Abstract
Schizothoracinae is a subfamily of naked carp which is widespread throughout the Qinghai-Tibet Plateau region. Cytogenetic analyses were performed on two sympatric species of naked carp, Gymnocypris chui and G. scleracanthus from the Lake Langcuo, Qinghai-Tibet Plateau. The results showed G. chui and G. scleracanthus had the same chromosome number (2n = 92), but different karyotypes:, (36m + 16sm + 12st + 28a) for G. chui and (26m + 12sm + 18st + 36a) for G. scleracanthus, respectively. The six loci of 45S ribosomal genes (rDNA) were detected through fluorescent in situ hybridization, and their locations were highly conserved between two species. This study depicted the chromosomal characteristics of the Schizothoracinae species and improved our understanding about the relationships and the evolution of two sympatric species.
Introduction
Karyological studies provide basic information on the number, size and morphology of chromosomes that is important to study evolution and diversification in fish (Almeida-Toledo et al. Citation2000; Borba et al. Citation2012; Poletto et al. Citation2010). The karyotype is unique for each species, therefore is widely used for distinguishing different organisms. Traditionally, fish karyotypes have been described using C-banding and N-banding. In the last few decades, fluorescence in situ hybridization (FISH) was introduced into cytogenetic studies of fish, which enables the chromosomal localization of specific genes or repetitive sequence. The different numbers and positions of the rRNA genes provided useful information for understanding the evolutionary relationships among closely related species (Rubert et al. Citation2011; Cardoso et al. Citation2013).
The fish of the subfamily Schizothoracinae (Family: Cyprinidae) are important members of the ichthyofauna in the Qinghai-Tibet Plateau and its surrounding areas, where they are widely distributed in a variety of rivers and lakes (Wu and Wu Citation1992; Chen and Cao Citation2000). Currently, this subfamily includes 15 genera and over a hundred species distributed all over the world (Mirza Citation1991; Wu and Wu Citation1992; Chen and CaoCitation 2000). The karyotypes of four Schizothoracinae fish were first reported by Zan et al (Citation1985). Available cytogenetic data for this subfamily showed a wide variation with tetraploid, hexaploid and octoploid numbers, ranging from 2n = 66 in Gymnocypris dobula to 2n = 417–470 in Ptychobarbus dipogon (Table ). However, cytogenetic research on Schizothoracinae lags behind that for other fishes because of the similar chromosomal organization, large number of chromosomes, and autotetraploidy, with four nearly identical genomes (Zan et al.Citation1985; Yu et al.Citation1987; Wu et al. 1998 ; Qi Citation2004; Li et al.Citation2010; Kong et al.Citation2011 ).
Table 1. A summary of the cytogenetic data available for the Schizothoracinae.
G. chui Tchang, Yueh et Hwang and G. scleracanthus Tsao et al (Cyprinidae: Schizothoracinae) are endemic fish that are sympatric distributed in Lake Langcuo in Tibet, China (Wu and Wu Citation1992). Morphological difference was investigated (Wu and Wu Citation1992), and more evidence was required to explain the relationship between these two sympatric fish. In the present work, we obtained G. chui and G. scleracanthus from the Lake Langcuo, Qinghai-Tibet Plateau and illustrated their different karyotypes, which helps to explore their karyotypic evolution and contributes to cytogenetic information for members of the subfamily Schizothoracinae.
Material and methods
Samples of G. chui (two males and six females) and G. scleracanthus (four males and three females) from the Lake Langcuo, Qinghai-Tibet Plateau, were analyzed. All necessary permits for collection and experimentation were acquired for the described field study from the Agriculture Department of Tibet Autonomous Region, China.
Chromosomal preparations followed the methods described by Lin (Citation1982) with some modifications. In brief, a live fish was administered an intraperitoneal injection of a 10 μg/1 g solution of phytohemagglutinin (PHA) to stimulate mitosis and kept alive for 20 h in a fully aerated aquarium. Subsequently the fish was injected with PHA again. Two hours later, a 0.025% colchicine solution was injected at the proportion of 0.5 ml/100 g of body weight. After 120 min the fish was euthanized and the visceral cavity was opened to collect the kidneys. The kidneys were macerated in a hypotonic solution of 0.04 M KCl and incubated at 37°C for 60 min. The cell solution was then suspended in a fixative (3:1 methanol: acetic acid) and centrifuged twice. The resulting pellet was then suspended in fresh fixative and dropped onto warmed slides. The slides were analyzed after conventional Giemsa staining with 6-diamidino-2-phenylindole (DAPI) (Pieczarka et al. Citation2006) and FISH with 45S ribosomal DNA (45S rDNA) probes. The 45S probes were labeled with tetramethyl-rhodamine-5-dUTP (Roche Diagnostics, Mannheim, Germany) by a nick-translation method. Images were acquired with a cooled CCD camera (Photometrics CoolSNAP, USA) under a fluorescence microscope (Leica, Wetzlar, Germany) and analyzed with the MetaVue imaging system (Universal Imaging Corporation, USA). Finally, images were adjusted with Adobe Photoshop (version 6.0; Adobe Inc. USA) for contrast and background optimization.
