitro germination of pollen in acc. no. 7; (e) in vitro germination of pollen in acc. no. 195; (f) in vitro germination of pollen in acc. no. 821. Scale bars represent 200 μm in (a–c) and 100 μm in (d–f); F, fertile pollen grain; S, sterile pollen grain.
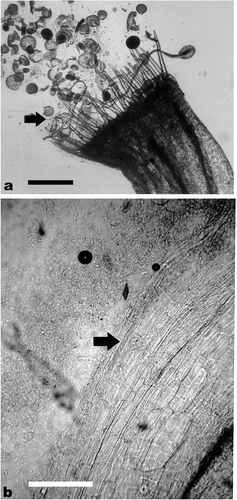
In vivo germination of a few pollen grains was observed on the stigmatic surface of the self pollinated flowers of acc. nos. 12, 82, 195 and 821 (Figure a). In the case of acc. no. 821 progression of pollen tube to style was observed in one instance (Figure b). The pollinated and undisturbed flowers have not resulted in any symptoms of fruit set or fruit development even after weeks. In all of them the labellum along with the anther, style and stigma fell off the day after pollination.
Figure 2. In vivo germination of pollen grains on stigmatic surface and progression of pollen tube in style on self pollination of acc. no. 821. (a) Germination of pollen grains on stigmatic surface (→); (b) progression of a pollen tube in the style (→). Scale bars represent 200 μm in (a) and 100 μm in (b).
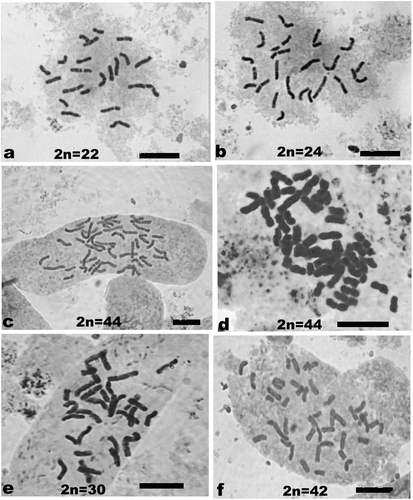
Chromosome number analysis showed normal number of 2n = 22 in root tip cells of 18 collections, namely acc. nos. 7, 12, 19, 21, 23, 26, 31, 35, 38, 45, 49, 53, 78, 82, 92, 106, 125 and 145. In acc. no. 147 somatic chromosome number was 2n = 24, an aneuploid number, while in acc. nos. 195 and 821 the chromosome number was 2n = 44, a tetraploid number. Further analysis of 64 metaphase plates from 10 root tips of acc. no. 195 showed 2n = 44 in 58 cells, 2n = 22 in three cells, and 2n = 34, 2n = 30 and 2n = 42 in one cell each. Occasionally late anaphase stages with lagging chromosomes were observed in root tip squashes of this accession. Analysis of 50 cells from 10 root tips of acc. no. 821 showed no variation in its tetraploid chromosome number of 2n = 44. Mitotic metaphase plates showing 2n = 22 in a diploid collection (acc. no. 12), 2n = 24 in acc. no. 147, 2n = 44 in acc. nos. 195 and 821 and two variant cells of acc. no. 195 showing 2n = 30 and 2n = 40 are presented in Figure .
Figure 3. Mitotic metaphase stages showing chromosome number in diploid, aneuploid and tetraploid collections of ginger. (a) Diploid collection acc. no. 12 showing 2n = 22; (b) aneuploid collection acc. no. 147 showing 2n = 24; (c) tetraploid collection acc. no. 195 showing 2n = 44; (d) tetraploid collection acc. no. 821 showing 2n = 44; (e) a variant cell of acc. no. 195 showing 2n = 30; (f) a variant cell of acc. no. 195 showing 2n = 42. Scale bars represent 10 μm.
