Abstract
This study has been carried out to assess and explore the sexual diversity in an important medicinal plant, Valeriana jatamansi. The species is gynodioecious and produces hermaphrodite and female flowered plants that grow sympatrically. It was observed that hermaphrodite and female flowered plants can be segregated only during the flowering phase. In addition to gynodioecy, the production of a sterile flower at each branching point (dichotomy) of inflorescence and the reduction in the length of peduncles in acropetal manner results in reduction of the angle of branches from first dichotomy to top of inflorescence, resulting in flagship effect. This crowding effect of flowers helps to attract pollinators and the fertilization of a flower by pollen from another flower on the same plant (known as geitonogamy) by means of staminal movement in hermaphrodite flowers. Female flowered plants did not set any seeds when isolated from bisexual flowered plants. Female seeds showed more seed viability (89.5%) than hermaphrodite ones (84.25%). The species also operates a unique mechanism of piercing of stamens through closed floral buds to facilitate cross pollination. Pollen/ovule ratio was in favor of Cruden’s xenogamous nature. In all populations of Valeriana jatamansi, we observed 16 bivalents at metaphase-I indicating the chromosome number to be 2n = 32 and normal meiosis without any abnormalities (e.g. laggards, bridges, and micronuclei). Valeriana jatamansi seed germination responded best to the nitrogenous compounds apart from scarification (85% with MGT 4.25 days), showing 80% in 1 mM of thiourea with MGT of 3.75 days. The paper discusses the multiple reproductive strategies employed by the species to increase its fitness and points out that the species follows unique pathways of evolution.
Introduction
Among the impressive array of reproductive systems in flowering plants, hermaphroditism that includes plants with bisexual flowers is the most common (Barrett et al. Citation2003). Monoecy (plants with male and female flowers on the same individual), androdioecy, (male and hermaphrodite flowers on separate individuals), gynodioecy (female and hermaphrodite flowers on separate individuals), and dioecy (male and female flowers on separate individuals) have all evolved from hermaphroditism (Delph and Wolf Citation2005; Charlesworth Citation2006). Dioecy is widely presumed to have evolved from gynodioecy. In some cases of gynodioecy and dioecy, hermaphrodite individuals were reproducing only via male function and others via both sex functions (Desfeux et al. Citation1996; Delph and Wolf Citation2005).
In the gynodioecy pathway to dieocy, females (male steriles) invade and persist in a population of hermaphrodites. To persist, the females must compensate for their loss of male function. Most models of the evolution of gynodioecy include compensation through increased female seed production and/or inbreeding avoidance (Lloyd Citation1975; Charlesworth and Charlesworth Citation1978). Females may reallocate resources not spent on pollen to seed production. The exact seed fertility advantage required of females depends on the inheritance of sex type (Charlesworth Citation1999). Evolution of dioecy from gynodioecy requires the evolution of males from hermaphrodites. However, the prediction that male allocation of hermaphrodites increases with female frequency has received very little support (Delph and Lloyd Citation1991; Ashman Citation2003). Field studies on closely related species with different reproductive systems have suggested that gynodioecy may be an intermediate stage along the pathway from hermaphroditism to dioecy (Arroyo and Raven Citation1975; Weller et al. Citation1990). Phylogenetic studies have recently proven to be valuable tools with which to test evolutionary pathways between reproductive systems (Karron et al. Citation2012; Barrett Citation2013). They help to determine how many times a particular reproductive system has evolved within a group, and can also reveal cases of reversion.
Valeriana jatamansi Jones is a perennial herb with a terminal flat-topped cluster of small white or pink-tinged flowers born on erect nearly leafless stems. It belongs to the family Valerianaceae, recently categorized in Caprifoliaceae (Reveal and Chase Citation2011). It is an important medicinal herb of NW Himalayas, being used in the treatment of epilepsy, leprosy, hysteria and asthma.
Valeriana jatamansi in Kashmir is a very important medicinal herb exhibiting considerable diversity in flower and fruit morphology (Eriksen Citation1989). The genus contains taxa adapted to a wide range of ecological conditions (Bell Citation2004; Hidalgo et al. Citation2004), but to date no work has been carried out on its mating system. Valeriana jatamansi is therefore an ideal plant species to investigate the evolution as well as the relationships between diverse sexual systems.
The present study has been carried out to assess various aspects of reproduction. The present investigation focused on the following issues: (a) the nature of sexual polymorphism exhibited by the species; (b) evidence for evolutionary transitions in reproductive system in the species; (c) the presence of diverse sexual systems in the species; and (d) the multiple and unique evolutionary pathways which facilitate the stable state in the species.
