Abstract
Dioxins (PCDDs, PCDFs and DL-PCBs) are a large family of congeners that are considered highly toxic and are reported to be teratogenic, mutagenic, carcinogenic, immunotoxic and hepatotoxic, also affecting the nervous and reproductive systems. Farm animals are particularly exposed to these chemicals when they are fed with grass produced close to polluted areas such as those located in vicinity of metallurgic factories. Cytogenetic tests can be very useful to check genetic damage occurring to domestic animal cells exposed to these chemicals. Fifty-two randomly selected Italian Friesian cows (Bos taurus, 2n = 60) from two farms located in the vicinity of and (as a control) far from the a metallurgic industrial area underwent cytogenetic investigations to ascertain possible differences in their chromosome fragility. One farm was under legal sequestration due to the presence in the milk mass of higher mean values of dioxins (24.78 ± 3.19 pg g−1 of fat as sum of PCDD + PCDF + DL-PCBs as WHO-TEQ (World Health Organization-Toxic Equivalent Quantity), with DL-PCBs being the main chemical component) than those permitted (5.5 pg g−1 of fat as WHO-TEQ). Cytogenetic analyses, performed by using both the chromosome abnormality (CA) test (chromosome and chromatid breaks) and sister chromatid exchange (SCE) test, revealed a significantly (p < 0.01) higher chromosome fragility in cells of exposed cows (26 cows) compared to those of the control (23 cows).
Introduction
Dioxins are a large family of congeners which can be fitted in three main groups: polychloro-dibenzo-dioxins (PCDDs), polychloro-dibenzo-furans (PCDFs) and dioxin-like polychlorobiphenyls (DL-PCBs). These chemicals are considered highly toxic, especially tetrachloro-dibenzo-p-dioxin (TCDD) which has been reported to be teratogenic, mutagenic, carcinogenic, immunotoxic, hepatotoxic and affecting also the nervous and reproductive systems (Mandal Citation2005; Bock and Kohle Citation2006; Steenland et al. 2014). Dioxins are also highly persistent in the environment, especially when entering in the human or animal body due to their ability to be absorbed by fat tissue where they can remain for long time; their half-life in the body varies from 7 to 11 years (Wolfe et al. Citation1994; Ogura Citation2004).
For this reason, international committees have established very low levels of permitted dioxins in both animal and fish fat, although these values vary among species and type of food as established in the most recent EC Regulation No.1259/2011. According to this EC regulation, in animal milk these values are 2.5 pg g−1 of fat for PCDDs + PCDFs as WHO-TEQ (World Health Organization-Toxic Equivalent Quantity) and 5.5 pg g−1 of fat as the sum of PCDDs + PCDFs + DL-PCBs as WHO-TEQ.
Most PCDDs and PCDFs are produced by both industrial processes and illegal waste burning, while DL-PCBs are produced during some industrial processes, e.g. steel production.
PCBs have been used in the past in various industrial applications. Due to their toxicity, many countries have forbidden their use (in Italy since 1985). PCB levels seem to be particularly high in the northern hemisphere, e.g. as revealed by studies performed in Barents Sea, which showed very high levels (36 mg g−1 of fat) in the Glaucous Gull (Larus hyperboreus) which is at the top of food chain in that area (Bustnes et al. Citation2003; Erikstad et al. Citation2011).
In Italy, after the famous Seveso disaster (10 July 1976) where a very high quantity of TCDDs (30 kg) were spread in the environment, many controls conducted in farm animals to check the transmission of dioxins to humans have established the presence of dioxins in animals raised in various regions such us Piedmont, Lombardy, Tuscany and Apulian regions (Biasioli and Ajmone-Marsan Citation2007; Ingelido et al. Citation2009; Di Meo et al. Citation2011).
