Abstract
In this research, the genotoxic and cytotoxic effects of the insecticide deltamethrin (DEL) on the root meristem cells of sunflowers (Helianthus annuus L.) were investigated. The lethal dose (LD50) value was determined as approximately 1 ppm in the sunflower root growth tests. The roots were treated with 1 (LD50), 0.25 (LD50/4), 0.5 (LD50/2) and 2 ppm (LD50 ×2) concentrations of DEL for 24, 36 and 48 h, with a control for each combination. Occurring morphological changes such as reduction of root elongation and discoloration in sunflower roots were observed with the using DEL concentrations. The mitotic index and mitotic abnormalities were determined in both control and test groups. Mitotic index reduced with increasing the insecticide concentration at each exposure time. The mitotic abnormalities were recorded as disturbed prophase, c-mitosis, stickiness, laggards and chromatid bridges. In addition, micronucleus was observed at interphase and its frequency was calculated in the test solutions.
Introduction
The use of pesticides is steadily increasing in agricultural practices, especially in economically important plants (Ajay and Sarbhoy Citation1987). However, there are potential negative effects of the pesticides. The main concern arising from the widespread use of pesticides in modern agriculture is that environmental contamination by these agents or their by-products could negatively impact ecosystem stability and human health (Bolle et al. Citation2004), as they can be mutagenic and/or carcinogenic to non-target organisms (Pavlica et al. Citation1998). Therefore, pesticides should be followed on living systems or in nature.
Negative effects on living organisms in the environment of the pesticides can be assessed precisely and consistently with using plant bioassays including root meristem tips (Grant Citation1978), e.g. with Vicia faba (Sang and Li Citation2004), Pisum sativum (Amer et al. Citation1999), Hordeum vulgare (Kluge and Podlesak Citation1985; Ozkara et al. Citation2011), Crepis capillaris (Gadeva and Dimitrov Citation2008), Allium cepa (Demir et al. Citation2014; Karaismailoglu Citation2015, Citation2016) and Helianthus annuus (Karaismailoglu et al. Citation2013)Citation. Most studies show that there is a unique relationship between chromosome abnormalities and toxic activity in plant roots and in mammalian cell systems. Hence, monitoring of the root tips of plants presents a quick and responsive process for follow-up of environmental events (Karaismailoglu Citation2013, 2014).
Deltamethrin (DEL) is a synthetic pyrethroid insecticide (Chauhan et al. Citation1999). It has detrimental effects on the environment, especially plants, animals, and human beings. Moreover, widespread use and high non-selective potency of DEL cause harmful effects to nature with supressing factors of the insect (Leake et al. Citation1985).
Sunflower is an important plant that belongs to the Asteraceae family. DEL is used to control insect pests such as Loxostega sticticalis and Heliothis armigera in sunflower fields at concentrations in the range of 0.25–0.75 ppm (MARA Citation2009). Although there is a study on the effect of DEL on non-target organisms (Chauhan et al. Citation1986), no studies are available, to our knowledge, on the genotoxic and cytotoxic effects of DEL on sunflowers. In the present study, we analyze the effects of DEL on the mitotic cell division and somatic chromosomes of sunflower for the first time.
Materials and methods
Test organism and growth conditions
Sunflower seeds were purchased from a trading market in Istanbul, Turkey. The seeds were put in Petri dishes and germinated at 22 ± 2 °C in the dark. Seedlings with approximately 1 cm extended roots were selected for the analysis.
Chemical material
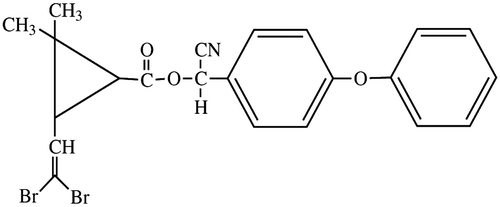
DEL [(S-alpha-cyano-3-phenoxybenzyl-(1R-3R)-3-(2, 2- dibromoviny)-2, 2 dimethylethyl cyclopropane carboxylate; CAS No: 52918–63-5] was used for genotoxic assays (Tomlin Citation2006; Figure ).