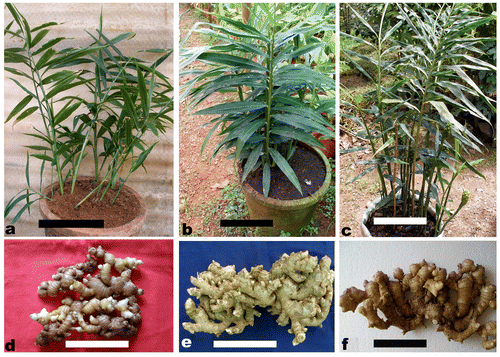
Morphologically both the tetraploid collections showed vigorous growth, greater plant height, leaf and inflorescence size, compared to the diploid collections and the aneuploid. Both the tetraploids produced very bold rhizomes; however, rhizomes of acc. no. 821 were bolder than those of acc. no. 195. Plant and rhizome morphologies of tetraploid collections in comparison to a diploid (acc. no. 7) are presented in Figure .
Discussion
Normal pollen fertility in flowering plants is the result of regular meiosis and balanced segregation of chromosomes unless otherwise influenced by genic or cytoplasmic factors specifically controlling pollen fertility. The role of chromosomal abnormalities such as inversion and translocation heterozygosity in producing duplications and deletions in paired chromosomes during meiosis, as a result of crossing over and its subsequent reflection as reduced pollen fertility, is well established (McClintock Citation1938; Burnham Citation1962; Sjodin Citation1971; Prasad Citation1975). The reason for poor pollen fertility in diploid ginger has been reported as hybridity for interchanges as well as inversion heterozygosity by Ramachandran (Citation1969, Citation1982). Later investigations by Ramachandran and Nair (Citation1992a, Citation1992b) revealed that interchange heterozygosity involving four chromosomes and heterozygosity for three paracentric inversions are present in diploid ginger. Similar observations were subsequently made by Adaniya and Shoda (Citation1998b) in ginger cultivars from Japan and Philippines.
The widely reported chromosome number of ginger is 2n = 2x = 22 and the basic number of the genus is suggested as x = 11 (Morinaga et al. Citation1929; Sugiura Citation1936; Raghavan and Venkatasubban Citation1943; Chakravorti Citation1948; Sato Citation1960; Ramachandran Citation1969; Ratnambal Citation1979; Omanakumari and Mathew Citation1985; Rai et al. Citation1997; Das et al. Citation1998a, Citation1998b, Citation1999a; Dhamayanthi Citation1998; Eksomtramage et al. Citation2001; Nayak et al. Citation2005; Wang et al. Citation2014). The results of the present investigation also showed that majority of germplasm collections have 2n = 22.
Staining with different dyes, namely acetocarmine (Pillai et al. Citation1978; Adaniya and Shirai Citation2001; Dhamayanthi et al. Citation2003; Ratnambal and Nair Citation1981), aceto-orcein (Adaniya and Shoda Citation1998a) and fluorescein diacetate (Subbaraudu et al. Citation2014) has been used to assess the percentage of fertile pollen grains in ginger. In the present study a mixture of 2% acetocarmine and glycerine in 1:1 ratio was used following the method of Ratnambal and Nair (Citation1981). For pollen germination studies the medium and culture conditions standardized by Pillai et al. (Citation1978) for ginger pollen were selected, after a preliminary comparison with Brewbaker and Kwack (Citation1963) and the medium formulated by Dhamayanthi et al. (Citation2003) using the pollen from a released variety of ginger namely Varada.
