Abstract
This work aims to establish some cytogenetic characteristics of Loricaria simillima from Paraná River. The obtained results show that L. simillima has a 2n = 64 diploid number, with 12 metacentric, 12 sub-metacentric and 40 sub-telo/acrocentric chromosomes. No chromosomal differences were found between sexes. The nucleolus organizer region (NOR) revealed by Ag-NOR was located next to the centromeric region in the short arm of pair 8. The C-bands were pale and detected in pericentromeric regions of the most chromosomes and interstitially on a sub-metacentric pair, coincident with secondary and NORs constriction. A conspicuous interstitial band on an acrocentric chromosome was also observed. Finally DAPI and CMA3 fluorescent bandings showed G-C richness at the NOR region. Present data evidence a great similarity among L. simillima, L. cataphracta and L. carinata, reinforcing the hypothesis that they constitute a closely related phylogenetic group.
Introduction
The Neotropical family Loricariidae is one of the most diversified groups among the Siluriformes.There is incomplete knowledge about this family due to its great diversity and taxonomic complexity. Although cytogenetics is an effective tool for generating basic information, only ~1000 Neotropical fish species have been karyotypically characterized (Nirchio and Oliveira Citation2006). Only around 17% of Loricariidae have been cytogenetically studied (Bitencourt et al. Citation2012). These studies reveal an accentuated variation in the diploid number and variable chromosome banding patterns, suggesting that different chromosomal rearrangements and/or polyploidization events could have occurred along the speciation process of this family (Artoni and Bertollo Citation2001).
The genus Loricaria is one of the smallest genera in the subfamily Loricariinae, with 17 valid species (Eschmeyer et al. Citation2016). These species exhibit very similar morphological features and great taxonomic complexity. Liotta (Citation2006) suggested that L. simillima is a synonym of L. carinata, while Reis et al. (Citation2003), Ferraris (Citation2007) and Eschmeyer et al. (Citation2016) consider L. simillima a valid species and L. carinata as a junior synonym of L. cataphracta.
Cytogenetic studies of the genus Loricaria are scarce and only three nominal species have been characterized: L. cataphracta shows 2n = 64 with polymorphism in localization and number of NORs (Porto et al. Citation2014a); L. carinata with 2n = 64 (Fenocchio et al. Citation2003); and finally Loricaria sp., which also exhibits 2n = 64 with 1–5 supernumerary chromosomes (Scavone and Ferreira-Julio Citation1994).
In view of the scarce available data for Loricaria and the potential of chromosome markers as additional tools for taxonomical identification, this work aims to characterize cytogenetically L. simillima and compare it with its studied congeners.
Materials and methods
Were analyzed 11 specimens of L. simillima, five males and six females. All of them were sampled from Paraná River (Argentina) from Corpus, Misiones (27°06′22.5ʺS; 55°31′20ʺW) to Ituzaingó, Corrientes (27°29′08.4ʺS; 56°40′35.35ʺW). The chromosome preparations were obtained by means of direct techniques (Moreira Filho and Bertollo Citation1991) and kidney cells cultures (Fenocchio et al. Citation1991). Slides were stained with Giemsa (10%, 10 min), analyzed with a Olympus CX-31 trinocular (Tokyo, Japan) and photographed with a Olympus C-5000 zoom digital camera. The diploid number was established by the analysis of 416 metaphases. Chromosomes were measured using the free software MicroMeasure 3.3 (Reeves Citation2001) and classified as suggested by Levan et al. (Citation1964) with modifications of Artoni and Bertollo (Citation2001). Karyotypes were organized in metacentrics (m), sub-metacentrics (sm) and sub-telo/acrocentric (st/a) types according to chromosome size and morphology (Klinkhardt Citation1998). The following were applied: differential staining, Ag-NORs according Howell and Black (Citation1980), C-banding (Sumner Citation1972), chromomycine A3 (CMA3) and 4–6-diamino-2-phenilindol (DAPI) (Schweizer Citation1976).
Results
The karyotype of L. simillima presents 2n = 64 with 12 metacentric, 12 sub-metacentric and 40 sub-telo/acrocentric chromosomes (Figure ). The calculated fundamental number was FN = 88 and no karyotypic differences between males and females were found. The sub-metacentric eighth pair is characterized by the presence of a conspicuous secondary constriction at the short arm (p), near to the centromere that could be seen clearly with conventional staining (Figure (a)).