Root growth test and determination of LD50 value
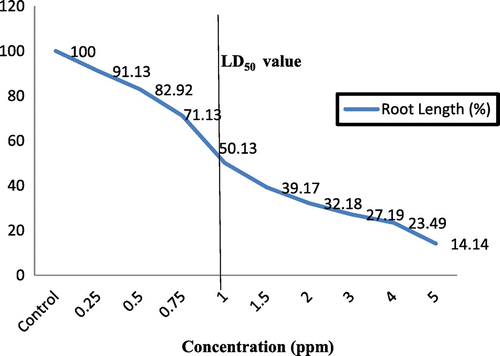
The seeds were treated with distilled water for 48 h at 22 ± 2 °C and permitted to generate roots. After, 10 roots (up to 1 cm in length) for each group were transferred to the control (distilled water) and different DEL insecticide concentrations (0.1, 0.2, 0.25, 0.4, 0.5, 0.75, 1, 1.5, 2, 2.5, 3, 4 and 5 ppm) for 72 h. At the end of this time, the root lengths from the control and each DEL concentration were evaluated; the percentage growth of exposed roots was determined compared to the control. Dose response curves were obtained using these values. The LD50 (lethal dose) value was found to be approximately 1.5 ppm, which showed c.50% decrement in root growth with respect to the control.
Cytogenetic assay protocol for somatic cells of sunflower
In addition to a control group, 1 (LD50 value), 0.25 (LD50/4), 0.5 (LD50/2) and 2 (LD50 × 2) ppm concentrations of DEL insecticide for cytological experiments were utilized. The concentrations were prepared with distilled water. The controls were put in only distilled water. The seeds were exposed to the insecticide concentrations for 24, 36 and 48 h.
Root tips obtained from the control and seedlings treated with DEL were fixed in ethanol-glacial acetic acid (3:1) and maintained at 4 °C overnight. Afterwards, they were hydrolyzed in 1 N HCl at 60 °C for 10–12 min and colored with Schiff’s reagent for 1–2 h at room temperature (Darlington and La Cour Citation1976). Five slides were taken randomly for each group and the mitotic index, micronucleus (MN) in interphase, and chromosome aberrations in dividing cells were investigated in the cytogenetic analysis. The mitotic index was calculated by scoring more than 4000 cells. MN frequency was determined by examination of more than 800 interphase cells per slide. The chromosomal abnormalities were defined in dividing cells.
Statistical analysis
Analysis of variance (one-way ANOVA) of the data was performed with the SPSS computer program. The Dunnet t (2-sided) multiple range test was used to identify the statistical importance of differentiations among the means. Statistical investigations are shown in Tables , , and (p = 0.05).
Table 1. The effect DEL insecticide on root growth in sunflower.
Table 2. Effects of deltamethrin on mitotic cell division of sunflower.
Table 3. Type and percentage of mitotic abnormalities in the root tips of sunflowers exposed to DEL.
Table 4. Results from genotoxicity testing of DEL in the sunflower micronucleus assay.
Results
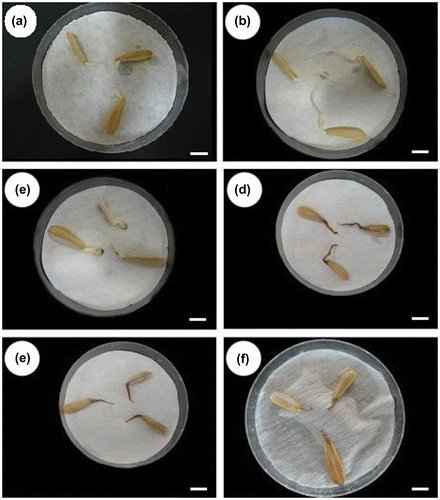
The average root lengths of Helianthus annuus and percentage reduction in root growth with DEL insecticide and the control at the application times are given in Table . Mostly, the root elongation was reduced at all the applied DEL insecticide concentrations. The highest growth rate was in sunflower roots exposed to low concentrations. A relation was obtained between the administered doses and responses. The LD50 value was determined as 1 ppm (Figure ). DEL concentrations of 2 ppm and over were found to be highly toxic. No growth was observed in the sunflowers after 48 h with application of over 2 ppm. Morphological alterations like blackout in the roots occurred with increasing insecticide concentrations (Figure ).