Wide variation in pollen fertility based on stainability as well as in vitro germination was observed among the different diploid genotypes tested. This indirectly indicates a variation in meiotic chromosome behavior of different cultivars which might have resulted from different levels of structural modifications in chromosomes accumulated in the course of domestication and continuous vegetative propagation. These variations among different accessions in the level of chromosome abnormalities may be reflected in variation of pollen fertility, as suggested by Adaniya and Shoda (Citation1998a, Citation1998b). Intraspecific variability for meiotic behavior has been investigated in 25 cultivars of ginger by Ratnambal and Nair (Citation1981) and they observed the presence of quadrivalents in most of the cultivars and hexavalents in a few, indicating the presence of translocation involving four to six chromosomes. They established a significant positive linear regression between pollen sterility, and chromosome aberration at anaphase-II and aberrant quartets, and concluded that the structural chromosome aberrations have a significant influence in lowering the pollen fertility in cultivars of Z. officinale. They observed high pollen sterility ranging from 54.4% to 90.2% based on carmine staining. Variation in pollen fertility among nine cultivars of ginger has been reported by Dhamayanthi et al. (Citation2003). They reported pollen stainability ranging from 14.7% to 28.5% in those cultivars. Recently Subbarayudu et al. (Citation2014) reported that 87.3–88.0% of microspores are viable in cultivar “Nadia”, based on FDA staining. This is in contradiction with the earlier report of Dhamayanthi et al. (Citation2003) that pollen fertility based on staining and in vitro germination is 23.3% and 17.0% respectively in cultivar “Nadia”.
The only aneuploid with 2n = 24 (acc. no. 147) identified in the present study showed low pollen fertility, indicated by 22.89% staining and 4.82% in vitro germination, suggesting that the additional chromosomes do not have a positive influence on pollen fertility. Whether this aneuploid is a tetrasomic or double trisomic is to be further verified by precise karyotype analysis. It is also possible that the additional chromosomes belong to the category of B-chromosomes, as indicated by Ramachandran (Citation1969). A somatic chromosome number of 2n = 24 has been reported earlier in a cultivar of ginger (Dhamayanthi and Zachariah Citation1998).
Two cultivars with higher pollen fertility (acc. nos. 195 and 821) were found to be tetraploids with 2n = 44, which indicates the influence of ploidy level in improving pollen fertility in ginger. Acc. no. 821 showed 2n = 44 in all the cells counted, indicating a more stable nature compared to acc. no. 195, which showed a few somatic cells with different chromosome numbers. These abnormal numbers in acc. no. 195 might have originated as a result of chromosome lagging occurring rarely during anaphase separation. Tetraploids with higher pollen fertility were identified from the germplasm collections of ginger for the first time. High pollen fertility in induced tetraploids of ginger has been reported earlier (Ramachandran Citation1982; Ramachandran and Nair Citation1992a, Citation1992b; Adaniya Citation2001; Adaniya and Shirai Citation2001). While comparing the cytology of diploids and induced tetraploids of ginger, Ramachandran and Nair (Citation1992b) suggested that the high pollen fertility in the induced tetraploid of ginger may be a consequence of high frequency of quadrivalents followed by the regular two-by-two anaphase-I separation of chromosomes. They observed that the most commonly observed configuration at metaphase-I was 10 IV + 2II and approximately 80% of the total of 124 PMCs at anaphase-I exhibited 22–22 disjunction. It is possible that few quadrivalents formed in diploids as a result of pairing between chromosomes involved in the structural heterozygosity may be creating higher imbalance in the meiotic products of diploids compared to tetraploids which results in poor pollen fertility in diploids. High pollen fertility associated with increasing quadrivalent frequency was reported in an autotetraploid of rye (Roseweir and Rees Citation1962). The tetraploids identified in the present study might have originated by spontaneous somatic mutations and survived and perpetuated through natural selection. Wang et al. (Citation2014) recently reported the existence of mixoploids having diploid–tetraploid cells in germplasm collections of ginger from China. This supports the possibility of spontaneous origin of tetraploids in the diploid populations of ginger.
In vivo self pollination studies using the diploid genotypes with comparatively higher in vitro pollen germination (acc. nos. 12, 82) and tetraploid lines with higher pollen germination (acc. nos. 195, 821), also has not resulted in fruit set. However, few pollen grains germinated on the stigmatic surface in all these cases. Thus, it is evident that the lack of seed set is not exclusively due to inhibition of pollen germinating at the stigmatic surface. But, a common phenomenon observed is that the ginger flower opens between 1.30 and 3.30 in the afternoon and the style and stigma of the pollinated flower along with the anther and labellum falls off the next morning, which may be affecting the process of fertilization even if the pollen tubes progress through the style. In view of the above observation the opinion of Ramachandran and Nair (Citation1992b) that the lack of fruit and seed set in ginger is due to sporophytic incompatibility is doubtful.