Figure 1. Giemsa stained karyotype of Loricaria simillima. The boxes show Ag-NOR, C-banding, CMA3 and DAPI staining of the eighth pair.
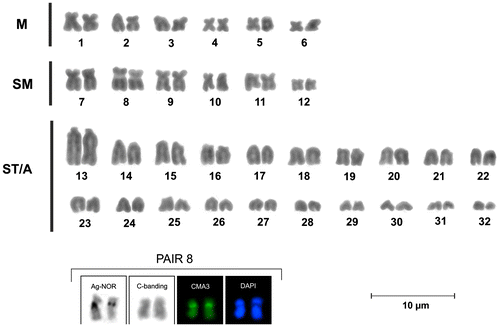
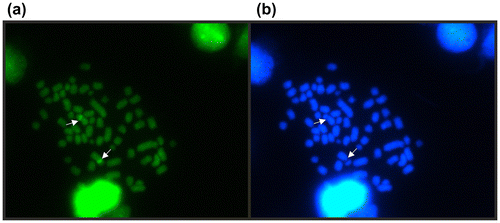
Figure 2. Loricaria simillima: (a) conventional Giemsa staining; arrows indicate the eighth pair. (b) C-banding technique; arrows indicate the NOR bearing pair and an acrocentric chromosome with conspicuous interstitial band. (c) Silver staining; arrows indicate the NOR bearing pair.
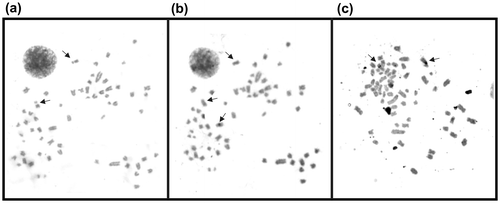
The C-banding revealed weak pericentromeric bands (Figure (b)) with the exception of an acrocentric chromosome with a conspicuous interstitial band. The NOR regions were also positive to C banding.
Staining with silver salts (Ag-NORs) revealed a pair of active nucleolus organizer regions (Figure (c)). They were located at the short arm of sub-metacentric eighth pair that is also characterized by the presence of a secondary constriction. In some cases only one chromosome of the pair showed argentic impregnation.
Fluorescent staining with CMA3 and DAPI was used to detect G-C and A-T rich regions. The CMA3 detected just a pair of bright marks located at the same chromosome pair than the NOR (Figure (a)). When the chromosome preparations were stained with DAPI, as expected, the bright CMA3 band show a depletion of coloration, appearing as a gap in the chromosome 8 (Figure (b)). No other bright marks were detected with DAPI.
Discussion
Cytogenetic studies in genus Loricaria are scarce and reveal the presence of 64 chromosomes in three nominal species: L. simillima (present study), L. cataphracta (Porto et al. Citation2014a) and L. carinata (Fenocchio et al. Citation2003). The karyotypic formulae and fundamental number is distinct among these species (Table ). Loricaria simillima presents the highest number of metacentric and sub-metacentric chromosomes (m/sm 24), similar to L. cataphracta (m/sm = 20) but distinct from Loricaria sp. and L. carinata which present 16 and 12 m/sm chromosomes respectively. A hypothesis to explain the differences in karyotype macrostructure but conservative diploid number within the species of the genus is based on the role of pericentric inversions. Meiosis analysis combined with telomeric hybridization probes will be useful to corroborate the hypothesis that inversion takes place in the karyotypic evolution of Loricaria.
Table 1. Cytogenetic studies in genus Loricaria.
Another feature reported is the presence of supernumerary chromosomes in one population of Loricaria sp. from Upper Parana River basin (Scavone and Ferreira Julio Citation1994); this numeric polymorphism is rare in Loricariinae and described only for Rineloricaria pentamaculata (Porto et al. Citation2011) and Harttia longipinna (Blanco et al. Citation2012).
