Abstract
Sporogenesis is an important phenomenon in bryophytes, occurring in the sporophyte, as reduction division takes place at this time and haploid spores with distinctive morphological features are formed. They can serve as important taxonomic tools. The present communication deals with taxonomical status of Physcomitrium eurystomum Sendtn. of family Funariaceae, along with sporophytic characterization and sporogenesis with detailed reporting of meiotic pattern. Our observations suggest that, at the onset of sporogenesis, the acentrically located interphase nucleus becomes central and then undergoes division. Spore mother cells were spherical and their size remained unchanged till the second telophase, after which there was enlargement and the tetrad separated into four spores. 26 bivalents were seen at diakinesis, which has already been reported by some workers. The sporophyte of this species had short pyriform capsule with spinose papillose ornamentation in spores.
1. Introduction
Bryophytes are non-flowering, small miniature cryptogams, without vascular elements, and are very distinct from other land plants. They are positioned between Algae and Pteridophytes, and play a major role in soil management, formation of fertile substrata for other plants, and are an important component of ecosystems. The bryoflora has been well studied taxonomically with reports of more than 20,000 species with 10,000–14,000 mosses, 5000 liverworts and 400 hornworts (Goffinet and Shaw Citation2009). They are early pioneers of life on land and maximum diversity of species has been shown in tropical and temperate rainforests. Under various ecological conditions, these plants comprise the dominant elements of the vegetation (Makinde and Fajuke Citation2009). Therefore, these plants are considered as an ancient group of plants that appear sister to protracheophytes and tracheophytes and their life cycle includes a functionally dominant, free living haploid phase (gametophyte) and a partially dependent diploid phase (sporophyte).
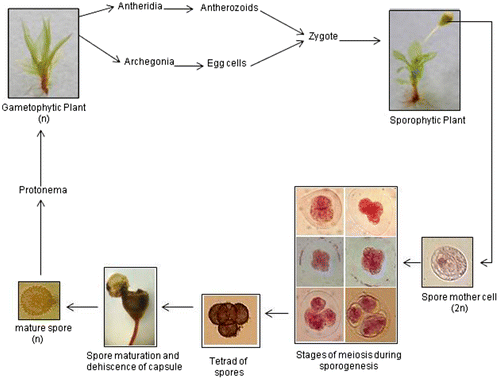
In bryophytes, sporogenesis is a unique developmental process by which the haploid cells (spores) are formed by meiosis and are covered with a complex desiccation-resistant, sporopollenin-impregnated wall known as sporoderm. Morphology and ultrastructure of the spores are significant in the taxonomy and phylogeny of bryophytes (Shaw et al. Citation2011). The spores serve as dispersal units and on favourable substrate, they germinate into a filamentous protonema which matures to form the leafy gametophores that bear antheridia and archegonia in modified apices. The haploid gametes fuse and the diploid sporophytic generation is initiated. Thus this alternation of generations with meiosis taking place in the sporophyte is the main feature in the life cycle of all bryophytes. Brown and Lemmon (Citation2013) have reported that in bryophytes, cytokinesis reflected by the quadrilobing of the cytoplasm occurs first, followed by the different stages of meiosis. The meiotic spindle is quadripolar with poles in the four future spore domains. The four cells produced after meiosis/cytokinesis becomes a tetrad of spores, each covered with the sporoderm.
The family Funariaceae comprises 16 genera of short-lived, weedy, soil-inhabiting mosses and has a worldwide distribution (Goffinet et al. Citation2012). It is represented by only three genera: Physcomitrium (Brid.) Fuernr., Entosthodon Schwaegr. and Funaria Hedw. in India. In contrast to other mosses, this family exhibits gametophytic uniformity and sporophytic plasticity i.e. variation from extremely reduced cleistocarpic genera to highly organised diplolepideous condition is observed.