Pillai et al. (Citation1978) reported that flower structure of ginger manifests an adaptation suitable for insect pollination. Different pollination strategies such as self pollination at the time of anthesis, bud pollination before 24 h of flower opening with pollen from a freshly opened flower, bud pollination before 24 h of flower opening with pollen from the same flower bud, self pollinating the freshly opened flower after removing the stigma and self pollinating the freshly opened flower after smearing the stigmatic surface with germination media, have been tested in cultivar “Rio-De-Janeiro” by Jayachandran and Vijayagopal (Citation1979). However, they failed to get any fruit or seed set. Presence of pin and thrum type incompatibility has been indicated by Das et al. (Citation1999b) and Dhamayanthi et al. (Citation2003) as a barrier for seed set in ginger. But both the groups failed to get any fruit or seed set either by crossing or selfing of different cultivars. They also failed to demonstrate the existence of two types of styles structurally. Dhamayanthi et al. (Citation2003) stated that pollen germination is inhibited either close to the stigmatic surface or towards the top of the style, and argued that structural incompatibility is a predominant factor in ginger. But, as it is a well-established fact that most ginger cultivars have poor pollen fertility, the validity of this argument is doubtful. These experiments might have failed due to the use of ginger genotypes with poor pollen fertility.
Adaniya (Citation2001) reported that in vivo pollen germination and pollen tube growth in a tetraploid clone of ginger, 4XSanshu is greatly influenced by temperature and relative humidity. According to him, for pollen to germinate and grow in the stylar tissue, a relatively low temperature (approximately 20°C) and 100% RH are essential. Thus, it is evident that a methodology has to be devised through physiological investigations to sustain the flowers until successful fertilization occurs. The cross compatibility of the tetraploids identified has to be tested further to explore the possibility of fruit and seed set by devising suitable pollination techniques. The possibility of bud pollination as attempted by Jayachandran and Vijayagopal (Citation1979) has to be tested in the tetraploids by selfing as well as crossing. The in vitro pollination techniques described by Valsala et al. (Citation1996) can also be examined using the tetraploid with high pollen fertility identified in the present study.
Vigor in morphological and agronomical characters is frequently observed in polyploids compared to diploids (Tal Citation1980). Similar observations were made by earlier workers who successfully induced tetraploids of ginger as well (Ramachandran Citation1982; Ratnambal and Nair Citation1982; Ramachandran and Nair Citation1992a, Citation1992b; Adaniya Citation2001; Adaniya and Shirai Citation2001; Smith et al. Citation2004). This is due to the larger cell size in tetraploids (Ramachandran Citation1982). The natural tetraploids identified in the present study also had taller leafy shoots, longer and broader leaves, larger inflorescences and bolder rhizomes compared to the other diploid genotypes.
Conclusion
Unlike many earlier studies, the present report compares pollen fertility in ginger by stainability as well as in vitro germination. The collections with higher in vitro pollen germination may be useful for in vivo and in vitro approaches to achieve fruit and seed set in ginger. The study clearly indicates that pollen fertility in ginger is more influenced by the ploidy level rather than the genotype of the cultivar.
Funding information
This work was supported by the Indian Council of Agricultural Research [Project of ICAR-IISR].
Disclosure statement
No potential conflict of interest was reported by the author.
Acknowledgements
The author is thankful to the Director, ICAR-Indian Institute of Spices Research for providing facilities for successful completion of this work and Mr P. A. Mathew, Principal Scientist (Horticulture) for providing rhizome material of acc. 821 for the study.
Notes
* Contribution no. 586 of ICAR Indian Institute of Spices Research
References
- Adaniya S. 2001. Optimal pollination environment of tetraploid ginger (Zingiber officinale Roscoe) evaluated by in vitro pollen germination and pollen tube growth in styles. Sci Hort. 90(3–4):219–226.