The Apulian region (southern Italy), and more specifically the metallurgic Taranto city industrial area, has been in the spotlight because it was indicated as responsible for the production of environmental pollution (i.e. air pollution, reviewed in Mangia et al. Citation2013), dioxins, especially DL-PCBs, increasing cases of mortality and cancers (Mitis et al. Citation2005; Martinelli et al. Citation2009; Casale Citation2011). For this reason, the veterinary service of the local health sanitary control performed analyses in search of dioxins in a farm raising Friesian cattle relatively close to the metallurgic industrial area (Figure ). In this farm, under legal sequestration for long time, levels of dioxins (mainly DL-PCBs) were found to be much higher than those permitted in the milk of dairy cattle (5.5 pg g−1 of fat as a sum of PCDDs + PCDFs and DL-PCBs).
Figure 1. Farm raising Friesian cattle very close to the metallurgic industrial area of Taranto city.

Cytogenetic tests are very useful to check the presence of chromosome damage due to the mutagens present in the environment (Lovreglio et al. Citation2014; Mrdjanovic et al. Citation2014), in the food chain and more specifically in livestock animals, which are below humans in the food chain (Iannuzzi et al. Citation2004; Perucatti et al. Citation2006; Di Meo et al. Citation2011). In addition, high frequencies of chromatid breaks have been found in blood cells from a high percentage of cancer patients (Bryant et al. Citation2004). Indeed, 40% of breast cancer cases show elevated “chromatid radiosensitivity” in contrast to only some 6% in a similar matched group of normal (non-cancer) surgical cases (Bryant et al. Citation2004).
PCBs have been found to produce sex chromosome disomy in sperms, especially YY and XY (McAuliffe et al. Citation2012). Indeed, dietary PCB exposure seems to have a negative impact on the sperm chromatin integrity of adult males (Spanò et al. Citation2005). A small chromosome region duplication has been found in humans exposed to PCB (in particular to PCB95) (Mitchell et al. Citation2012). In vitro studies, using some types of PCBs, have revealed a very high percentage of tetraploid cells and significant increasing number of sister chromatid exchanges, compared to those achieved in the control (Flor and Ludewig Citation2010). Some PCBs, especially the volatile ones (PCBs 28 and 52), reduced significantly the chromosome telomerase activity (Senthilkumara et al. Citation2011).
In previous reports we found chromosome fragility in cells of livestock naturally exposed to dioxins (PCDDs + PCDFs only), i.e. sheep (Iannuzzi et al. Citation2004; Perucatti et al. Citation2006) and to both PCDDs + PCDFs and DL-PCBs (i.e. cattle, northern Italy – Piedmont-Valdostana hybrid cattle breeds) (Di Meo et al. Citation2011); or river buffalo (Genualdo et al. Citation2012) compared to that found in cell populations of control animals.
In the present paper we have studied, for the first time, the most famous dairy cattle breed in the world (Friesian) by comparing two groups of sample cows raised very close to, and (as a control) far from the largest European metallurgic factory, which is located in the industrial area of Taranto city (Apulian region, southern Italy). We performed the study using two different cytogenetic tests and found a significant higher chromosome fragility in cells of the exposed cows compared to that of the control group.
Materials and methods
Animals and dioxin analyses
We studied 52 Friesian cows (3–5 years old) randomly sampled from two different farms: one (29 animals) located close to the steel manufacturing industrial area of Taranto city, Apulian region (southern Italy) and the other one (23 animals) 65 km away in the same province and used as control. Both farms used the same animal feeding: natural pasture in the area of the farm and additional food in the box, especially during lactation.
The farm located close to the industrial area of Taranto city was under legal sequestration for a long time due to the presence in the milk mass of higher dioxin values (sum of DCDDs + DCFFs + DL-PCBs as WHO-TEQ) than those permitted. Chemical analyses in search of dioxins were also extended to both pasture and soil of the farm located close to the industrial area. All chemical analyses were performed by specialized laboratories under local veterinary sanitary health control.