Figure 3. Effects of deltamethrin insecticide on sunflower roots: (a) chosen roots for the cytogenetic tests; (b) roots in control for 48 h; (c) roots in 1 ppm DEL treatment for 48 h; (d) roots in 2 ppm DEL treatment for 48 h; (e) roots in 4 ppm DEL treatment for 48 h; (f) roots in 5 ppm DEL treatment for 48 h (scale bars: 1 cm).
The effects of DEL insecticide on the mitotic index and the frequency of mitotic phases are presented in Table . The mitotic index clearly decreased at all insecticide concentrations when compared to the control at each application time. There were significant differences between the different concentrations of DEL and the control group at all application times (p=0.05). However, mitotic index was not significantly different to the control at 0.25 ppm at all times, and 0.5 ppm for 24 h. The percentage of mitotic index was markedly lower at 2 ppm than other concentrations at 24, 36 and 48 h.
The effects of the applied DEL concentrations on the mitotic cell division in the root tips of sunflower are shown in Table . Almost all of the DEL applications significantly affected the periodicity of mitotic phases. As the percentage of prophase increased, the periodicity of metaphase and ana-telophase reduced in the majority of the root cells. Furthermore, the percentages of anomaly mitotic phases were shown to increase with the increasing DEL concentrations at all application times.
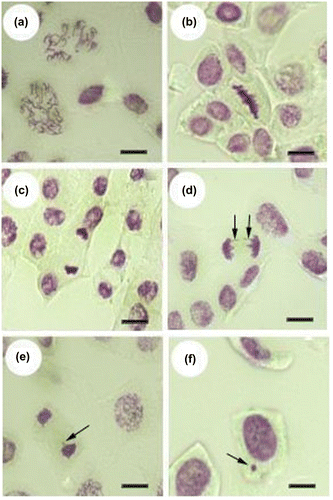
The outcomes of mitotic anomalies are given in Table and Figure . The alterations in the organization of the chromosomes in the root tips were monitored and five types of the most common anomalies were recorded: disturbed prophase, stickiness, c-mitosis, laggards, and chromatid bridges. This indicates that as the DEL concentration increased, the percentage of total anomalies markedly increased.
Figure 4. Mitotic abnormalities induced by deltamethrin in root meristem cells of sunflowers: (a) disturbed prophase; (b, c) stickiness; (d) chromatid bridge; (e) laggards; (f) micronucleus (scale bars: 5 μm).
As well as the types of chromosome abnormalities, the presence or absence of MN in interphase cells was investigated; percentages of micronuclei are given in Table . MN formation gradually increased with increasing DEL concentrations when compared to control at each application time. It was clearly higher at 2 ppm than the other DEL concentrations at all the exposure times.
Discussion
The harmful effects in the environment of the pesticides can be monitored macroscopically with root growth tests in some plants, and microscopically with cytological parameters including types and frequencies of chromosome abnormalities (Smaka-Kincl et al. Citation1996). Based on this information, effects of DEL insecticide on sunflower root growth in terms of macroscopic evaluation are given in Table . Root growth of sunflower decreased with increasing DEL insecticide concentration at each application time compared to the control. When we evaluated our outcomes, we observed a high detrimental effect of DEL on meristematic cells that had compromised morphology. These cells looked to be unstructured, with a fragile cellular envelope, increased cell volume and in several cases rupture of the cellular membrane when observed under a light microscope. These observations are indicative of cell death (Lerda et al. Citation2010). Also, browning and obscuration in the roots occurred with 48 h treatment of >2 ppm DEL insecticide. This event was interpreted as sporadic morphological changes in plant section owing to the physiological instability stimulated by DEL.