- Adaniya S, Shirai D. 2001. In vitro induction of tetraploid ginger (Zingiber officinale Rosc.) and its pollen fertility and germinability. Sci Hort. 88(4):277–287.
- Adaniya S, Shoda M. 1998a. Variation in pollen fertility and germinability in ginger (Zingiber officinale Roscoe). J Japanese Society Horti Sci. 67(6):872–874.
- Adaniya S, Shoda M. 1998b. Meiotic irregularity in ginger (Zingiber officnale Roscoe). Chromosome Sci. 2(1):141–144.
- Brewbaker JL, Kwack BH. 1963. The essential role of calcium ion in pollen germination and pollen tube growth. Am J Bot. 50(9):859–865.
- Burnham CR. 1962. Discussions in cytogenetics. Minneapolis, MN: Burgress.
- Chakravorti AK. 1948. Multiplication of chromosome numbers in relation to speciation in Zingiberaceae. Sci Cult. 14(4):137–140.
- Das AB, Rai S, Das P. 1998a. Estimation of 4C DNA and karyotype analysis in ginger (Zingiber officinale Rosc.) II. Cytologia. 63(2):133–139.
- Das AB, Rai S, Das P. 1998b. Karyotype analysis and 4C DNA content in some cultivars of ginger (Zingiber officinale Rosc.). Cytobios. 93(1):175–184.
- Das AB, Rai S, Das P. 1999. Karyotype analysis and cytophotometric estimation of nuclear DNA content in some members of the Zingiberaceae. Cytobios. 97(1):23–33.
- Das P, Rai S, Das AB. 1999. Cytomorphological barriers in seed set of cultivated ginger (Zingiber officinale Rosc.). Iran J Bot. 8(1):120–129.
- Dhamayanthi KPM. 1998. Comparative karyomorphology and DNA estimation studies in ginger cultivars (Zingiber officinale Rosc). Cytologia. 63(3):311–315.
- Dhamayanthi KPM, Sasikumar B, Remashree AB. 2003. Reproductive biology and incompatibility studies in ginger (Zingiber officinale Rosc.). Phytomorphology. 53:123–131.
- Dhamayanthi KPM, Zachariah TJ. 1998. Studies on karyology and essential oil constituents in two cultivars of ginger. J Cytol Genet. 33(2):195–199.
- Eksomtramage L, Sirirugsa P, Jivanit P, Maknoi C. 2001. Chromosome counts of Zingiberaceous species from Thailand. Songklanakarin J Sci Technol. 24(2):311–319.
- Jayachandran BK, Vijayagopal PD. 1979. Attempts on breaking self incompatibility in ginger (Zingiber officinale Rosc.). Agri Res J Kerala. 17(3):256–257.
- McClintock B. 1938. The fusion of broken ends of sister half–chromatids following chromatid breakage at meiotic anaphases. MO Agr Exp Stn Res Bull. 290:1–48.
- Morinaga T, Fukushina E, Kanui T, Tamasaki Y. 1929. Chromosome numbers of cultivated plants. Bot Mag (Tokyo). 43(515):589–594.
- Nayak S, Naik PK, Acharya L, Mukherjee AK, Panda PC, Das P. 2005. Assessment of genetic diversity among 16 promising cultivars of ginger using cytological and molecular markers. Z Naturforsch. 60c(5-6):485–492.
- Omanakumari N, Mathew PM. 1985. Karyomorphological studies on four species of Zingiber Adns. Cytologia. 50(3):445–451.
- Parthasarathy VA, Srinivasan V, Nair RR, Zachariah TJ. 2012. Ginger: botany and horticulture. Hort Rev. 39:273–388.
- Pillai PKT, Vijayakumar G, Nambiar MC. 1978. Flowering behavior, cytology and pollination in ginger (Zingiber officinale Rosc.). J Plantation Crops. 6(1):12–13.
- Prasad G. 1975. Cytological studies of paracentric inversion in barley. In: Gaul H, editor. Barley Genetics III, Proceedings of the 3rd International Barley Genetics Symposium; 7–12 July 1975, Garching. p. 282–288.