Blood samples and cell cultures
Peripheral blood samples were collected by veterinarians of the local health unit by using sterile vacutainer tubes containing sodium heparin. Cell cultures from whole blood were performed at 37.8°C in RPMI (Roswell Park Memorial Institute) medium, enriched with fetal calf serum (10%), L-glutamine (1%), antibiotic-antimycotic mixure (1%) and concanavalin A (15 μg ml−1) as mitogen. Two different cell cultures were performed: normal cultures (duration time 48 h) and cultures treated with 5-bromodeoxyuridine (BrdU) 28 h before harvesting (duration time 72 h). Colcemid (0.01 μg ml−1) lasted 1.5 h for both cell cultures. Slides obtained from normal cultures were used for chromosome abnormality (CA) tests such as chromatid breaks and chromosome breaks, while those treated with BrdU were used for sister chromatid exchange (SCE) tests. Slides from both types of cell cultures were stained for 10 min with acridine orange (0.01% in phosphate buffer pH = 7.0), washed with tap and distilled water, mounted in P-buffer and sealed under slide coverslips. The slides were observed a day later (or more) under a fluorescence microscope NIKON E-1000 connected to a digital camera. At least 50 cells for the CA test and 35 for the SCE test were studied for each animal (exposed and control animals). All images were recorded and later carefully examined by two expert cytogeneticists.
Statistical analysis
Mean values and standard deviations of both CA and SCE were calculated for single animals and animal groups. Statistical analyses were performed between the two cow groups by using a non-parametric test (Mann–Whitney), and differences were considered significant if p ≤ 0.05.
Results
Chemical analysis
Chemical analysis in search of dioxins revealed higher levels of dioxins (24.78 pg g−1 of fat as the sum of PCDDs + PCDFs + DL-PCBs as WHO-TEQ) in the milk mass of cows raised in the farm located close to the metallurgic area than those permitted (5.5 pg g−1 of fat as WHO-TEQ) (Table 1). The same situation was found in the dioxins in the grass, with higher levels (2.37 pg g−1) than those permitted (1.5 pg g−1) (Table ). Conversely, levels of dioxins in the soil were lower (2.72 pg g−1) than those permitted (10 pg g−1) (Table ).
Table 1. Levels of PCDD + PCDFs, DL-PCBs and sum of PCDD + PCDF + DL-PCBs in the milk mass of the farm under legal sequestration, as well as in the grass and soil of the same farm. Permitted values of dioxins are reported between parentheses.
Of the different dioxin types, DL-PCBs are the main chemical component in both milk mass (20.56 ± 2.53 pg g−1) and grass (1.54 pg g−1), while PDDDs and PCDF are prevalent in the soil (2.49 ± 0.53), although levels of total dioxins in the soil are below those permitted (10 pg g−1) (Table ). No analyses of dioxins have been performed in the control farm by the local veterinary health control because the area where it was located was very far from sources of pollution, including dioxins.
Cytogenetic analyses
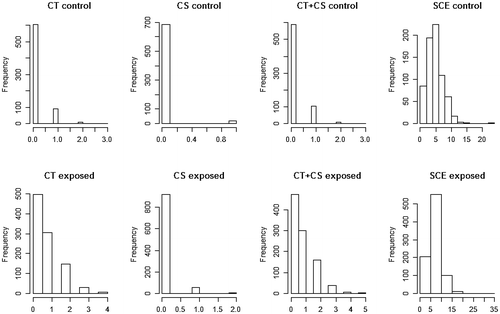
The mean value of abnormal cells, with at least one chromatid break or chromosomal break (Figure ), was significantly higher (p < 0.01) in the exposed cows (0.52 ± 0.49) than those of the control (0.16 ± 0.37) (Table ). This is due to the presence of a significantly (p < 0.01) higher mean number of chromatid breaks (CT) (0.72 ± 0.86) and chromosome breaks (CS) (0.07 ± 0.28) in the exposed cows, compared to those (0.15 ± 0.39 and 0.02 ± 0.15, respectively) achieved in the control (Table ).
Figure 2. Female cattle metaphase plate showing a chromatid break (arrow). Slides were stained with acridine orange and later observed under a fluorescence microscope connected with a digital camera.