Physcomitrium, our experimental plant, has a cosmopolitan distribution with about 80 species distributed over moist and cool regions of the globe (Fife Citation1985) and eight species in India (Lal Citation2005). Among the Indian bryogeographical zones, the Gangetic Plain houses the maximum (six) number of species. Eastern Himalaya has all the species reported from the Gangetic Plain except P. cyathicarpum and P. indicum. The West Himalayan region has only three species, whereas South India, Central India, Rajasthan and Punjab plains comprises only two species each. Previously Gangulee (Citation1974–1977) reported seven species of this genus, but P. insigne was also reported from South India (Foreau Citation1964, Daniels Citation2010) and P. delicatulum from Delhi (Lal and Menon Citation1971). Subsequently, Dandotiya et al. (Citation2011) listed 13 species of this genus from various parts of India including P. eurystomum from Eastern and Western Himalaya, the Gangetic Plain and the Rajasthan Plain, but the species was also reported from Kerala, South India (Rajeevan Citation1990).
There are reports of chromosome numbers from n = 9 to n = 72 in Physcomitrium (Fritsch Citation1991). Pande and Chopra (Citation1957) reported nine chromosomes in P. pyriforme; Chopra (Citation1959) reported n = 3, 6 in another Physcomitrium sp. and 18 chromosomes count in P. japonicum collected from Nainital. Gangulee and Chatterji (Citation1960) reported 12 chromosomes in the same species collected from Eastern India. Subsequently, Khanna (Citation1959, Citation1960) reported n = 51+1–2f (51 bivalents with additional 1–2 fragments) in P. cyathicarpum and P. repandum. Verma and Kumar (Citation1980) listed n = 52 in both the above two species.
The life cycle of P. eurystomum with focus on its sporophyte is presented in this paper. A detailed account of the meiotic events leading to the formation of spores is reported here.
2. Materials and methods
2.1. Sampling
P. eurystomum is a common winter moss growing in and around Lucknow (26°51′0ʺ N; 80°55′0ʺ E with altitude 128 m above sea level) from November to March. The fresh gametophytes bearing sporophytes were collected from Lucknow University where these species grow luxuriantly during December–February.
2.2. Taxonomical studies
Morphological characters of plants were examined under a stereo-zoom dissecting microscope. Size and shape of leaves and thin hand-cut sections of stem and leaves were examined under a compound microscope. All measurements were made from wet material. Laminal width was measured at the broadest part of the leaf, costal width in the lower third and plant length refers only to the gametophyte. Photographs were taken with a digital camera.
2.3. Cytological preparations
Capsules in which the operculum was not yet pigmented were selected for the meiotic study, as revealed by preliminary studies. For cytological analysis, young capsules were fixed in Carnoy’s fluid (1 part glacial acetic acid + 3 parts ethanol) for 24 h and then preserved in 70% ethanol. Fixation and slide preparation was done by the protocol of Sharma and Sharma (Citation1980). Meiotic stages were studied in acetocarmine squash preparations of sporocytes from the capsule tip. The observations were recorded under low and high power of the microscope. A Nikon Eclipse 600 light microscope was used for slides observations and microphotographs were taken with a Nikon Cool Pix 950 digital camera. The percentage meiotic index and phase indices were calculated by using the classical formula:
3. Results
3.1. Taxonomical observations
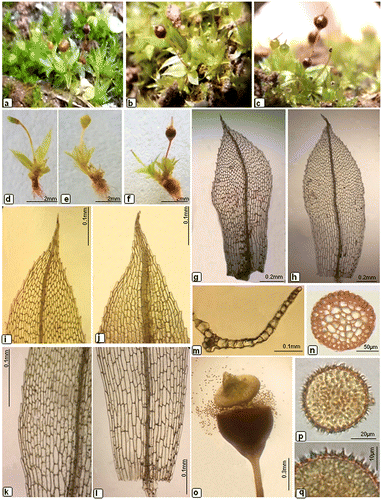