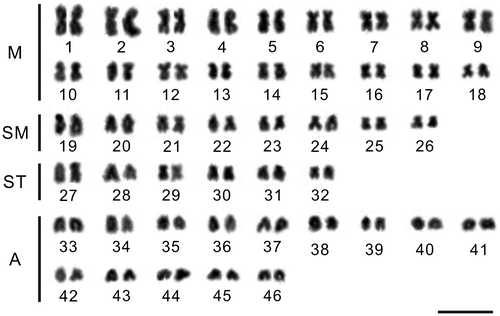
Chromosome morphology was determined on the basis of arm ratio, as proposed by Levan et al. (Citation1964) and chromosomes were classified as metacentrics (M), submetacentrics (SM), subtelocentrics (ST) and acrocentrics (A). All analyzed specimens were preserved in 95% ethyl alcohol, and deposited in Northwest Institute of Plateau Biology, Chinese Academy of Sciences.
Results
Conventional cytogenetic analysis
A total of 250 metaphasic cells were analyzed for the two species, 100 for G. chui and 150 for G. scleracanthus. G. chui showed 2n = 92 chromosomes, a 36m + 16sm + 12st + 28a karyotypic formula and a fundamental number (FN) of 144 (Figure ). The chromosome number of G. scleracanthus was 2n = 92, with the karyotype formula of 26m + 12sm + 18st + 36a and FN of 130 (Figure ). Both males and females were analyzed for G. chui and G. scleracanthus, but no sex chromosomes were identified.
Chromosomal localization of 45S rDNA by fluorescence in situ hybridization
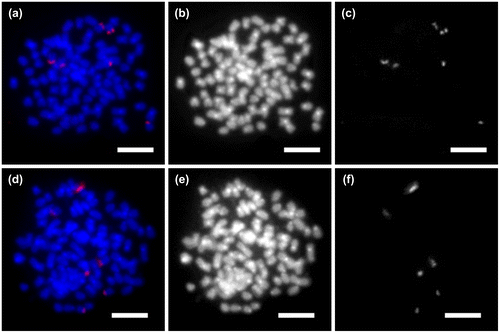
45S rDNA signals were physically mapped on the chromosomes of G. chui and G. scleracanthus (Figure (a, d)). Six 45S rDNA signals were detected at the NOR region of chromosomes in G. chui (Figure (a, c)). Similarly, the same number of 45s rRNA genes were found in three pairs of chromosomes in the G. scleracanthus (Figure (d, f)). No signals were detected outside these regions. Differences of hybridization signals were not observed between G. chui and G. scleracanthus. The chromosomes of these two fish appeared painted with shades of bands after DAPI staining (Figure (b, e)). The pericentromere of most chromosomes showed bright bands. No obvious difference was observed between the two fish.
Figure 3. Physical localization of ribosomal genes on metaphase chromosomes in (a–c) Gymnocypris chui: (a) fluorescent in situ hybridization with 45S rDNA probe; (b) metaphase showing DAPI staining; and (c) sites of 45S rDNA; and (d–f) G. scleracanthus: (d) fluorescent in situ hybridization with 45S rDNA probe; (e) metaphase showing DAPI staining; (f) sites of 45S rDNA.
Discussion
This is the first report of the karyotypes of G. chui and G. scleracanthus from the Lake Langcuo. The chromosome number of G. chui and G. scleracanthus is consistent with some species of the Schizothoracinae (Table ). Nearly 40 Schizothoracinae species have already been submitted to some cytogenetic study, and show considerable interspecific chromosomal diversity (Table ). A karyotype comprising 50 chromosomes could be considered as the ancestral state for the cyprinid fish; it is frequently found and widespread across all of the family (Arai Citation1982). Consequently, we speculate that G. chui and G. scleracanthus may be derived from another non-polyploid cyprinid with 2n = 50 chromosomes, probably with some merged or lost chromosomes after polyploidization. Polyploidization is the process by which Schizothoracinae fishes respond to the complicated environment during the uplift of the Qinghai-Tibet Plateau.
Differences in karyotypic formula were found between G. chui and G. scleracanthus, showing a variation in their karyotypic macrostructure. These species have the same chromosome number but different karyotype formula probably because of structural chromosomal rearrangements. The karyotypic differences may play a role in post-zygotic reproductive isolation during the speciation process (Cardoso et al. Citation2013). Therefore, hybridizations between G. chui and G. scleracanthus are unlikely to occur because of the identified karyotypic differences. However, a deeper insight into the reproductive isolation will probably be achieved by studies of reproductive biology and ethology.