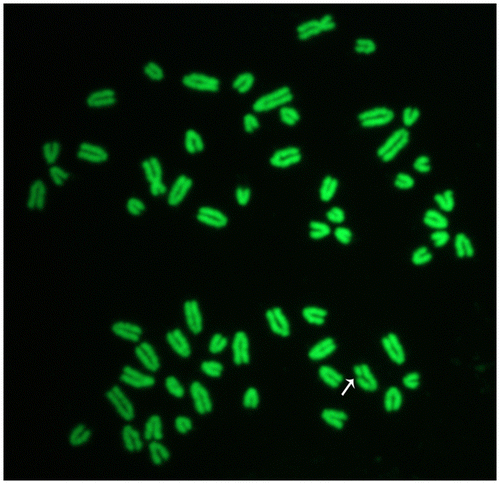
Table 2. Number of animals studies, examined cells, abnormal cells (AC), chromatid breaks (CT), chromosome breaks (CS), and total CT + CS in cattle reared in dioxin-contaminated and control areas of Apulian region (southern Italy).
SCE/cell mean value (Figure ) was significantly higher (p < 0.01) in the exposed cows (7.41 ± 3.02) compared to the control group (5.30 ± 2.61) (Table ).
Figure 3. Female cattle metaphase plate showing several SCEs (arrows). Slides were stained with acridine orange and later observed under a fluorescence microscope connected with a digital camera.
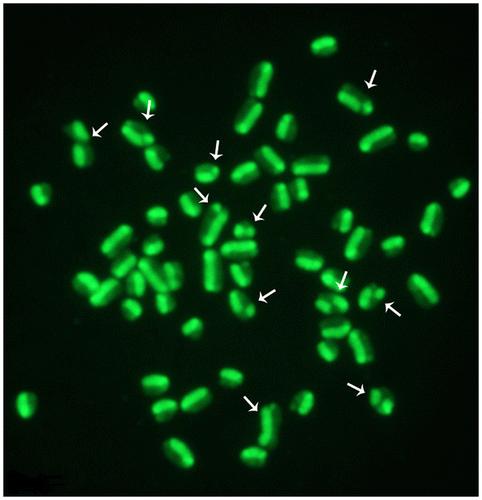
Table 3. Number of examined cells and SCE mean values in Friesian dairy cows reared in dioxin-contaminated and control areas of Apulian region (southern Italy).
Data analysis
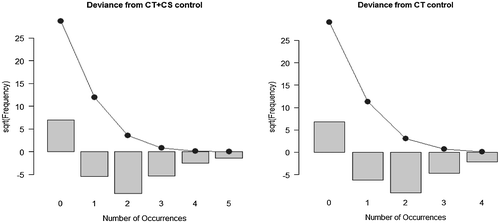
Table provides some descriptive statistics for our variables. They are all count variables and descriptive statistics show consistently larger values in the exposed animals compared with the control sample, as also shown in Figure which demonstrates a no normal distribution of variables.
Table 4. Descriptive statistics.
To confirm that data are not normally distributed, we test for normality with the Shapiro–Wilk test (Table ). The hypothesis H0 is rejected for all variables both for control and for exposed animals. In this case, a non-parametric test is more appropriate than using a t-test. The Mann–Whitney test (Table ) is implemented to verify whether means of CS, CT, CT + CS and SCE of exposed cell animals are larger than those achieved in the control sample. All H0 are rejected and the exposed cell variable shows a larger effect. This means that exposed cell variables are always greater in the mean than in the control. We can also test whether other sample statistics are different between exposed and control samples. It is interesting to verify whether the two samples have different dispersion parameters by using a permutation test (Table ). We use the ratio of the mean deviance as a statistic. In this case, H0 is rejected in all cases. This means that not only is there a larger effect on the variable in the exposed cell samples, but also a higher variance compared with the control. In two cases there are similar values for mean and variance in the samples. Therefore, we decided to test the goodness-of-fit for the variables as a Poisson distribution. CT and CS + CT have a Poisson distribution for the control sample, but the variables measured in the exposed samples reject always the H0 to be distributed as a Poisson. This is important because we can also implement a parametric test that can show differences in all distributions between exposed and control samples (Figure ).