The mitotic activity is frequently used for following of the toxic materials (Linnainmaa et al. Citation1978). The effect of DEL on mitotic activity of the sunflower somatic cells is shown in Table . Mitotic index decreased significantly with increasing DEL concentrations when compared to the control at each exposure time (p=0.05). The decrease in the mitotic efficiency might be due to the reduction of DNA synthesis or an obstacle in the G2 phase of the cell cycle, preventing the cell from entering mitosis (Sudhakar et al. Citation2001). Rank and Nielsen (Citation1997) suggested that if the highest concentration used for the toxicity analysis is assumed to be below LD50 value, then the mitotic index would not decrease below 50% of the control. In the present study, the decrease percentage was c.61% at the 2 ppm concentration at 48 h in comparison with the control (Table ). Nevertheless, the mitotic index values in 1 ppm (LD50 value) concentration of DEL at all application times were over 50% of control. Similar influences on mitotic index have been found for cypermethrin and fenvalerate insecticides (Chauhan et al. Citation1999), ceresan, agrosan and mercuric chloride fungicides (Nandi Citation1985) in Allium cepa, atrazine (Badr Citation1986) in Vicia faba, and cypermethrin insecticide (Inceer et al. Citation2009) and quizalofop-P-ethyl herbicide (Karaismailoglu et al. Citation2013) in Helianthus annuus. Additionally, DEL induced an alteration in the frequencies of the distinct mitotic phases. These alterations in the phases of mitosis show that DEL has influenced each phase at all application periods in comparison with the control. Similar outcomes were acquired after application of Garlon-4 (El-Khodary et al. Citation1989) on A. cepa root tip cells and quizalofop-P-ethyl herbicide (Karaismailoglu et al. Citation2013) on H. annuus root tip cells. The outcomes indicate that as the concentration of the insecticide increased, the percentage of total abnormalities increased. After application of the highest DEL concentration for each treatment time, genotoxicity was higher than expected (Table ). Spindle failure induced abnormalities on chromosomes, which caused chromosome abnormality types such as unequal distribution, stickiness, c-mitosis, chromatid bridges, and laggards. Some of these abnormalities were observed because of chromatid erosion and breaks which are determined as disturbed prophase, stickiness and chromatid bridges. These alterations can form an irreversible and genotoxic influence (Fiskesjö and Levan Citation1993). In addition, c-mitosis was generated as a result of the inhibition of spindle fiber form. These outcomes are compatible with that of Inceer et al. (Citation2004), which indicates an influence like colchicine. The lowest percentage of chromosome abnormalities was found in the control groups. The percentage of chromosome abnormalities increased with increasing insecticide concentration. This finding was in agreement with previous studies (Yildiz and Arikan Citation2008; Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014, Citation2015, Citation2016), suggesting that the genotoxicity of the applied pesticide depends on its concentration.
MN tests have a key role in identifying of the genotoxicity potency of materials (Gebel et al. Citation1997). MN formation and frequency in treated groups (Table ) are clearly enhanced with increasing DEL concentrations. The percentage of micronucleated cells was markedly higher at the highest DEL concentration (2 ppm) than others. Micronuclei may be a result of acentric fragments and/or entire chromosomes not united with the major nucleus throughout the cell cycle (Fenech and Crott Citation2002). Hence genotoxic materials which have clastogenic effect cause increment of MN formation (Fernandes et al. Citation2006). This information shows that increased MN percentage caused proliferation of the chromosome aberrations in the observed dividing cells.
This investigation found that the presence of DEL in the environment has possible detrimental effects on the genetic material of exposed living organisms. Widespread implementation of insecticides for the control of insects in agricultural applications is a possible danger to genetic information of economically important plants like H. annuus. For this reason, it is essential to analyze of the genotoxic influences of insecticides on plants and other systems before considering their implementation in agriculture.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
This study is a part of the Master thesis of the first author and he thanks Professor Sema Hayırlıoglu-Ayaz for helping during the thesis.
References
- Ajay KJ, Sarbhoy RK. 1987. Cytogenetical studies on the effect of some chlorinated pesticides I. Effect on somatic chromosomes of lens and pisum. Cytologia. 52:47–53.