Plants bright green to yellowish-green (Figure (a–c)), 3–6 mm long, with radicles below. Stems very short, slender, simple, with many rhizoids at base. Leaves crowded, at the top of stem only, erectopatent to erectospreading when moist, shrunk when dry, oblong–obovate (Figure (g), (h)), ±3.4–5.2 × 1–1.7 mm larger at upper stem, whereas smaller at lower part, acute or acuminate at apex, margins serrulated in the upper part (Figure (i), (j)), entire in the basal part. Apical (45–54 × 18.5–27.7 μm) and median (46.8–74.1 × 18.5–23.1 μm) leaf cells hexagonal to oblong–hexagonal (Figure (k)), basal cells (125.4–156.4 × 34–37.4 μm) large rectangular (Figure (l)); marginal cells narrow elongated, ±139.5–148 × 10.2–12.7 μm, in one or two rows, sublinear. Costa strong, slender, extending to the apex or short excurrent. In a cross-section near the base, the costa (Figure (m)) showed a layer of wide cells on the upper and lower surfaces and a steroidal band. Seta are slender, 3–6 mm long, somewhat flexuose, yellowish brown. In transverse section seta (Figure (n)) appeared to be massive and uniform with rounded shape. Capsules green when young (Figure (d), (e)), short pyriform, reddish brown at maturity Figure (f)), 1.2–1.4 mm long and 0.9–1 mm in diameter with a short apophysis and wide mouth (Figure (o)). Operculum clearly differentiated, conical convex. Calyptra very small, conical, long–rostrate and with a smooth circular rim, easily falling off long before the capsules became fully mature. Peristome absent. Spores irregularly spherical, blackish-brown (Figure (p)), spinulosely papillose (Figure (q)), 26.5–30.4 μm in diameter.
Figure 1. Morphological details of Physcomitrium eurystomum Sendtn. (a–c) plants; (d–f) developmental stage of capsule; (g–h) leaves; (i–j) apical laminal cells; (k) middle laminal cells; (l) basal laminal cells; (m) cross-section of leaf; (n) cross-section of seta; (o) dehisced capsule; (p) spores; (q) enlarged view of spore.
The sporophyte of P. eurystomum was compared to other species of the genus and was found to be very similar to P. pyriforme having almost similar seta and capsule size, whereas spores of P. pyriforme was much larger than that of former species. The spore size was similar to P. immersum, P. pulchellum and P. pyriforme (Table ).
Table 1. Sporophytic details of Physcomitrium spp.
3.2. Sporogenesis
The dominant generation in life cycle of P. eurystomum included a functionally dominant, free-living haploid gametophyte followed by a short-lived sporophyte which was differentiated into foot, seta and capsule. The meiotic events in the diploid spore mother cells in the sporophyte, responsible for the formation of haploid spores were investigated (Figure ). Sporophytes belonging to different age groups as reflected by their relative size were observed after suitable preparation and division stages were found in green and young capsules with shorter seta while mature spores were observed in reddish-brown capsules with 4–7 mm long seta.
3.3. Meiotic observations
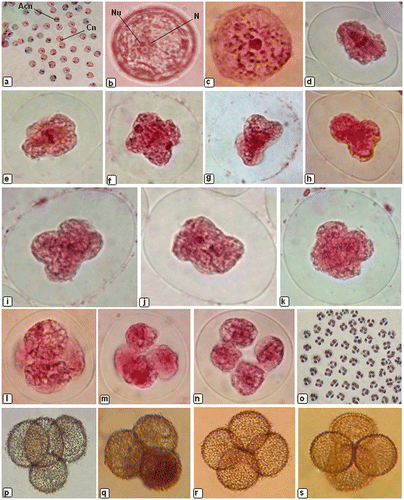
Spore formation is a major event in the life cycles of land plants which allows the transition from diploid sporophyte generation to the haploid gametophyte generation. At the time of sporogenesis, meiotic behaviour in P. eurystomum showed that the spore mother cells were spherical (Figure (a)), and their size remained unchanged till the second telophase after which there was a rapid phase of growth before the tetrad separated into four spores. The nucleus was located acentrically (Figure (b)) in the cell during interphase. At pachytene, the nucleus became centrally located (Figure (c)), marking the onset of division. In metaphase I the spindle was bipolar with poles for first meiotic division located in opposite furrows (Figure (d), (e)) between lobes of the cytoplasm. During division the bivalents showed a tendency to clump together, which made counting and interpretation difficult. At anaphase the chromosomes separated and moved towards the opposite poles (Figure (f)), whereas during prophase II, sporocytes showed extreme quadripolarity (Figure (g–i)) of the developing spindle. In metaphase II, the spindle again drew the chromosomes to the metaphase plate (Figure (j)) and this was followed by separation of the sister chromatids of each chromosome during the next phase of meiosis i.e. anaphase II and their movement towards opposite poles (Figure (k)). At telophase II, nuclei are delivered to opposite poles. Cleavage furrows were observed (Figure (l), (m)) and a flattened tetrad of spores (Figure (n)) formed. The tetrad of young spores was observed to be tetrahedral (Figure (p), (q)), isobilateral (Figure (r)) or decussate (Figure (s)).