The heterochromatin distribution pattern observed in L. simillima is characterized by a few blocks in pericentric regions and coincident with secondary constriction at NOR bearing pair. This pattern is similar to that described in Loricaria sp. (Scavone and Ferreira-Julio Citation1994), the only species with C-banding characterization. Porto et al. (Citation2014a) claim that NOR bearing chromosomes were due to positive C-banding but they do not make comments on the heterochromatin distribution at the whole complement of Loricaria cataphracta. According to Takagui et al. (Citation2014) the presence of a few heterochromatic blocks, mainly in the pericentric region, is a remarkable feature of armored catfishes of the subfamily Loricariinae, present in species of the basal genus Harttia (Kavalco et al. Citation2005; Blanco et al. Citation2012) as well as in the derived genus Rineloricaria (Rosa et al. Citation2011; Porto et al. Citation2014b). The exceptions to this pattern are Farlowella amazonum (Fernandes et al. Citation2015) and Loricaria cataprhacta (Porto et al. Citation2014a), which have additional conspicuous terminal heterochromatin blocks in different chromosomes.
In Loricariidae NORs in a terminal position do not occur frequently; however, Kavalco et al. (Citation2005) mentioned that in the family around 25% of the studied species show interstitial NORs, as was reported in Loricaria sp., L. cataprhacta and in the present study (Table ). According to Ziemniczak et al. (Citation2012), NOR sites in only one chromosome pair in an interstitial position is a plesiomorphic condition in Loricariidae, because this pattern is common in the sister group of the superfamily Loricarioidea (Trichomycteridae), as well as in Neoplecostominae and Hypoptopomatinae, which are basal subfamilies of Loricariidae (Andreatta et al. Citation1994, Armbruster Citation2004, Alves et al. Citation2005). In Loricariinae interstitial NORs occur in a few groups, included basal species of genus Harttia (Centofante et al. Citation2006, Blanco et al. Citation2012, Blanco et al. Citation2013, Blanco et al. Citation2014), Loricariichthys anus (Takagui et al. Citation2014), Rineloricaria latirostris (Giuliano-Caetano Citation1998) and Loricaria (Porto et al. Citation2014a; present study).
Comparisons of CMA3 and DAPI banding with other studied species of the genus Loricaria are not possible, because there is no reference of this analysis in them. However, correspondence among Ag-NORs, CMA3 positive marks and DAPI negative marks was observed in L. simillima. This is expected due the guanine and cytosine richness in the NOR region in vertebrates.
Taxonomists currently agree that L. carinata is a synonym of L. cataphracta. Although L. cataphracta is considered to be present in the Upper Paraguay River, according to Mirande and Koerber (Citation2015) this species is not reported in Argentina. Differences on karyotypic formulae between L. cataphracta from Paraguay River and L. carinata reported by Fenocchio et al. (Citation2003) from Paraná River are probably due a misidentification of the specimens. Two other sympatric species of Loricaria are cited in the Paraná River, so it would be possible that what Fenocchio et al. (Citation2003) reported as L. carinata is instead L. tucumanensis or L. luciae.
Present data show a great similarity among L. simillima, L. cataphracta and L. carinata, reinforcing the hypothesis that they constitute a closely related phylogenetic group.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The authors are grateful for the “Proyecto Biología Pesquera Regional” for collaboration and assistance during the fieldwork; and Facultad de Ciencias exactas, químicas y naturales (Universidad Nacional de Misiones) and Instituto de Biología subtropical for permanent support. MB is supported by a doctoral grant given by Consejo nacional de investigaciones científicas y técnicas Res. D Número [grant number 3424/2014].
References
- Artoni RF, Bertollo LAC. 2001. Trends in the karyotype evolution of Loricariidae fish (Siluriformes). Hereditas. 134:201–210.
- Andreatta AA, Almeida-Toledo LF, Oliveira C, Almeida-Toledo FS. 1994. Cytogenetic studies on the subfamily Hypoptopomatinae (Pisces, Siluriformes, Loricariidae). III Analysis of seven species. Caryologia. 47(1):27–37.
- Armbruster JW. 2004. Phylogenetic relationships of the suckermouth armoured catfishes (Loricariidae) with emphasis on the Hypostominae and the Ancistrinae. Zool J Linn Soc. 141(1):1–80. doi:10.1111/zoj.2004.141.issue-1.