Materials and methods
Study site
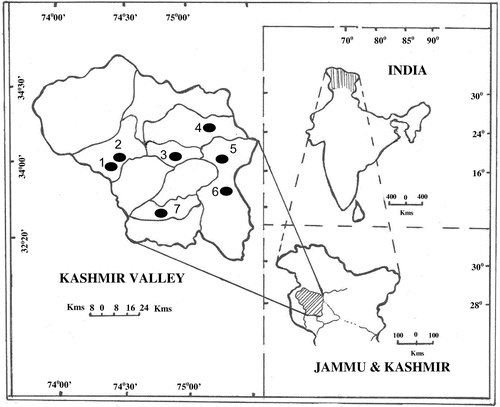
The study was carried out in a strip of sub-temperate and temperate vegetation, where this plant species thrives best in moist shady slopes and rocky slopes with an altitude gradient of 1200–3000 m above mean sea level, located in the valley of Kashmir which is situated in the northern fringes of the Indian sub-continent between 33°22′ and 34°50′ N and 73°55′ and 73°33′ E covering an area of about 16,000 km2. The valley is surrounded by a girdling chain of Himalayan Mountains: Pir Panjal in the south and the Great Himalayan range in the southeast to northeast and west. During the present survey, the plant species was collected from Ferozpora, Gulmarg, Sonamarg, Dara, Aharbal, Pahalgam and Daksum (Figure ).
Sampling of plants along with floral and vegetative traits
A random sample of 100 flowers on 50 plants from the selected sites was used for studying the quantitative and qualitative characters. The floral structure and characteristics of floral parts (sepals, petals, stamens and carpel) and inflorescence architecture was observed by using magnifying lenses (10× and 20×) and Zeiss stereo Discovery V8 trinocular microscope (Carl Zeiss made from Germany). Sampling was done by comparing the ratio of hermaphrodites to females at each site by laying a quadrant size of 1 × 1 m.
Determination of functional gender
For quantification of gender patterns the methods of Lloyd (Citation1979a); Lloyd and Bawa (Citation1984) were followed. The method records the expenditure a given plant makes on pollen (Pi) and ovules (Oi) (or seeds) relative to the average ratio of expenditure in the population. The number of ovule bearing flowers (Oi) and polliniferous flowers (Pi) were used to estimate the standardized phenotypic gender of individual plants (Gp) in each population by the formula:
where E is the equivalence factor that measures the ratio of ovule bearing to polliniferous flowers in the population as a whole. A Gp value of 1.0 represents plants that produce only ovules and 0.0, plants that produce only pollen. Values of Gp were calculated for plants sampled in all populations.
Pollen viability
To test viability, pollen grains from ready-to-dehisce anthers of 10 flowers per population were stained in 1% acetocarmine and 1% aniline blue-lactophenol (Swanson and Sohmer Citation1976). The stained, healthy and plump pollens were recorded as viable.
Pollen/ovule ratio
Fifteen mature flowers just about to anthesize were collected at random for estimating the pollen/ovule ratio (P/O). The average number of ovules per pistil was counted using a dissection microscope.
P/O was calculated following Cruden’s (Citation1977) method as follows:
Bagging experiments
In order to analyze the breeding system operating in the species the following experiments were carried out:
| (1) | Open pollination: | ||||
| (a) | Hermaphrodite and female flowered plants were tagged and allowed to open pollinate. | ||||
| (2) | Forced pollination: | ||||
| (a) | Hermaphrodite flowers were emasculated by removing the anthers and were allowed to cross pollinate. | ||||
| (b) | Hermaphrodite flowers were bagged at bud stage to ascertain geitenogamy. | ||||
| (c) | Female flowers were bagged at bud stage. | ||||
Pollen mother cell meiosis
For investigation of pollen mother cell meiosis, buds were collected from all seven study sites in the morning (7–9 am) and were fixed in Carnoy’s fixative – absolute alcohol, chloroform and glacial acetic acid 6:3:1, respectively – for a period of 24 h. After the fixation time the plant material was washed with 70% alcohol to remove all traces of the fixative. Then the fixed material was stored in freshly prepared 70% alcohol at 4°C. 2% propionocarmine was prepared by taking 45 ml of 45% propionic acid with 55 ml of distilled water to which 2 g of carmine powder was added to prepare 100 ml of 2% propionocarmine. Anthers were squashed to record stages with countable chromosomes numbers (metaphase and anaphase).