Karyotypic variability can be detected by the chromosomal localization of specific genes, such as 45S rDNA and 5S rDNA. In fish, the location of the 45S rDNA (18S + 5.8S + 28S) is an important cytogenetic marker. This technique has not been applied previously for chromosome studies in Schizothoracinae, and the present study is the first to report the number of nuclear ribosomal loci in Schizothoracinae using FISH. The location of 45S rDNA loci indicated G. chui and G. scleracanthus both had multiple NORs. Little difference was observed between the two species, presumably for the following reasons. First, it is difficult to study the karyotype of Schizothoracinae because of the small chromosome size, high chromosome number, and autotetraploid nature of its chromosomes. Moreover, G. chui and G. scleracanthus, as closely related Schizothoracinae species, have similar chromosome karyotypes. Therefore, some new FISH chromosome markers would be needed in future to identify chromosomes in Schizothoracinae.
In conclusion, accurate nuclear DNA measurements and further information about chromosome banding in these two sympatric species are needed to clarify their karyotypic evolution.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding information
This work was supported by the National Natural Science Foundation of China [grant numbers 31172070, 31572258].
Acknowledgments
We thank Dr Feng Yu for the assistance with the laboratory work. We sincerely thank Dr Fei Tian for her insightful comments and suggestions. We are also very grateful to Prof. Quanwen Dou for providing 45S rDNA.
References
- Almeida-Toledo LF, Foresti F, Toledo-Filho SA. 2000. Karyotypic evolution of neotropical freshwater fish. In: Olmo E, Redi CA, editors. Chromosomes Today. Basel: Birkhäuser Verlag; p. 169–182.
- Arai R. 1982. Chromosome study on two cyprinid fishes, Acrossocheilus labiatus and Pseudorasbora pumila pumila, with notes on Eurasian cyprinids and their karyotypes. Bull Nat Sci Mus Ser A: Zool. 8(3):131–192.
- Borba RS, Silva EL, Pacheco ACS, Parisi-Maltempi PP, Alves AL. 2012. Trends in the karyotypic evolution of the Neotropical catfish family Heptapteridae Bockmann 1998 (Teleostei: Siluriformes). Rev Fish Biol Fisher. 22(2):509–518.
- Cardoso AL, Sales KAH, Nagamachi CY, Pieczarka JC, Noronha RCR. 2013. Comparative cytogenetics of two species of genus Scobinancistrus (Siluriformes, Loricariidae, Ancistrini) from the Xingu River. Brazil. Comp Cytogenet. 7(1):43–51.
- Chen YF, Cao WX. 2000. Schizothoracinae. In: Yue PQ, editor. Fauna Sinica, Osteichthyes, Cypriniformes II. Beijing: Science Press; p. 273–335.
- Chen YQ, Yang C, Zhao J, Li KM, Tang WJ, Wang ZJ. 2006. The chromosome karyotype of Yellow River Schizothoracin (Schizopygopsis pylzovi). Fisheries Sci. 25(11):577–580.
- Chen YX. 2013. The genetic characterization and population genetic diversity of Schizothorax kozlovi (Nikolsky) [PhD thesis]. [Ya'an (China)]: Sichuan Agricultural University.
- Collares-Pereira MJ. 1994. The karyology of barbins and the possible pleisiomorphic condition of polyploidy in Cyprinidae. Bull Fr Pêche Pisc. 67(334):191–199.
- Ganai FA, Dar SA, Yousuf AR, Tripathi NK, Wani S. 2012. Karyoevolutionary and karyosystematic considerations on Schizothorax curvifrons and Schizothorax niger (Teleostei: Cyprinidae): Important hill-stream food fishes of Kashmir Himalaya. Afr J Biotechnol. 11(57):11,998–12,004.
- Ganai FA, Wani S, Ahmad S, Yousuf AR, Tripathi NK. 2014. Coupled biochemical genetic and karyomorphological analyses for taxonomic classification-A case study of Schizothorax species complex (Teleostei: Cyprinidae). Afr J Biotechnol. 13(15):1623–1630.
- Ganai FA, Yousuf AR, Dar SA, Tripathi NK, Wani SU. 2011. Cytotaxonomic status of schizothoracine fishes of Kashmir Himalaya (Teleostei: Cyprinidae). Caryologia. 64(4):435–445.
- Kalbassi MRK, Hosseini SV, Tahergorabi R. 2008. Karyotype analysis in Schizothorax zarudnyi from Hamoon lake. Iran. Turk J Fish Aquat S. 8(2):335–340.