- Amer SM, Mohammed FI, Ashry ZM. 1999. Cytogenetic effects of the fungicide benomyl on Vicia faba and Pisum sativum. Bull Nat Res Cent. 24(4):481–494.
- Badr A. 1986. Effect of the s-triazine herbicide terbutryn on mitosis chromosomes and nucleic acids in root tips of Vicia faba. Cytologia. 51:571–578. 10.1508/cytologia.51.571
- Bolle P, Mastrangelo S, Tucci P, Evandri MG. 2004. Clastogenicity of atrazine assessed with the Allium cepa test. Envıron Mol Mutagen. 43(2):137–141. 10.1002/(ISSN)1098-2280
- Chauhan LKS, Dikshith TSS, Sundararaman V. 1986. Effect of deltamethrin on plant cell. I. Cytological effects on the root meristem cells of Allium cepa. Mutat Res. 171(1):25–30. 10.1016/0165-1218(86)90005-4
- Chauhan LKS, Saxena PN, Gupta SK. 1999. Cytogenetic effects of cypermethrin and fenvalerate on the root meristem cells of Allium cepa. Environ Exp Bot. 42(3):181–189. 10.1016/S0098-8472(99)00033-7
- Darlington CD, La Cour F. 1976. The handling of chromosomes. London: Allen and Unwin.
- Demir E, Kaya N, Kaya B. 2014. Genotoxic effects of zinc oxide and titanium dioxide nanoparticles on root meristem cells of Allium cepa by comet assay. Turk J Biol. 38(1):31–39. 10.3906/biy-1306-11
- El-Khodary S, Habib A, Haliem A. 1989. Cytological effect of the herbicide garlon-4 on root mitosis Allium cepa. Cytologia. 54(3):465–472. 10.1508/cytologia.54.465
- Fenech M, Crott JW. 2002. Micronuclei, nucleoplasmic bridges and nuclear buds induced in folic acid deficient human lymphocytes-evidence for breakage-fusion bridge cycles in the cytokinesis-block micronucleus assay. Mutat Res. 504(1–2):131–136. 10.1016/S0027-5107(02)00086-6
- Fernandes TCC, Mazzeo DEC, Marin-Morales MA. 2006. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pest Biochem Physiol. 88(3):252–259.
- Fiskesjö G, Levan A. 1993. Evaluation of the first ten MEIC chemicals in the Allium-test. ALTA. 2:139–149.
- Gadeva P, Dimitrov B. 2008. Genotoxic effects of the pesticides Rubigan, Omite and Rovral in root-meristem cells of Crepis capillaris. Genet Toxicol Environ Mutagen. 652(2):191–197. 10.1016/j.mrgentox.2008.02.007
- Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H. 1997. In vivo genotoxicity of selected herbicides in the mouse bone-marrow micronucleus test. Arch Toxicol. 71(3):193–197. 10.1007/s002040050375
- Grant WF. 1978. Chromosome aberrations in plants as a monitoring system. Environ Health Perspect. 27:37–43. 10.1289/ehp.782737
- Inceer H, Eryigit HN, Beyazoglu O. 2004. Effects of the herbicide Linuron on somatic chromosomes of Helianthus annuus L. Caryologia. 57(2):127–132. 10.1080/00087114.2004.10589381
- Inceer H, Hayirlioglu-Ayaz S, Ozcan M. 2009. Genotoxic effects of the insecticide cypermethrin on the root meristem cells of sunflowers (Helianthus annuus L.). Bull Environ Contam Toxicol. 83(5):652–656. 10.1007/s00128-009-9856-8
- Karaismailoglu MC. 2013. Deltamethrin ve quizalofop-p-etil pestisitlerinin Helianthus annuus L. (Ayçiçeği) kök ucu hücreleri üzerine mutajenik etkilerinin araştırılması [Investigation of mutagenic effects of the pesticides Deltamethrin and Quizalofop-p-ethyl on root tip cells of Helianthus annuus L. (Sunflower)] [ MSc thesis]. Trabzon: Karadeniz Technical University.