Seed set
Seed set was calculated following Lubbers and Christensen’s (Citation1986) formula:
The number of flowers per plant was recorded at each selected site. The number of seeds per fruit and per plant was estimated. Finally, percentage fruit/seed set was calculated.
Seed viability test
Seeds were soaked overnight at room temperature and cut longitudinally to expose the embryos. After preparing the desired number of seeds (mean: 20), they were soaked in 1% tetrazolium solution (TZ) at pH 6–7 and kept in the dark at 30°C for 3–4 h. After developing color, the TZ solution was drained by rinsing seeds 2–3 times with water. In a healthy seed the intensity of color is expected to be greater (Moor Citation1973) and hence percentage viability is determined.
In vitro seed germination
In vitro seed germination studies were carried out on randomly collected seeds from natural as well as ex situ populations. Each replicate (total: 26) was composed of 20 seeds. These were washed with 0.1% mercuric chloride for 5–7 min followed by washing 4–5 times with distilled water. They were subjected to different physical and chemical treatments at an average temperature of 15–200°C in Petri dishes on moist Whatman filter paper (Table ). For scarification treatments a small number of seeds (10) in one layer were put on one piece of sand paper. Then a second piece of sand paper was moved back and forwards. The seed coat was thus scratched by rubbing in the sand paper. Such treatments were given to seeds collected on different days from their maturity.
Table 1. Physical and chemical treatments to test seed germination of Valeriana jatamansi.
For each treatment, six replicates, each with a set of control (without any physical and chemical treatment) were used to compare the percentage germination and mean germination time (MGT). MGT was calculated following the equation of Joshi and Dhar (Citation2003):
where n number of seeds germinated after each incubation period in days and dN total number of seeds germinated at the end of experiment.
Data analysis
ANOVA using SPSS v 11.5 was used to calculate and compare the final means within and across different populations at various study sites (at p ≤ 0.001). The chi-square test was carried out to compare the ratio of individuals at seven different populations.
Results
Gynodioecy
Valeriana jatamansi is a gynodioecious plant species. Hermaphroditic and female plants show similarity in floral axis and type of inflorescence. The ratio of hermaphrodite and female individuals differ significantly in all studied populations as depicted in Table . The present study revealed that floral traits differ significantly in the two aforementioned types of plants (Figure ). The female flowers are always smaller in size than bisexual flowers (p ≤ 0.001). Also it was found that females have brighter color than hermaphrodites. It was also observed that hermaphrodite and female flowers show asynchrony in anthesis and anther dehiscence from flower to flower in a ramet (4–6 days) or in different flowers of a genet (10–12 days) in a population. This protracted asynchronous pollen presentation assures pollen availability for long periods to ensure effective pollination and also ensures survival of female plants.
Table 2. Ratio of hermaphrodites and females in different populations.
Figure 2. Comparison of quantitative flower characters of hermaphrodite and female plants. *Mean ± SD; PL, petal length; PB, Petal breadth; StL, stamen length; OL, ovary length; AL, anther length; StyL, style length; WFL, whole flower length; HP, hermaphrodite plant; FP, female plant.
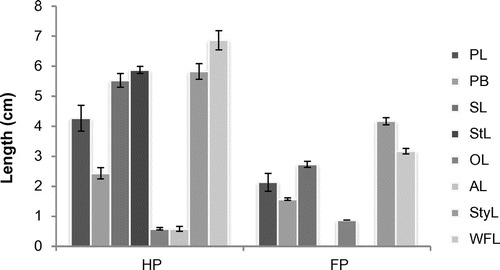
Temporal separation of opposite sexes was observed (dichogamy) during the present observation. The flowers of hermaphrodite plants are protandrous, with anther dehiscence occurring 1–2 days after anthesis; however, the stigmas were not found matured until 4–5 days, which prevents hermaphrodite flowers from autogamy and inbreeding depression; the concealed stigmas emerge and become receptive (with germinating pollen grains) within 4–5 days. In contrast, the stigmas of female flowers are receptive on the day of anthesis. The mean ovary length in female flowers is greater than that of hermaphrodite flowers and the difference is statistically significant.