Table 5. Shapiro Wilk test for normality.
Table 6. Mann–Whitney non-parametric test for equality of means.
Table 7. Permutation test for equality of deviance.
Discussion
Chromatin damage can be induced by several environmental mutagens (Bryant et al. Citation2004). Although cytogenetic tests applied to both human and animals exposed to dioxins have generated contradictory results (reviewed in Iannuzzi et al. Citation2004; Perucatti et al. Citation2006), all studies performed so far in domestic animals revealed an higher chromosome fragility in animal cells naturally exposed to dioxins (PCDD, PCDF and DL-PCBs), compared to that achieved in the controls (same species, breed, age and dietary habits) (Iannuzzi et al. Citation2004; Perucatti et al. Citation2006; Di Meo et al. Citation2011; Genualdo et al. Citation2012). In addition, chromatin revealed the presence of localized and discontinuous changes due to dioxin (TCDD) (Okino and Whitlock Citation1995). In particular, PCBs were found to produce sex chromosome disomy in sperms, especially YY and XY (McAuliffe et al. Citation2012), probably because PCB exposure has been reported to have a negative impact on the sperm chromatin integrity of adult males (Spanò et al. Citation2005). Furthermore, small chromosome region duplications have been found in humans exposed to PCBs (Mitchell et al. Citation2012), while in vitro studies on male Chinese hamster V79 lung fibroblasts revealed a very high percentage of tetraploid cells and significant increasing number of sister chromatid exchanges using some types of PCBs, compared to those achieved in the control (Flor and Ludewig Citation2010). In addition, the chromosome telomerase activity appeared significantly reduced in human skin keratinocytes when using some PCBs, especially the volatile ones (PCBs 28 and 52) (Senthilkumara et al. Citation2011).
Considering that chromatin is the main component of chromosomes, damage at the chromosomal level, especially when double DNA breakages occur, may denote chromosome fragility with a subsequent increasing probability of originating unbalanced gametes during meiosis, and unbalanced embryos, which can die in early embryonic life. Alternatively, the animal may have an abortion or abnormal fetuses as those occurring in sheep exposed to relatively high levels of dioxins (Perucatti et al. Citation2006).
Several effects of dioxins, including those of DL-PCBs, are mediated by the aryl hydrocarbon receptor (AhR), an intracellular protein which binds the dioxin molecule and transports them into the nucleus where AhR forms a complex with ARNT (AhR nuclear translator), which is able to promote and regulate the transcription of specific genes (i.e. ARNT, AHR, CYP1A1, CYP1A2, CYP1B1 and AHRR) (Mandal Citation2005; Beischlag et al. Citation2008; Hung et al. Citation2013). These loci have recently been FISH mapped in domestic bovids, including cattle (Genualdo et al. Citation2011). AHR-KO rats had lower basal expression of transcripts for these genes and also accumulated ~30–45-fold less TCDD in the liver at seven days post-exposure. In untreated animals, AHR-KO mice, but not AHR-KO rats, had alterations in serum analytes indicative of compromised hepatic function, patent ductus venosus of the liver and persistent hyaloid arteries in the eye. Furthermore, AHR-KO rats, but not AHR-KO mice, displayed pathological alterations to the urinary tract: bilateral renal dilation (hydronephrosis), secondary medullary tubular and uroepithelial degenerative changes and bilateral ureter dilation (hydroureter) (Harrill et al. Citation2013).
As shown in Table , the main component of dioxins present in the milk mass of the exposed cows are DL-PCBs, further supporting their origin from industrial processes such as those producing steel in the Taranto city industrial area (metallurgic factory).