- Khuda-Bukhsh AR, Nayak K. 1982. Karyomorpological studies in two species of hill stream fishes from Kashmir, India: occurrence of a high number of chromosomes. Chromosome Inf Serv. 33:12–14.
- Kong L, Hu W, Wang J. 2011. Primary study on karyotype and C-banding of Gymnodiptychus dybowskii kessler. J Biol. 28(1):34–36.
- Lakara WS, John G, Barat A. 1997. Cytogenetic studies on endangered and threatened fishes 2. Karyotypes of two species of snow-trout, Schizothorax richardsonii (Gray) and S. kumaonensis (Menon). Proc Natl Acad Sci India Biol Sci. 67(1):79–81.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52(2):201–220.
- Li X, Xu Y, Fang Y, Tao L, Li G, Zhan H. 2010. Preliminary study on chromosome and isozyme of Schizothorax prenanti. Freshw Fish. 40(1):34–39.
- Li Y, Li K, Gui J, Zhou D. 1987. Studies on the karyotypes of Chinese cyprinid fishes XI. karyotypes of two species of schizothoracinae and three species of gobiobotinae. Acta Hydrobiol Sin. 11(2):184–186.
- Lin Y. 1982. A PHA injection method in vivo for the rapid obtainment of large number of metaphase figures from kidney cells in teleosts. J Fish China. 6(3):201–208.
- Mazik EJ, Toktosunov A, Ráb P. 1989. Karyotype study of four species of the genus Diptychus (Pisces, Cyprinidae), with remarks on polyploidy of schizothoracine fishes. Folia zool. 38(4):325–332.
- Mirza M. 1991. A contribution to the systematics of the schizothoracine fishes (Pisces: Cyprinidae) with the description of three new tribes. Pak J Zool. 23 (4):339–341.
- Pieczarka JC, Nagamachi CY, Souza ACP, Milhomem SSR, Castro RR, Nascimento AL. 2006. An adaptation to DAPI-banding to fishes chromosomes. Caryologia. 59(1):43–46.
- Poletto AB, Ferreira IA, Cabral-de-Mello DC, Nakajima RT, Mazzuchelli J, Ribeiro HB, Venere PC, Nirchio M, Kocher TD, Martins C. 2010. Chromosome differentiation patterns during cichlid fish evolution. BMC Genet. 11(1):50.
- Qi D. 2004. Preliminary studies on chromosome karyotype and polyploidy of Qinghai-lake naked carp. J Qinghai Univ. 22(2):44–47.
- Rishi KK, Singh J, Kaul MM. 1983. Chromosomal analysis of Schizothoraichthys progastus (McClell.) (Cyprinidae: Cypriniformes). Chromosome Inf Serv. 34:12–13.
- Rubert M, Rosa R, Jerep FC, Bertollo LAC, Giuliano-Caetano L. 2011. Cytogenetic characterization of four species of the genus Hypostomus Lacépède, 1803 (Siluriformes, Loricariidae) with comments on its chromosomal diversity. Comp Cytogenet. 5(5):397–410.
- Wu Y, Kang B, Men Q, Wu C. 1999. Chromosome diversity of Tibetan fishes. Zool Res. 20(4):258–264.
- Wu Y, Wu C. 1992. The fishes of the Qinghai-Xizang Plateau. Chengdu: Sichuan Publishing House of Science & Technology.
- Yan X, Shi J, Sun X, Liang L. 2007. Study on the karyotype of Gymnocypris przewalskii. J Northeast Agr Univ. 38(5):645–648.
- Yu X, Li Y, Zhou T. 1990. Karyotype studies of cyprinid fishes in China-comparative study of the karyotypes of 8 species of schizothoracine fishes. J Wuhan Univ. (2):97–104.
- Yu X, Yu X. 1990. A schizothoracine fish species, Diptychus dipogon, with a very high number of chromosomes. Chromosome Inf Serv. (48):17–18.
- Yu X, Zhou T, Li K, Li Y, Zhou M. 1987. On the karyosystematics of cyprinid fishes and a summary of fish chromosome studies in China. Genetica. 72(3):225–236.
- Zan R, Liu W, Song Z. 1985. Tetraploid-hexaploid relationship in schizothoracinae. Acta Genet Sin. 12(2):137–142.
- Zan RG, Song Z, Liu WG. 1986. Studies on karyotypes and nuclear DNA contents of some cyprinid fishes, with notes on fish polyploids in China. In: Uyeno T, Arai R, Taniuchi T, Matsuura K, editors. Indo-Pacific fish biology. Tokyo: Ichthyological Society of Japan; p. 887–885.
- Zou XJ. 2009. Study on karyotype and genetic diversity in the population of Schizothorax (Racoma) kozlovi [Master's thesis]. [Guiyang (China)]: Guizhou University.