Gender pattern
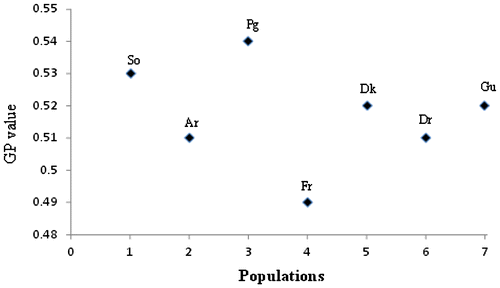
Gender pattern (Gp) was quantified in all the selected populations for tagged hermaphrodite individuals and it has a range of 0.49–0.54. The Gp value points towards the complete hermaphroditic nature of hermaphrodite individuals (Figure ).
Pollen viability
Valeriana jatamansi produces large quantity of healthy, plump and stainable tricolpate pollen grains, showing 90% pollen viability. This serves as an indicator of a good seed set and the strategy to materialize maximum reproductive success. The data analyzed on pollen viability are summarized in Table .
Table 3. Pollen viability and pollen ovule ratio of Valeriana jatamansi.
Pollen/ovule ratio
The flower of Valeriana jatamansi produces enormous number of pollen grains as compared to single basal ovule per flower. The P/O has been worked out per flower, per ramet as well as per genet. The data are summarized in Table .
Ovule number per ramet as well as per genet varies with respect to number of flowers per ramet and per genet as there is single basal ovule per flower.
Pollen mother cell meiosis
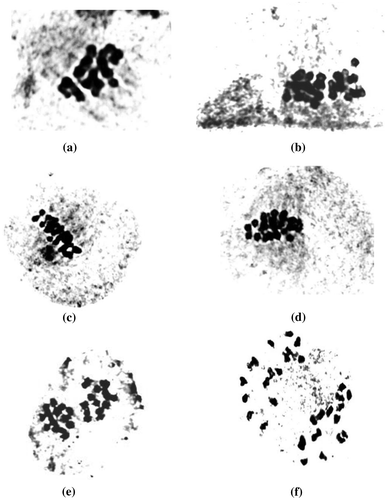
Pollen mother cell meiosis in Valeriana jatamansi was found regular with small chromosomes which are suitably countable at metaphase-I and anaphase-I. Segregation of chromosomes was normal with 16 chromosomes clearly seen at two opposite poles at anaphase. Sixteen bivalents at metaphase-I were observed in all populations of V. jatamansi studied, indicating the chromosome number to be 2n = 32. Meiosis was completely normal and no abnormalities, e.g. laggards, bridges, and micronuclei, were observed (Figure ). This is also supported by the fact that seed germination and pollen viability were quite normal in the present studies.
Staminal movement and production of sterile flowers
The present study revealed that a sterile flower is produced at each branching point (dichotomy) of the inflorescence and the reduction in the length of peduncles in an acropetal manner results in reduction of the angle of branches from first dichotomy to the top of the inflorescence. The reduction of angle from the base (600–1500) to the top (1000–1100) and the decrease in length of peduncles mean that all the inflorescences come to lie in the same plane to give it a head-like appearance. Crowding of flowers at top of inflorescence results in physical contact of anthers and stigmas of two different flowers. Physical contact is facilitated by bending of stamens towards straight styles. The anthers are positioned in such a way that wind or insect visitation (ambophilly) results in pollination success in hermaphrodite individuals. However, it will also facilitate cross pollination meant for separated female plants. The crowding of flowers results in a flagship, by which flowers of hermaphrodite plants are easily visualized by insects for cross pollination. This mode of pollination is also favored by the protandry and asynchronous anthesis of flowers in an inflorescence. Hence, the architecture of inflorescences and staminal movement are important in successful mating (Rather et al. Citation2014).
Piercing of stamens
The present study revealed that in 17–20/1000 hermaphrodite flowers, stamens break through the petals and dehisce earlier (4–6 days) to pollinate the female flowered individuals within the population. It was also observed that out of 50 female individuals, 15–20% of the flowers were fully open and functional earlier than hermaphrodite ones. The unique mechanism of dichogamy and herkogamy in hermaphrodite flowers and earlier anthesis of female flowers ensure successful cross pollination.
Sex advantage
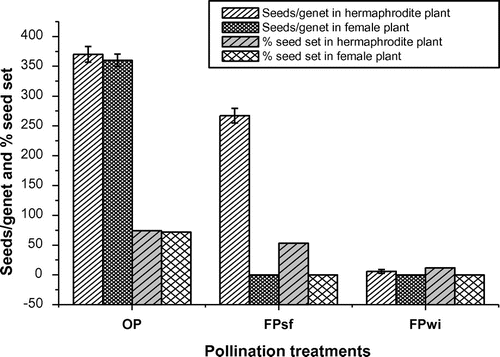
The results of the bagging experiments revealed that the un-emasculated hermaphrodites and female flowers which were allowed to open pollinate produce seeds. The inflorescences of hermaphrodite flowers which were bagged at bud stage also produced ample amounts of seeds, while bagged female plants did not set seed (Figure ).