Cytogenetic analyses performed in the exposed Friesian cows revealed a significantly higher number of abnormal cells in exposed animals (0.52 ± 0.59) than those of the control (0.16 ± 0.37) (Table ). This is due to a significantly increasing mean number of both chromatid and chromosome breaks per cell of exposed cows compared to those of the control (Table ). Indeed, significant differences (p < 0.01) were also found when comparing the two groups of cows by examining the total mean number of CA (0.79 ± 0.92 in the exposed cows and 0.17 ± 0.42 in the control), as well as by examining the chromatid breaks (0.72 ± 0.86 in the exposed cows and 0.15 ± 0.39 in the control) and chromosome breaks (0.07 ± 0.28 in the exposed cows and 0.02 ± 0.15 in the control) alone (Table ).
The CA values found in the present study in both exposed and control animals are similar to those found in cells of other breeds (cattle) and species (sheep and river buffalo) naturally exposed to dioxins (reviewed in Genualdo et al. Citation2012). The mean CA value in control animal cells of the present study does not differ greatly from those reported in some human control lymphocytes (Bryant et al. Citation2004), although most studies in human lymphocytes (Liou et al. Citation1999; Bonassi et al. Citation2008; Costa et al. Citation2014) reported lower CA values than those achieved in the present study and in other previous studies on the same topic in domestic animals (reviewed in Genualdo et al. Citation2012). The higher CA values found in both exposed and control animal cells, compared to those achieved in human control cells, can in part be explained considering that (i) the animals are below humans in the food chain; and (ii) the soil may be present in the diet, especially during pasturage. In the soil matrix the mutagens are present in higher quantities than in the plants (grass). Dioxins are permitted at higher values in the soil (10 ng kg−1) than in grass (0.75 ng kg−1) (Table ). Also the acridine orange staining used in this and our previous studies can in part explain the higher CA values compared to CA values reported using Giemsa staining. However, only using acridine orange staining in human cells we can draw final conclusions about this technical aspect.
The chromosome fragility found in the Friesian cows in the present study using the CA test was also confirmed when applying the SCE test, as the SCE mean value was significantly higher (p < 0.01) in the exposed cows (7.41 ± 3.02) than in the control (5.30 ± 2.61) (Table ).
In conclusion, both cytogenetic tests used for the first time on Friesian cows exposed to dioxins (mainly DL-PCBs) showed a pronounced chromosome fragility in the cells of exposed cows compared to that found in the control group cells. It is possible that increasing chromosome damage observed in Friesian cows could originate from synergic action of both dioxins (mainly PCBs in the present case) and other mutagens present in the industrial area of Taranto city. This suggests that farm animals should be raised far from industrial areas to avoid accumulation of toxic chemicals in their products. At the same time, domestic animals can be considered important environmental sentinels of the food chain. Indeed, systematic controls of animal products for the presence of chemicals, accompanied by genetic tests, like those performed in this study, are very useful to organize a correct food chain so to reduce the risk for human populations, especially those living close to industrial areas.
Funding information
The study has been supported in part by “Progetto premiale CNR 2013” and in part by [PON 2007-2013, 01_01145], Project “Sviluppo tecnologico e innovazione per la sostenibilità e competitività della cerealicoltura meridionale” (ISCOCEM).
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Beischlag TV, Morales JL, Hollingshead BD, Perdew GH. 2008. The aryl hydrocarbon receptor complex and the control of gene expression. Crit Rev Euk Gene Express. 18(3):207–250.
- Biasioli M, Ajmone-Marsan F. 2007. Organic and inorganic diffuse contamination in urban soils: the case of Torino (Italy). J Environ Mon. 9(8):862–868.
- Bock KW, Kohle C. 2006. Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol. 72(4):393–404.
- Bonassi S, Norppa H, Ceppi M, Stromberg U, Vermeulen R, Znaor A, Cebulska-Wasilewska A, Fabianova E, Fucic A, Gundy S, Hansteen I-L, Knudsen LE, Lazutka J, Rossner P, Sram RJ, Boffetta P. 2008. Chromosomal aberration frequency in lymphocytes predicts the risk of cancer: results from a pooled cohort study of 22 358 subjects in 11 countries. Carcinogenesis. 29(6):1178–1183.
- Bryant PE, Gray LJ, Peresse N. 2004. Progress towards understanding the nature of chromatid breakage. Cytogenet Genome Res. 104(1−4):65–71.