- Luìs Alves AL, Oliveira C, Foresti F. 2005. Comparative cytogenetic analysis of eleven species of subfamilies Neoplecostominae and Hipostominae (Siluriformes: Loricariidae). Genetica. 124(2–3):127–136. doi:10.1007/s10709-004-7561-4.
- Bitencourt JA, Affonso PRAM, Giuliano-Caetano L, Carneiro PLS, Dias AL. 2012. Population divergence and peculiar karyoevolutionary trends in the loricariid fish Hypostomus aff. unae from northeastern Brazil. Genet Mol Res. 11(2):933–943. doi:10.4238/2012.April. 13.1.
- Blanco DR, Vicari MR, Artoni RF, Traldi JB, Moreira-Filho O. 2012. Chromosomal characterization of Armored catfish Harttia longipinna (Siluriformes, Loricariidae): first reporto f B chromosomes in the genus. Zoolog Sci. 29(9):604–609. doi:10.2108/zsj.29.604.
- Blanco DR, Vicari MR, Lui RL, Carlos Bertollo Luiz Antônio, Baccarin Traldi Josiane, Moreira-Filho O. 2013. The role of the Robertsonian rearrangements in the origin of the XX/XY1Y2 sex chromosome system and in the chromosomal differentiation in Harttia species (Siluriformes, Loricariidae). Rev Fish Biol Fisheries. 23:127–134. doi:10.1007/s10709-014-9759-4.
- Blanco DR, Vicari MR, Lui RL, Artoni RF, Almeida MC, Traldi JB. 2014. Origin of the X1X1X2X2/X1X2Y sex chromosome system of Harttia punctata (Siluriformes, Loricariidae) inferred from chromosome painting and FISH with ribosomal DNA markers. Genetica. 142(2):119–126. doi:10.1007/s10709-014-9759-4.
- Centofante L, Bertollo LAC, Moreira-Filho O. 2006. Cytogenetic characterization and description of a XX/XY1Y2 sex chromosomes system in catfish Harttia carvalhoi (Siluriformes, Loricariidae). Cytogenet Genet Res. 112:320–324. doi:10.1159/000089887.
- Eschmeyer WN, Fricke R, Van der Laan R. 2016. Catalog of fishes: genera, species, references; [cited 2016 Mar 26]. Available from: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
- Fenocchio AS, Venere PC, Cesar ACG, Dias AL, Bertollo LAC. 1991. Short term culture from solid tissues of fishes. Caryologia. 44(2):161–166. doi:10.1080/00087114.1991.10797181.
- Fenocchio AS, Pastori MC, Roncati HA, Moreira-Filho O, Bertollo LAC. 2003. A Cytogenetic Survey of the Fish Fauna from Argentina. Caryologia. 56(2):197–204. doi:10.1080/00087114.2003.10589325.
- Fernandes CA, Alves DS, Guterres Z, Martins-Santos IC. 2015. Cytogenetic analysis of two locariid species (Teleostei, Siluriformes) from Iguatemi River (Parana River drainage) in Brazil. Comp Cytogenet. 9(1):67–78. doi:10.3897/CompCytogen.v9i1.8804.
- Ferraris CJ. 2007. Checklist of catfishes, recent and fossil (Osteichthyes: Siluriformes), and catalogue of siluriform primary types. Auckland, New Zealand: Magnolia Press; p. 628.
- Giuliano-Caetano L. 1998. Polimorfismo Cromossômico Robertsoniano em populações de Rineloricaria latirostris (Pisces, Loricariidae) [Robertsonian chromosomal polymorphism in Rineloricaria latirostris populations]. [ PhD Thesis]. Universidade Federal de São Carlos; p. 678.
- Howell WM, Black DA. 1980. Controlled silver staining of nucleolus organizer regions with a colloidal developer: a one step method. Cell Mol Life Sci. 36(8):1014–1015. doi:10.1007/BF01953855.
- Kavalco KF, Pazza R, Bertollo LAC, Moreira-Filho O. 2005. Karyotypic diversity and evolution of Loricariidae (Pisces, Siluriformes). Heredity. 94:180–186. doi:10.1038/sj.hdy.6800595.
- Klinkhardt MB. 1998. Some Aspects of Karyoevolution in Fishes. Anim Res Dev. 47:7–36.
- Levan A, Fredga K, Sandberg AA. 1964. Nomenclature for centromeric position on chromosomes. Hereditas. 52:201–220.