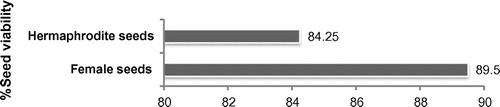
Seed viability
Seed viability was checked in both hermaphrodite and female individuals (Figure ) and it was observed that female seeds showed more viability (89.5%) than hermaphrodite ones (84.25%).
Seed germination
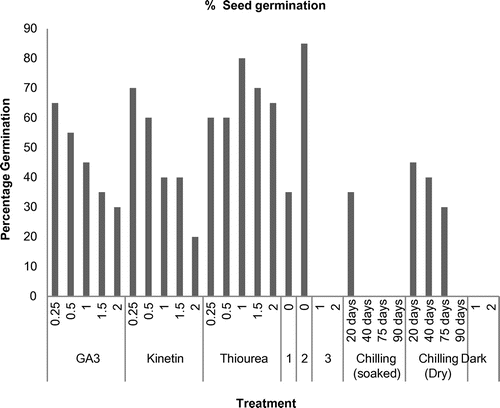
Valeriana jatamansi responded best in scarification (85% with MGT 4.25 days), showing 80% seed germination in 1 mM of thiourea with mean germination time of 3.75 days followed by 70% germination in 0.25 mM of kinetin with MGT of 6.30 days, as against the control (35%). The prolonged chilling has been observed to be ineffective with respect to germination of such seeds. The results of seed germination are summarized in Figure.
Discussion
Sexual diversity in Valeriana jatamansi
Valeriana jatamansi is gynodioecious plant species. A similar case of gynodioecy has been observed in Plantago lanceolata L. and Pachycereus pringlei (S. Watson) Britton & Rose by Poot (Citation1997) and Sosa and Fleming (Citation1999) respectively. The number of female flowers is higher than hermaphrodite flowers in V. jatamansi and anthesize before the hermaphrodite flowers, which favors high seed set in female plants. Fleming et al. (Citation1994) also observed that seed production was higher in female plants in Pachycereus pringlei. It has also been observed during the present study that female flowers are always smaller in size than bisexual flowers. This sexual dimorphism in flower size has been hypothesized to be advantageous because the smaller size of female flowers allows re-allocation to greater seed production, or because greater size in hermaphrodite flower may result in better pollination attraction and pollen dispersal (Miller and Venable Citation2003; Shykoff et al. Citation2003) and hence keeps the pollen grains available for longer durations for females. The high seed set in females is also attributed to the bright color of the flowers which are easily visualized by pollinators, resulting in increased insect visiting efficiency compared to the hermaphrodite plants. The bright flowers enhance the attractiveness of individual plants to increase the approach frequency of pollinators (Gori Citation1983, Citation1989; Delph and Lively Citation1989). The present study revealed that the GP value of hermaphrodite plants was almost equal to 0.5 in all the studied populations, which indicates that the nature of these plants is completely hermaphroditic (Lylod Citation1979b; Lylod and Bawa Citation1984; Sarkissian et al. Citation2001). The formation of ample amounts of seeds by both hermaphrodite and female plants in all the studied populations and the complete hermaphroditic nature of hermaphrodite plants points that the species has evolved a mechanism which seems to be advanced and stable.
Floral movements: exploring pathway towards cross pollination
Floral movements have previously been viewed as a mechanism to avoid self-pollination (Darwin Citation1862), but increasing evidence suggests that it may also act as a mechanism to reduce interference between the reproductive functions of female and male organs (Sun et al. Citation2007). However, it is very difficult to distinguish the reduction in intrafloral male–female interference from the avoidance of self-pollination. Features which are of adaptive significance towards successful pollination include formation of sterile flowers (Morales et al. Citation2012). Protandry and asynchrony in anthesis of flowers favors geitonogamy. Hence, hermaphrodites are both self and cross compatible, although cross pollination is more efficient (as per the bagging experiments). In female individuals seeds are produced by means of cross pollination only.
Piercing of stamens
The stamens of hermaphrodite plants break through the flower buds to pollinate the female flowers in order to ensure earlier emerging female plants to ascertain cross pollination. The present study revealed that both cross pollination (to produce genetic variability) and selfing (to ensure recruitment) are simultaneously at work in Valeriana jatamansi.