- Bustnes JO, Bakken V, Skaare JU, Erikstad KE. 2003. Age and accumulation of persistent organochlorines: a study of arctic breeding glaucous gulls (Larus hyperboreus). Environ Toxicol Chem. 22(9):2173–2179.
- Casale A. 2011. Relationship between the incidence of cancer and the presence of metallurgic factory in Taranto. Italy. The Health. 2(2):64–65.
- Costa C, García-Lestón J, Costa S, Coelho P, Silva S, Valdiglesias V, Mattei F, Dall’Armi V, Bonassi S, Laffon B, Snawder J, Teixeira JP. 2014. Is organic farming safer to farmers’ health? A comparison between organic and traditional farming. Toxicol Lett. 230(2):166–176.
- Di Meo GP, Perucatti A, Genualdo V, Caputi-Jambrenghi A, Rasero R, Nebbia C, Iannuzzi L. 2011. Chromosome fragility in dairy cows exposed to dioxins and dioxin-like PCBs. Mutagenesis. 26(2):269–272.
- EC Regulation No. 1259/2011. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:320:0018:0023:EN:PDF
- Erikstad KE, Moum T, Bustnes JO, Reie TK. 2011. High levels of organochlorines may affect hatching sex ratio and hatchling body mass in arctic glaucous gulls. Funct Ecol. 25(1):289–296.
- Flor S, Ludewig G. 2010. Polyploidy-induction by dihydroxylated monochlorobiphenyls: structure-activity-relationships. Environ Int. 36(8):962–969.
- Genualdo V, Perucatti A, Iannuzzi A, Di Meo GP, Spagnuolo S, Caputi-Jambrenghi A, Coletta A, Vonghia G, Iannuzzi L. 2012. Chromosome fragility in river buffalo cows exposed to dioxins. J Appl Genet. 53(2):221–226.
- Genualdo V, Spalenza V, Perucatti A, Iannuzzi A, Di Meo GP, Caputi-Jambrenghi A, Vonghia G, Rasero R, Nebbia C, Sacchi P, Iannuzzi L. 2011. Fluorescence in situ hybridization mapping of six loci containing genes involved in the dioxin metabolism of domestic bovids. J Appl Genet. 52(2):229–232.
- Harrill JA, Hukkanen RR, Lawson M, Martin G, Gilger B, LeCluyse EL, Budinsky RA, Rowlands JC, Thomas RS. 2013. Knockout of the aryl hydrocarbon receptor results in distinct hepatic and renal phenotypes in rats and mice. Toxicol Appl Pharmacol. 272(2):503–518.
- Hung W-T, Lambert GH, Huang P-W, Patterson DG, Guo YL Jr. 2013. Genetic susceptibility to dioxin-like chemicals’ induction of cytochrome P4501A2 in the human adult linked to specific AhRR polymorphism. Chemosphere. 90(9):2358–2364.
- Iannuzzi L, Perucatti A, Di Meo GP, Polimeno F, Ciotola F, Incarnato D, Peretti V, Caputi-Jambrenghi A, Pecoraro A, Manniti F, D’Alessandro A, Vonghia G. 2004. Chromosome fragility in two sheep flocks exposed to dioxins during pasturage. Mutagenesis. 19(5):355–359.
- Ingelido AM, Abballe A, Di Domenico A, Fochi I, Iacovella N, Saragosa A, Spagnesi M, Valentini S, De Felip E. 2009. Levels and profiles of polychlorinated dibenzo-p-dioxins, polychlorinated dibenzofurans, and polychlorinated biphenyls in feedstuffs and milk from farms in the vicinity of incineration plants in Tuscany. Italy. Arch Environ Contam Toxicol. 57(2):397–404.
- Liou S-H, Lung J-C, Chen Y-H, Yang T, Hsieh L-L, Chen C-J, Wu T-N. 1999. Increased chromosome-type chromosome aberration frequencies as biomarkers of cancer risk in a blackfoot endemic area. Cancer Res. 59(7):1481–1484.