Figure 3. Sporogenesis in Physcomitrium eurystomum Sendtn.: (a) sporocytes showing centric (Cn) and acentric (Acn) nucleus; (b) pre-meiotic sporocyte showing acentric nucleus (N) and nucleolus (Nu); (c) prophase showing sporocyte with centrally positioned nucleus and bivalents; (d) metaphase I showing wall ingrowths coincide with the equator; (e) quadrilobed metaphase sporocyte; (f) as chromosomes separate in anaphase the spindle becomes more elliptical and terminates adjacent to polar cleavage furrows; (g) telophase I; (h) sporocyte showing the extreme quadripolarity of the developing spindle during prophase II; (i) quadrilobed sporocyte during prophase II; (j) metaphase II in the undivided cytoplasm; (k) anaphase II showing separation and movement of sister chromatids toward opposite poles; (l) pair of phragmoplasts in slightly flattened sporocyte; (m) each phragmoplast consists of opposing arrays of microtubules emanating from telophase nuclei; (n) a flattened tetrad of spores; (o) tetrad of spores showing 100% tetrad index; (p–s) tetrad of young spores.
The value of meiotic index (MI) and the frequency of the meiotic phases/phase indices (% of prophase, metaphase and anaphase) were also calculated to determine the progress of division. It was observed that at a given time the value of MI for the species P. eurystomum was 24.4%. At the time of interphase, 72.9% cells were acentric in which nucleus was situated near the periphery, whereas 16.7% cells had centrally located nucleus during pachytene stage of prophase (Figure (a)). The stages progressed differently in the spore mother cells giving variation in phase index at a particular time. Metaphase I index was 50%, anaphase index 14.3% and telophase index was 25%. During second division, phase index (PI) was 35.7%, metaphase index 7.2% and tetrad index was 100% (Figure (o)).
4. Discussion
The sporocytes in P. eurystomum followed a precise coordinated pathway in which quadripolarity for the eventual quadripartitioning into the tetrad of spores was established very early, as reported in Andreaea, Sphagnum, Polytrichum sp. and bryopsid spp. (Brown and Lemmon Citation2013). Our observations suggest that, in P. eurystomum, sporogenesis was normal like other similar species and four viable spores were formed from single spore mother cell. During meiosis, 26 bivalents were seen at diakinesis in the spore mother cell, as in the report of Kumar et al. (Citation1987). Pande and Chopra (Citation1958) stated the basic number for Physcomitrium is n = 3, which evolved independently and without any cytological inter-generic relationship whatsoever. Therefore, on the basis of cytological data provided by earlier workers, Uniyal (Citation1998) suggested that cytological evolution in the family Funariaceae has proceeded along two lines, n = 9 in Physcomitrium and n = 14 in Funaria.
The occurrence of P. eurystomum is quite interesting since it is taxonomically well defined and reproductively isolated from the other Physcomitrium–Physcomitrella species complexes. However, McDaniels et al. (Citation2009) suggested its hybrid origin by assuming an event between two early diverging lineages in the complex, and that the ancestral population size of these lineages was much smaller than the current population sizes. The only reliable and significant character distinguishing P. eurystomum from other allied species are oblong-obovate leaves with bluntly denticulate margin in the upper part and distinctly bordered by one or rarely two rows of elongate cells. The costa is percurrent to shortly excurrent and the operculum has a conical-rounded apiculus.