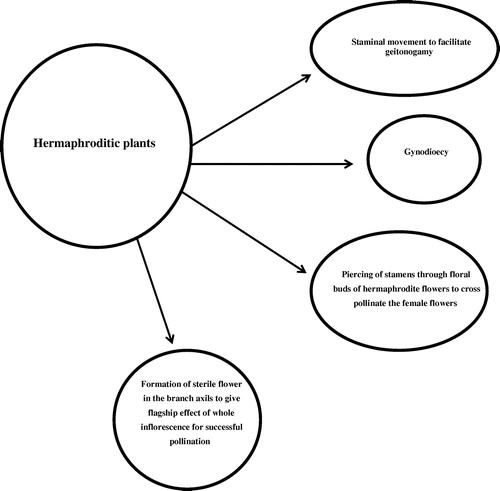
During the course of evolution it seems that Valeriana jatamansi Jones has adapted multiple pathways for successful pollination and fitness (Figure ).
Reproductive biology traits
As observed in the present study, Valeriana jatamansi displays a very high P/O ratio, indicating its out-breeding nature. In populations, pollen grain load may vary greatly and have the potential to effect the intensity of pollen competition for ovules. Pollen competition can in turn, affect seed production, fruit set, and progeny vigour, with important ecological and evolutionary consequences (Barbara and Nancy Citation2003).
Pollen mother cell meiosis
The present observation of 2n = 32 in Valeriana jatamansi confirms the earlier reports of Mehra and Sobti (Citation1955). Also during present studies no cytotype based on x 7 (2n 28) was observed, henceforth is in conformity with the results of Stebbins (Citation1971) who reported 8 as base number in Valeriana jatamansi. The high percentage of pollen viability and normal seed germination also supports normal meiotic behavior in this species as observed in the present study. The newly reports on cytology of Valeriana jatamansi showed tetraploid cytotype with chromosome numbers 2n = 32, which is in conformity with the earlier reports of the species from different parts of the world. An octoploid cytotype (2n = 64) makes a new addition for the species on a worldwide basis, whereas the diploid cytotype (2n = 16) is new to India and has been reported for the first time here. These cytotypes (2n = 16, 32, 64) show significant variations with respect to morphology as well as geographical distribution in the Western Indian Himalayas (Rani et al. Citation2015).
Seed viability
During the present study, female flowered plants did not set any seeds when isolated from bisexual flowered plants. However, bisexual flowered plants set seed under both open and self-pollination conditions. Hermaphroditic plants of Valeriana jatamansi are both self as well as cross compatible while the female plants are only cross compatible. This view is also supported by Charlesworth and Charlesworth (Citation1978), Ganders (Citation1978) and Kesseli and Jain (Citation1984), i.e. that females are at an advantage because seeds produced by females are a product of obligate out-crossing while the seeds produced by hermaphrodites are at least partly likely to be the product of self-fertilization.
Seed germination
The physiological and chemical treatments have been found to improve seed germination, seedling growth and nitrogen metabolization in plants (Joshi and Dhar Citation2003; Mohi-ud-din et al. Citation2005). Among many treatments used to increase percentage germination and reducing MGT, scarification is the most successful, with germination enhanced to 85% followed by 1 mM thiourea and 0.25 mM kinetin. In Valeriana jatamansi the seed coat, which is thin and papery, interferes with seed germination (showing non-deep physiological dormancy) because of inhibitors contained in the seed coat.
Conclusion
During the course of the present study we concluded that the V. jatamansi has adapted many floral modifications. This helps to improve its reproductive behavior under diverse environment conditions. Hence, future investigations need to include the molecular facts underlying these adaptive mechanisms. These improved modifications open up various pathways and links which are given in Figure .
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgments
We are very grateful to the Head, Department of Botany, University of Kashmir, Srinagar, for providing necessary facilities. We are also thankful to Dr. Anzar A. Khuroo for his contribution, by providing necessary advices about the study during field visits. We are thankful to Prof. K.S. Bawa (University of Massachusetts, Boston) and Prof. Spencer Barrett (Department of Ecology and Evolutionary Biology University of Toronto, Canada) for providing valuable inputs during the preparation of manuscript.