- Lovreglio P, Maffei F, Carrieri M, D’Errico MN, Drago I, Hrelia P, Bartolucci GB, Soleo L. 2014. Evaluation of chromosome aberration and micronucleus frequencies in blood lymphocytes of workers exposed to low concentrations of benzene. Mutat Res. 770:55–60.
- Mandal PK. 2005. Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J Compar Physiol B. 175(4):221–230.
- Mangia C, Gianicolo EAL, Bruni A, Vigotti MA, Cervino M. 2013. Spatial variability of air pollutants in the city of Taranto, Italy and its potential impact on exposure assessment. Environ Mon Assess. 185(2):1719–1735.
- Martinelli D, Mincuzzi A, Minerba S, Tafuri S, Conversano M, Caputi G, Lopalco PL, Quarto M, Germinario C, Prato R. 2009. Malignant cancer mortality in Province of Taranto (Italy). Geographic analysis in an area of high environmental risk. J Prevent Med Hygiene. 50(3):181–190.
- McAuliffe ME, Williams PL, Korrick SA, Altshul LM, Perry MJ. 2012. Environmental exposure to polychlorinated biphenyls and p, p′-DDE and sperm sex-chromosome disomy. Environ Health Persp. 120(4):535–540.
- Mitchell MM, Woods R, Chi L-H, Schmidt RJ, Pessah IN, Kostyniak PJ, LaSalle JM. 2012. Levels of select PCB and PBDE congeners in human postmortem brain reveal possible environmental involvement in 15q11-q13 duplication autism spectrum disorder. Environ Mol Mutagen. 53(8):589–598.
- Mrdjanovic J, Šolajic S, Dimitrijevic S, Dan I, Nikolic I, Jurišic V. 2014. Assessment of micronuclei and sister chromatid exchange frequency in the petroleum industry workers in province of Vojvodina. Republic of Serbia. Food Chem Toxicol. 69:63–68.
- Mitis F, Martuzzi M, Biggeri A, Bertollini R, Terracini B. 2005. Industrial activities in sites at high environmental risk and their impact on the health of the population. Int J Occup Environ Health. 11(1):88–95.
- Ogura I. 2004. Half-life of each dioxin and PCB congener in the human body. Organohalogen Compounds. 66:3329–3337.
- Okino ST, Whitlock JP. 1995. Dioxin induces localized, graded changes in chromatin structure: implications for Cyp1A1 gene transcription. Mol Cell Biol. 15(7):3714–3721.
- Perucatti A, Di Meo GP, Albarella S, Ciotola F, Incarnato D, Caputi-Jambrenghi A, Peretti V, Vonghia G, Iannuzzi L. 2006. Increased frequencies of both chromosome abnormalities and SCEs in two sheep flocks exposed to high dioxin levels during pasturage. Mutagenesis. 21(1):67–75.
- Senthilkumara PK, Klingelhutzd AJ, Jacobusa JA, Lehmlera H, Robertsona LW, Ludewiga G. 2011. Airborne polychlorinated biphenyls (PCBs) reduce telomerase activity and shorten telomere length in immortal human skin keratinocytes (HaCat). Toxicol Lett. 204(1):64–70.
- Spanò M, Toft G, Hagmar L, Eleuteri P, Rescia M, Rignell-Hydbom A, Tyrkiel E, Zvyezday V, Bonde JP. 2005. INUENDO: exposure to PCB and p, p′-DDE in European and Inuit populations: impact on human sperm chromatin integrity. Human Reprod. 20(12):3488–3499.
- Steenland K, Bertazzi P, Baccarelli A, Kogevinas M. 2004. Dioxin revisited: developments since the 1997 IARC classification of dioxin as a human carcinogen. Environ Health Persp. 112(13):1265–1268.
- Wolfe WH, Michalek JE, Miner JC, Pirkle JL, Caudill SP, Patterson DG, Needham LL. 1994. Determinants of TCDD half-life in veterans of operation ranch hand. J Toxicol Environ Health. 41(4):481–488.