Abstract
Anchusa L., with four species, is one of the weedy species of Boraginaceae in Iran. These species are distributed in different habitats. Species delimitation is mainly based on corolla lobes, corolla tube condition and fruit shape. In this study chromosome number and meiotic behavior of 10 populations of Anchusa taxa have been considered. Due to the large number of studied populations of A. italica we have considered the intra-specific variation in this species. Statistical multivariate methods were done by use of PAST software ver. 5. 2012. Although all populations showed the chromosome number 2n = 4x = 32, population of A. arvensis subsp. orientalis showed the presence of n = 8 (2n = 2x = 16). This is the first meiotic study for Anchusa. Variation in meiosis stages such as synozytic knot was observed. Meiotic irregularities such as cytomyxis, stickiness, anaphase bridge, laggard chromosomes, micronucleus, triad and unreduced pollen grains were observed.
Keywords:
Introduction
Anchusa L. (Boraginaceae) is a large genus with about 170 annual and perennial species in temperate and sub-temperate zone of the Old World (Akcin et al. Citation2010). Southern parts of Balkan Peninsula are the main center of diversification of this genus (Selvi and Bigazzi Citation2003). Anchusa species are distributed in Europe, north and south of Africa and West of Asia. These weedy plants (annual, biennial or perennial species) are covered with robust hairs and rough nodes (Judd et al. Citation1999). Leaves are ovate or lanceolate and sessile on top of the stem. Blue or white flowers aggregate in cymes. The fruit is composed of four nutlets with wrinkled surface.
Anchusa is composed of four species and five taxa (A. italica has two varieties: italica and kurdica) in Iran, which is distributed in the north, northwest, center, northeast and south of Iran. A. italica var. italica is more frequent than the others (Khatamsaz Citation2002). Anchusa species have ornamental, medicinal and edible uses in different parts of Europe and Asia. In Iran these species are used as a substitution for the medicinal Borago (Zargari Citation1989). Moreover, Anchusa roots have a special resinous component as a coloring agent. Some species are also weeds of different cultivations (Judd et al. Citation1999). Riedl (Citation1967) and Khatamsaz (Citation2002) and many other researchers mentioned different classifications at infra-generic and infra-specifics levels in Anchusa (Guşuleac Citation1927; Chater Citation1972; Greuter et al. Citation1984; Selvi and Bigazzi Citation1998).
No research on gametophyte content has been reported on Anchusa species. Only Ghaffari (Citation1996) reported n = 16 for A. italica. Several cytogenetic studies have been performed on Anchusa from different parts of the world (Luque Citation1980; D’Amato and Trojani Citation1985; D’Amato Citation1989; Diaz lifante et al. Citation1992; Constantinidis and Kamari Citation1995; Markova and Goranova Citation1995; Ghaffari Citation1996; Lövkvist and Hultgård Citation1999), but such studies are not available for the Anchusa species growing in Iran. In this study accessions of Anchusa are collected from different locations of Iran and chromosomal behavior and gametophyte content of Anchusa species are reported for the first time.
Methods and materials
Plant material
Meiotic studies were performed in 10 populations of four Anchusa species and varieties of Iran (Table ). The studied species are: (1) A. italica Retz. var. italica (six populations); (2) A. italica var. kurdica Gusuleac. (two populations); (3) A. strigosa Labill. (one population); and (4) A. arvensis subsp. orientalis (L.) Nordh. (one population). The voucher specimens are deposited in Herbarium of Alzahra University (AUH).
Table 1. List of Anchusa species studied.
Cytological studies
Young flower buds were collected from 10 randomly selected plants of each population, fixed in glacial acetic acid: ethanol (1:3) for 24 h, and then washed and preserved in 70% ethanol at 4°C until use, following Sheidai et al. (Citation2003). Cytological preparations were done using squash technique and 2% aceto-orcein. Between 50 and 100 pollen mother cells (PMCs) were analyzed for chiasma frequency and distribution at diakinesis/metaphase stage, and 500 PMCs were analyzed for chromosome segregation during the anaphase and telophase stages.
Pollen stainability as a measure of fertility was determined by staining minimum 1000 pollen grains with 2% acetocarmine:50% glycerin (1:1) for about 30 min. Round complete pollens which were stained were taken as fertile, while incomplete, shrunken pollens with no stain were considered as sterile (Sheidai et al. Citation2003).
Statistical analyses
Grouping the populations of A. italica var. italica was performed using un-weighted paired group with arithmetic mean (UPGMA) as well as ordination based on principal component analysis (PCA) (Sheidai et al. Citation2002). For numerical analyses meiotic characteristics such as chiasmata frequency and distribution (terminal and intercalary chiasmata) and chromosome association (ring and rod bivalents) as well as univalents were used.