References
- Arroyo MTK, Raven PH. 1975. The evolution of sub-dioecy in morphologically Gynodioecious species of Fuchsia sect. Encliandra Onagraceae. Evolution. 29(3):500–511.10.2307/2407262
- Ashman TL. 2003. Constraints on the evolution of dioecy and sexual dimorphism: field estimates of quantitative genetic parameters for reproductive traits in three populations of gynodioecious Fragaria virginiana. Evolution. 57:2012–2025.10.1111/evo.2003.57.issue-9
- Barbara MN, Nancy L. 2003. pollen deposition, pollen tube growth, seed production and seedling performance in Natural populations of Clarkia unguiculata (Onagraceae). Int J Plant Sci. 164(1):153–164.
- Barrett SCH, Cole WW, Herrera CM. 2003. Mating systems and genetic diversity in wild daffodil Narcissus longispathus (Amaryllidaceae). Heredity. 92(5):459–465.
- Barrett SCH. 2013. The evolution of plant reproductive systems: how often are transitions irreversible? Proc R Soc B. 280:20130913.10.1098/rspb.2013.0913
- Bell CD. 2004. Phylogeny and biogeography of Valeriana (Dipsacales). [ PhD Thesis], Yale University.
- Charlesworth B, Charlesworth D. 1978. A model for the evolution of dioecy and gynodioecy. Am Nat. 112:975–997.10.1086/283342
- Charlesworth D. 1999. Theories of the evolution of dioecy. In: Geber MA, Dawson TE, Delph LF, editors. Gender and sexual dimorphism in flowering plants. New York (NY): Springer. p. 33–60.10.1007/978-3-662-03908-3
- Charlesworth D. 2006. Evolution of plant breeding systems. Curr Biol. 516:726–735.10.1016/j.cub.2006.07.068
- Cruden RW. 1977. Pollen-ovule ratio: a conservative indicator of breeding system in flowing plants. Evolution. 31:32–46.10.2307/2407542
- Darwin C. 1862. On the various contrivances by which British and foreign orchids are fertilized by insects. London: John Murray.
- Delph LF, Lively MS. 1989. Floral color changes in response to pollinator visitation. Nature. 45:212–223.
- Delph LF, Lloyd DG. 1991. Environmental and genetic control of gender in the dimorphic shrub hebe subalpine. Evolution. 45(8):1957–1964.10.2307/2409844
- Delph LF, Wolf DE. 2005. Evolutionary consequences of gender plasticity in genetically dimorphic breeding systems. New Phytol. 166:119–128.10.1111/j.1469-8137.2005.01339.x
- Desfeux C, Maurice S, Henry JP, Lejeune B, Gouyon PH. 1996. Evolution of reproductive systems in the genus Silene. Proc R Soc Lond Biol. 263:409–414.10.1098/rspb.1996.0062
- Eriksen B. 1989. Note on the generic and infrageneric delimitation in the Valerianaceae. Nordic J Bot. 9:179–187.10.1111/j.1756-1051.1989.tb02113.x
- Fleming TH, Maurice S, Buchmann SI, Tuttle MD. 1994. Reproductive biology and relative male and female fitness in a trioecious Cactus, Pachycereus pringlei (Cactaceae). Amr J Bot. 81:858–867.10.2307/2445767
- Ganders FR. 1978. The genetic and evolution of gynodioecy in Nemophila menziesii (Hydrophyllaceae). Can J Bot. 56:1400–1408.10.1139/b78-162
- Gori DF. 1983. Post pollination phenomena and adaptive floral changes. In: Jones CE, Little RJ, editors. Handbook of experimental pollination biology. New York (NY): Van Nostrand Reinhold. p. 31–49.
- Gori DF. 1989. Floral color change in Lupinus arenteus (Fabaceae). Why should plants advertise the location of unrewarding flowers to pollinators. Evolution. 43:870–882.10.2307/2409314
- Hidalgo O, Garnatje T, Susana A, Mathez J. 2004. Phylogeny of Valerianaceae based on matk and ITS markers, with reference to matK individual polymorphisms. Ann Bot. 93:283–293. doi:10.1093/aob/mcr062
- Joshi M, Dhar U. 2003. Effect of various presuming treatments on seed germination of Heracleum condicans wall. Ex Dc: a high value medicinal plant. Seed Sci Technol. 31:737–743.10.15258/sst
- Karron JD, Ivey CT, Mitchell RJ, Whitehead MR, Peakall R, Case AL. 2012. New perspectives on the evolution of plant mating systems. Ann Bot. 109(3):493–503.10.1093/aob/mcr319
- Kesseli R, Jain SK. 1984. An ecological genetic study of gynodioecy in Limnathes dauglassii (Limnathaceae). Am J Bot. 71:775–786.10.2307/2443468
- Lloyd DG. 1975. The maintenance of gynodioecy and androdioecy in angiosperms. Genetica. 45:325–339.10.1007/BF01508307
- Lloyd DG, Bawa KS. 1984. Modification of gender seed plants in varying conditions. Evol Biol. 17:255–338.