- Purseglove JW, Brown EG, Green CL, Robbins SRJ. 1981. Ginger. Spices. vol 2. London: Longman; p. 447–531.
- Raghavan TS, Venkatasubban KR. 1943. Cytological studies in the family zingiberaceae with special reference to chromosome number and cytotaxonomy. Proc Indian Acad Sci. 17B(4):118–132.
- Rai S, Das AB, Das P. 1997. Estimation of 4C DNA and karyotype analysis in ginger (Zingiber officinale Rosc.) – 1. Cytologia. 62(2):133–141.
- Ramachandran K. 1969. Chromosome numbers in zingiberaceae. Cytologia. 34(2):213–221.
- Ramachandran K. 1982. Polyploidy induced in ginger by colchicine treatment. Curr Sci. 51:288–289.
- Ramachandran K, Nair PNC. 1992a. Induced tetraploids of ginger (Zingiber officinale Rosc.). J Spices and Aromatic Crops. 1(1):39–42.
- Ramachandran K, Nair PNC. 1992b. Cytological studies on diploid and autotetraploid ginger (Zingiber officinale Rosc.). J Spices Aromatic Crops. 1(2):125–130.
- Ratnambal MJ. 1979. Cytogenetical studies in ginger (Zingiber officinale Rosc.) [PhD thesis]. [Bombay]: University of Bombay.
- Ratnambal MJ, Nair MK. 1981. Microsporogenesis in ginger (Zingiber officinale Rosc.). In: Proceedings of PLACROSYM IV, CPCRI; Kasaragod, 3–8 December 1981, India. p. 42–57.
- Ratnambal MJ, Nair MK. 1982. Colchicine induced tetraploids in ginger (Zingiber officinale Rosc.). J Plantation Crops. 10(1):57–61.
- Ravindran PN, Nirmal Babu K, Shiva KN. 2005. Botany and crop improvement of ginger. In: Ravindran PN, Nirmal Babu K, editors. Ginger – the genus Zingiber. Boca Raton (FL): CRC Press. p. 15–85.
- Roseweir J, Rees H. 1962. Fertility and chromosome pairing in autotetraploid rye. Nature. 195(4837):203–204.
- Sasikumar B, Ravindran PN, George JK. 1994. Breeding ginger and turmeric. Cocao Arecanut Spices J. 18(1):10–12.
- Sato D. 1960. The karyotype analysis in zingiberales with special reference to the protokayotype and stable karyotype. Sci Papers Coll Educ Univ Tokyo. 10:225–243.
- Shivanna KR, Rangaswamy NS. 1993. Pollen biology – a laboratory manual. New Delhi: Narosa.
- Sjodin J. 1971. Induced paracentric and pericentric inversions in Vicia faba L. Hereditas. 67(1):39–54.
- Smith M, Hamill KSD, Goel BJ, Severn-Ellis AA. 2004. Ginger (Zingiber officinale) autotetraploids with improved processing quality produced by an in vitro colchicine treatment. Australian J Exp Agri. 44(10):1065–1072.
- Subbarayudu S, Naik BS, Devi HS, Bhau BS, Khan PSV. 2014. Microsporogenesis and pollen formation in Zingiber officinale Roscoe. Plant Syst Evol. 300(4):619–632.
- Sugiura T. 1936. Studies on the chromosome numbers in higher plants, with special reference to cytokinesis. I. Cytologia. 7(4):437–595.
- Tal M. 1980. Physiology of polyploids. In: Lewis WH, editor. Polyploidy. New York, NY: Plenum Press; p. 61–75.
- Valsala PA, Nair GS, Nazeem PA. 1996. Seed set in ginger (Zingiber officinale Rosc.) through in vitro pollination. J Tropical Agri. 34(1):81–84.
- Wang L, Gao F, Xu K, Li X. 2014. Natural occurrence of mixploid ginger (Zingiber officinale Rosc.) in China and its morphological variations. Sci Hortic. 172(1):54–60.