The species has many features in common with other Physcomitrium species and related genera of the family Funariaceae. Sometimes, the specimens of P. eurystomum are most likely to be confused with P. pyriforme in several morphological features such as leaf shape, denticulation in upper parts, percurrent costa and presence of spinosely papillose spores, but differs from each other in having short pyriform capsule with 25–28 μm spores, whereas in P. pyriforme capsules are pear shaped and globose pyriform with larger spores ranging from 28 to 36 μm in diameter. A closely allied species, P. pulchellum, has been found growing in loose tufts and differs in having one to two rows bordered leaves with entire leaf margin, however in P. eurystomum leaf margin bordered with one row of elongate cells and denticulations are present in upper part only. Presence of larger leaves on fertile shoot and narrower capsule mouth in P. pulchellum also separates this species from P. eurystomum. Beike et al. (Citation2014) provided molecular evidence for sympatric speciation involving convergent evolution and allopolyploidization into the global genetic diversity of Physcomitrium–Physcomitrella species complex and they suggested that P. eurystomum and P. collenchymatum are hybrid species, putatively produced from hybridizations between ancestors of modern P. sphaericum and P. pyriforme.
5. Conclusions
Our investigation highlights a major aspect of the life cycle of P. eurystomum, with focus on the meiotic pattern of sporogenesis, thereby adding to the information available for this genus. Further work is required to elucidate the life cycle and reproductive mechanism in other species of the Physcomitrium–Physcomitrella species complex.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by University Grants Commission (UGC), New Delhi [grant number F.4-2/2006(BSR)/BL/13-14/0375].
References
- Al-Aish M, Anderson LE. 1960. Chromosome numbers of some Arizona mosses. Bryologist. 63(1):17–25.
- Anand S, Kumar SS. 1986. IOPB chromosome number reports XCI. Taxon. 35(2):404–410.
- Bassi G, Kumar SS. 1996. Cytology of some mosses from the Western Himalayas. Indian J Genet. 56(4):406–411.
- Beike AK, von Stackelberg M, Schallenberg-Rüdinger M, Hanke ST, Follo M, Quandt D, McDaniel SF, Reski R, Tan BC, Rensing SA. 2014. Molecular evidence for convergent evolution and allopolyploid speciation within the Physcomitrium-Physcomitrella species complex. BMC Evol Bio. 14(158):1–19.
- Brown RC, Lemmon BE. 2013. Sporogenesis in Bryophytes: patterns and diversity in meiosis. Bot Rev. 79(2):178–280. 10.1007/s12229-012-9115-2
- Chopra N. 1959. Cytological studies in Indian mosses. V. Physcomitrium japonicum (Hedw.) Mitt. and Physcomitrium sp. Curr Sci. 28(3):114–115.
- Dandotiya D, Govindapyari H, Suman S, Uniyal PL. 2011. Checklist of the bryophytes of India. Arch Bryol. 88:1–126.
- Daniels AED. 2010. Checklist of the bryophytes of Tamil Nadu, India. Arch Bryol. 65:1–117.
- Fife AJ. 1985. A generic revision of the Funariaceae (Bryophyta: Musci), part 1. J Hattori Bot Lab. 58:149–196.
- Foreau G. 1964. Miscellaneous notes: some South Indian mosses. J Bomb Nat Hist Soc. 61(1):223–226.
- Fritsch R. 1991. Index to bryophyte chromosome counts. vol 40. Berlin, Stuttgart: Cramer J/Gebrueder Borntraeger.
- Gangulee HC 1974–1977. Mosses of Eastern India and adjacent regions. Vol. II (Fasc. 4–6). Calcutta: Books and Allied Limited.
- Gangulee HC, Chatterji NK. 1960. Cytological studies in the mosses of eastern India. II Nucleus. 3:165–176.
- Goffinet B, Shaw AJ. 2009. Bryophyte biology. 2nd ed. New York (NY): Cambridge University Press.
- Goffinet B, Shaw AJ, Buck WR. 2012. Classification of the Bryophyta. [cited 2016 Jun 17]. Available from: http://www.eeb.uconn.edu/people/goffinet/Classificationmosses.html.