- Lloyd DG. 1979a. Parental strategies in angiosperms. New Zeal J Bot. 17:595–606.10.1080/0028825X.1979.10432573
- Lloyd DG. 1979b. Some reproductive factors affecting self-fertilization in angiosperms. Am Nat. 113:67–79.10.1086/283365
- Lubbers AE, Christensen NL. 1986. Intraseasonal variation in seed production among flowers and plants of Thalictrum thalctroides (Ranunculaceae). Am J Bot. 73:190–203.10.2307/2444172
- Mehra PN, Sobti SN. 1955. Cytology of Indian medicinal plants. II. Aconites, Valeriana and Senna. Res Bull Punjab Univ. 80:159–171.
- Miller JS, Venable DL. 2003. Floral morphometrics and the evolution of sexual dimorphism in Leum (Solanaceae). Evolution. 57:74–86.10.1111/evo.2003.57.issue-1
- Mohi-ud-din GG, Nawchoo IA, Wafai BA. 2005. Seed germination studies on Saursurea Costus (Falc.) Litch ‒ a threatened medicinal plant of N.W. Himalaya. Indian J Plant Physiol. 10:394–396.
- Moor RP. 1973. Tetrazolium staining for assessing seed quality. In Heydecker W, editor. Seed ecology. London: Butterworth. p. 347–366.
- Morales CL, Traveset A, Harder LD. 2012. Sterile flowers increase pollinator attraction and promote female success in the Mediterranean herb Leopoldia comosa. Ann Bot. 111(1):103–111.
- Poot P. 1997. Reproductive allocation and resource compensation in male-sterile and hermaphroditic plants of Plantago lanceolata. Am J Bot. 84:1256–1265.10.2307/2446050
- Rani S, Sharma TR, Kapila R, Chahota RK. 2015. Identification of new cytotypes of Valeriana jatamansi Jones, (Valerianaceae) from North-Western Himalayan region of India. Comp Cytogenet. 9(4):499–512.
- Rather AM, Nawchoo IA, Ganie AH, Wani AA. 2014. Inflorescence architecture and staminal movement as contrivance measures in reproductive assurance and survival of Valeriana jatamansi Jones (=Valeriana wallichii DC). Curr Sci. 107(4):568–570.
- Reveal JL, Chase MW. 2011. APG III: bibliographical information and synonymy of magnoliidae-monograph. Phytotaxa 19: 71–134. www.mapress.com/phytotaxa.10.11646/phytotaxa.19.1
- Sarkissian TS, Barrett SCH, Harder LD. 2001. Gender variation in Sagittaria latifolia (Alismataceae): is size all that matters? Ecology. 82:360–373.10.1890/0012-9658(2001)082[0360:GVISLA]2.0.CO;2
- Shykoff JA, Kolokotronis SO, Collin CL, Villavicencio M. 2003. Effects of male sterility on reproductive traits in gynodioecious plants: a meta-analysis. Oecologia. 135:1–9.10.1007/s00442-002-1133-z
- Sosa VJ, Fleming. 1999. Seedling performance in a trioecious Cactus Pachycerus Pringlei: effects of maternity and paternity. Plant Syst Evol. 218(1‒2):145–151.10.1007/BF01087042
- Stebbins GL. 1971. Chromosome evaluation in higher plants. London: Arnold.
- Sun S, Gao JY, Liao WJ, Li QJ, Zhang DY. 2007. Adaptive significance of flexistyly in Alpinia blepharocalyx (Zingiberaceae): a hand-pollination experiment. Ann Bot. 99:660–661.
- Swanson SD, Sohmer SH. 1976. The biology of Podophyllum peltatum L. (Berberidaceae), the May apple II. The transfer of pollen and success of sexual reproduction. Bull Torrey Bot Club. 103:223–226.
- Weller SG, Sakai AK, Wagner WL, Herbst DR. 1990. Evolution of dioecy in Scheidea (Caryophyllaceae) in the Hawaiian Islands: biogeographical and ecological factors. Syst Bot. 15:266–276.10.2307/2419182