- Liotta, J. 2006. Distribución geográfica de los peces de aguas continentales de la República Argentina [Geographic distribution of freshwater fishes in Argentina]. ProBiotA, Serie Documentos N°3. La Plata, Buenos Aires, Argentina. 367–370.
- Mirande JM, Koerber S. 2015. Checklist of the freshwater fishes of Argentina (CLOFFAR). Ichthyological Contrib PecesCriollos. 36: 1–68. Available from: http://www.pecescriollos.de/
- Moreira Filho, Bertollo. 1991. Extraction and use of the cephalic kidney for chromosome studies in small fish. Rev bras Genética. 14(4):1085–1090.
- Nirchio M, Oliveira C. 2006. Citogenética de peces [Fish cytogenetics]. Porlamar, Estado Nueva Esparta, Venezuela: Coordinación de publicaciones del Rectorado de la Universidad de Oriente; p. 212.
- Porto FE, Portela-Castro ALB, Martins-Santos IC. 2011. Chromosome polymorphism in Rineloricaria pentamaculata (Loricariidae, Siluriformes) of the Paraná River basin. Ichthyological Res. 58(3):225–231. doi:10.1007/s10228-011-0215-5.
- Porto FE, Gindri BS, Vieira MMR, Borin LA, Portela-Castro ALB, Martins-Santos IC. 2014a. Polymorphisms of the nucleolus organizing regions in Loricaria cataphracta (Siluriformes, Loricariidae) of the upper Paraguay River basin indicate an association with transposable elements. Genet Mol Res. 13(1):1627–1634. doi:10.4238/2014.March.12.15.
- Porto FE, Vieira MM, Barbosa LM, Borin-Carvalho LA, Vicari MR, Portela-Castro AL, Martins-Santos IC. 2014b. Chromosomal polymorphism in Rineloricaria lanceolata Gunther 1868 (Loricariidae:Loricariinae) of the Paraguay basin (Mato Grosso do Sul, Brazil): evidence of fusions and their consequences in the population. Zebrafish. 11(4):318–324. doi:10.1089/zeb.2014.0996.
- Reeves A. 2001. MicroMeasure: a new computer program for the collection and analysis of cytogenetic data. Genome. 44:239–243.
- Reis RE, Kullander SO, Ferraris CJ Jr. 2003. Check list of the freshwater fishes of South and Central America. Porto Alegre, Brasil: EDIPUCRS; p. 330–349.
- Rosa KO, Ziemniczak K, Barros AV, Nogaroto V, Almeida MC, Cestari MM, Artoni RF, Vicari MR. 2011. Numeric and structural chromossome polymorfism in Rineloricaria lima (Siluriformes: Loricariidae): fusions points carrying 5S rDNA or telomere sequence vestiges. Rev Fish Biol Fish. 22(3):739–749. doi:10.1007/s11160-011-9250-6.
- Scavone, MDP, Ferreira-Julio, H. 1994. Cytogenetics analysis and probable supernumerary chromosomes of Loricaria prolixa and Loricaria sp. females (Loricariidae-Siluriformes) from the Paraná River basin. Rev. de Ictiología 2/3(1/2); pp. 41–47.
- Schweizer D. 1976. Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma. 58(4):307–324. doi:10.1007/BF00292840.
- Sumner AT. 1972. A simple techinique for demostrating centromeric heterochomatin. Exp Cell Res. 75:304–306. doi:10.1016/0014-4827(72)90558-7.
- Takagui FH, Venturelli NB, Dias AL, Swarça AC, Vicari MR, Fenocchio AS, Giuliano-Caetano L. 2014. The importance of pericentric inversions in the karyotypic diversification of the species loricariichthys anus and loricariichthys platymetopon. Zebrafish. 11(4):300–305. doi:10.1089/zeb.2014.0985.
- Ziemniczak K, Barros AV, Rosa KO, Nogaroto V, Almeida MC, Cestari MM, Moreira-Filho O, Artoni RF, Vicari M. 2012. Comparative cytogenetic of Loricariidae (Actinopterygii: Siluriformes): emphasis in neoplecostominae and hypoptopomatinae. Ital J Zool. 79:492–501. doi:10.1080/11250003.2012.676677.