- Karaismailoglu MC. 2014. Evaluation of potential genotoxic effect of trifluralin in Helianthus annuus L. (sunflower). Caryologia. 67(3):216–221. 10.1080/0144235X.2014.974348
- Karaismailoglu MC. 2015. Investigation of the potential toxic effects of prometryne herbicide on Allium cepa root tip cells with mitotic activity, chromosome aberration, micronucleus frequency, nuclear DNA amount and comet assay. Caryologia. 68(4):323–329. 10.1080/00087114.2015.1109927
- Karaismailoglu MC. 2016. The evaluation of the genotoxic and cytotoxic effects of pyriproxyfen insecticide on Allium cepa somatic chromosomes with mitotic activity, chromosome abnormality and micronucleus frequency. Turk J Life Sci. 1(2):65–69.
- Karaismailoglu MC, Inceer H, Hayirlioglu-Ayaz S. 2013. Effects of quizalofop-p-ethyl herbicide on the somatic chromosomes of Helianthus annuus (Sunflower). Ekoloji. 22(89):49–56. 10.5053/ekoloji
- Kluge R, Podlesak W. 1985. Plant critical levels for the evaluation of boron toxicity in spring barley (Hordeum vulgare L.). Plant Soil. 83(3):381–388. 10.1007/BF02184450
- Leake LD, Buckley DS, Ford MG, Salt DW. 1985. Comparative effects of pyrethroids on neurones of target and non-target organisms. Neurotoxicology. 6(2):99–116.
- Lerda D, Biagi Bistoni M, Pelliccioni P, Litterio N. 2010. Allium cepa as a biomonitor of ochratoxin A toxicity and genotoxicity. Plant Biol. 12(4):685–688.
- Linnainmaa K, Meretoja T, Sorsa M, Vainto H. 1978. Cytogenetic effects of styrene and styrene oxidei. Mutat Res. 58(2–3):277–286. 10.1016/0165-1218(78)90020-4
- [MARA] Ministry of Agricultural and Rural Affairs of Republic of Turkey. 2009. General Directorate of Protection and Control. Technical Instructions in Agricultural combat (Ziraii mücadele teknik talimatları), Ankara; p. 152.
- Nandi S. 1985. Studies on the cytogenetic effect of some mercuric fungucides. Cytologia. 50(4):921–926. 10.1508/cytologia.50.921
- Ozkara A, Akyıl D, Erdogmus SF, Konuk M. 2011. Evaluation of germination, root growth and cytological effects of wastewater of sugar factory (Afyonkarahisar) using Hordeum vulgare bioassays. Environ Monitor Assess. 183(1–4):517–524. 10.1007/s10661-011-1936-7
- Pavlica M, Vasilevska J, Paes D. 1998. Genotoxity of pentachlorophenol revealed by Allium chromosome aberation assay. Acta Biol Crac Ser Bot. 40:85–90.
- Rank J, Nielsen MH. 1997. Allium cepa anaphase-telophase root tip chromosome aberation assay N-methyl-N-nitrosourea, malic hydradize, sodium azide, ethyl meyhyl sulfonate. Mutat Res. 390(1–2):121–127. 10.1016/S0165-1218(97)00008-6
- Sang N, Li G. 2004. Genotoxicity of municipal landfill leachate on root tips of Vicia faba. Mutat Res. 560(2):159–165. 10.1016/j.mrgentox.2004.02.015
- Smaka-Kincl V, Stegnar P, Lovka M, Toman MJ. 1996. The evaluation of waste, surface and ground water quality using the Allium test procedure. Mutat Res. 368:171–179. 10.1016/S0165-1218(96)90059-2
- Sudhakar R, Ninge Gowda KN, Venu G. 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 66(3):235–239. 10.1508/cytologia.66.235
- Tomlin CDS. 2006. The pesticide manual: a world compendium. 14th ed. Farnham, (UK): British Crop Protection Council; p. 286–287.
- Yildiz M, Arikan ES. 2008. Genotoxicity testing of quizalofop-P-ethyl herbicide using the Allium cepa anaphase-telophase chromosome aberration assay. Caryologia. 61(1):45–52.