- Kapila S, Kumar SS. 1997. Cytological observations on some West Himalayan mosses. Hikobia. 12(3):215–219.
- Khanna KR. 1959. Cytology of some Himalayan mosses II. Curr Sci. 28(12):497–498.
- Khanna KR. 1960. Cytological studies in some Himalayan mosses. Caryologia. 13(3):559–618. 10.1080/00087114.1960.10797100
- Kumar SS, Koponen T, Uniyal PL. 1987. Chromosome number reports 97. Taxon. 36(4):766–767.
- Kumar SS, Bassi G, Talwani S. 1988a. Some new reports of chromosome numbers in Western Himalayan mosses. SOCGI plant chromosome number reports VI. J Cytol Genet. 23(1–2):39–40.
- Kumar SS, Uniyal PL, Sharma C. 1988b. Some new reports of chromosome numbers in Western Himalayan mosses. SOCGI plant chromosome number reports VI. J Cytol Genet. 23(1–2):40–41.
- Lal J. 2005. A checklist of Indian mosses. Dehra Dun: Bishen Singh Mahendra Pal Singh.
- Lal M, Menon MKC. 1971. Physcomitrium delicatulum, new to the moss flora of India. Bryologist. 74(1):51–54. 10.2307/3241760
- Makinde A, Fajuke AA. 2009. Adaptive strategies of mosses to desiccation. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 37(1):191–193.
- McDaniels SF, Stackelberg M, Richardt S, Quatrano RS, Reski R, Rensing SA. 2009. The speciation history of the Physcomitrium-Physcomitrella species complex. Evolution. 64(1):217–231.
- Pande SK, Chopra N. 1957. Cytological studies in Indian mosses. I. Pogonatum microstomum (R. Br.) Brid., P. stevensii Ren. & Card., Bryum nitens Hook. and Physcomitrium pyriforme (L.) Brid. J Indian Bot Soc. 36(3):241–247.
- Pande SK, Chopra N. 1958. Cytological studies in Indian mosses. III. Funaria calvescens Schw., Bryum cellulare Hook., B. ramosum (Hook.) Mitt., B. pseudopachytheca C. Müll. Proc Nat Inst Sci India. 24B(2):94–99.
- Rajeevan B. 1990. Studies on the Bryophyte flora of the Idukki District, Kerala [ Ph.D. Thesis]. Coimbatore: Bharathiar University.
- Sannomiya M. 1957. Chromosome studies of mosses II. J Hattori Bot Lab. 18:98–117.
- Schmidt M. 1931. Experimentelle analyse der genom und plamonwirkung bei moosen. Zeitschrift fur indukt Abstammungs - und Vererbungeslehr. 57:306–342.
- Sharma AK, Sharma A. 1980. Chromosome technique - theory and practice. London: Butterworths.
- Shaw AJ, Szövényi P, Shaw B. 2011. Bryophyte diversity and evolution: windows into the early evolution of land plants. Am J Bot. 98(3):352–369. 10.3732/ajb.1000316
- Smith AJE. 1978. Cytogenetics, biosystematics and evolution in Bryophyta. In: Woolhouse HW, editor. Botanical research. London: Academic Press; p. 196–277.
- Smith AJE, Newton ME. 1968. Chromosome studies on some British and Irish mosses III. Trans Brit Bryol Soc. 5(3):463–522. 10.1179/006813868804146908
- Uniyal PL. 1996. Chromosome numbers in Entosthodon Schwaegr and Physcomitrium (Brid.) Fuernr. from the Garhwal Himalaya. Lindbergia. 21(2):85–88.
- Uniyal PL. 1998. Cytogenetics of Bryophytes. In: Chopra RN, editor. Topics in Bryology. New Delhi: Allied Publishers; p. 125–164.
- Verma SK, Kumar SS. 1980. Cytological observations on some West Himalayan mosses. J Bryol. 11(2):343–349. 10.1179/jbr.1980.11.2.343
- Vysotskaya EI. 1975. New data on chromosome numbers of Bryopsida in Ukraine. Ukrayins’kyi Botanichnyi Zhurnal. 32(4):498–503.