For grouping populations of four species of Anchusa, cluster analysis and PCA ordination were performed using relative meiotic characters (i.e. dividing the mean values of chiasmata frequency and bivalents by the chromosome number to obtain meiotic values per chromosome) unaffected by the chromosome number (Sheidai et al. Citation2002). Euclidean distance was used for cluster analysis. Statistical analyses were done using SPSS ver. 9 (1998). An ANOVA table is provided to show the significance variation (Table ).
Table 2. ANOVA test of quantitative cytological features of the populations studied of Anchusa.
Results
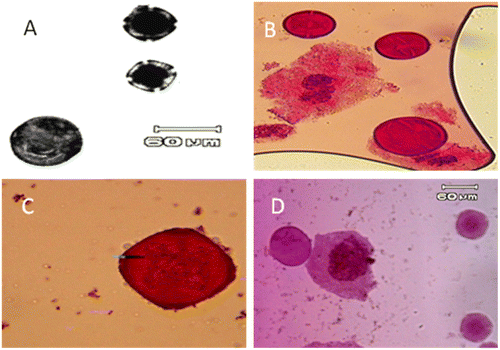
Studied Anchusa species showed 90% (A. italica var. italica, Hamedan population) to 99.7% (A. italica var. kurdica, Kilak village population) pollen fertility. Six populations of A. italica var. italica possessed n = 16 (2n = 4x = 32) chromosome number (Figure (A)), supporting an earlier report (Ghaffari Citation1996). More total and terminal chiasmata were observed in the Evin population and maximum ring bivalents were observed in the Darband population of A. italica var. italica (Table ).
Table 3. Meiotic characters in Anchusa species studied.
Two populations of A. italica var. kurdica studied possessed n = 16 (2n = 4x = 32; Figure (B), Table ). Higher total and terminal chiasmata as well as ring bivalents were observed in the Kamyaran population (2.44, 1.57 and 0.90 respectively; Table ) than in the Kilak village population. The only studied population of A. strigosa showed the presence of n = 16 (2n = 4x = 32), supporting the report of Diaz Lifante et al. (Citation1992). The studied population of A. arvensis subsp. orientalis showed the presence of n = 8 (2n = 2x = 16; Figure (D)), supporting the earlier report of Luque (Citation1980). Variation in chiasmata frequency has been reported in different accessions of some grasses, e.g. Aegilops, Lolium and Festuca (Rees and Dale Citation1974). Such variations could result in different kinds of recombinants, influencing the variability within natural populations in a possible adaptive manner (Rees and Dale Citation1974). Possible reasons for low pollen fertility in some accessions may be chromosome stickiness, laggard formation, triad and cytomixis.
Synezetic knot stage in meiosis-I prophase
The meiotic analysis of the studied Anchusa species showed variation in the prophase sub-stages of meiosis-I (Figure (A–C)). In the first sub-stage instead of the leptotene and zygotene stages a synezetic knot occurred, consisting of thin chromatin strands surrounding the nucleolus and eventually totally covering it (Figure (A)). Paired chromosomes unraveled from the knot and appeared as thick strands, ushering in the pachytene stage (Figure (B)), of which end-to-end attachment of chromosomes is a feature reported in taxa showing a synezetic knot stage (John et al. Citation1989; Sheidai and Inamdar Citation1991).
Figure 1. Representative pollen mother cells of Anchusa species showing gametic chromosome number in diakinesis-metaphase I. (A) Evin population of A. italica var. italica (n = 16); (B) Kilak population of A. italica var. kurdica (n = 16); (C) A. strigosa (n = 16); (D) A. arvensis subsp. orientalis (n = 8) (scale bar = 20 μm).
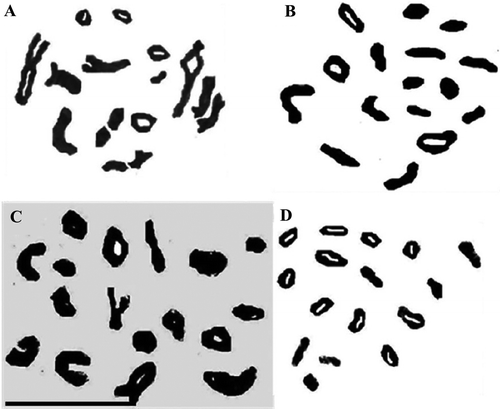
Figure 2. Representative pollen mother cells of Anchusa species showing prophase-I sub-stages of meiosis. (A) synezetic knot in Kilak population of A. italica var. kurdica; (B) pachytene stage in Evin population of A. italica var. italica; (C) diplotene stage in Evin population of A. italica var. italica (scale bar = 20 μm).
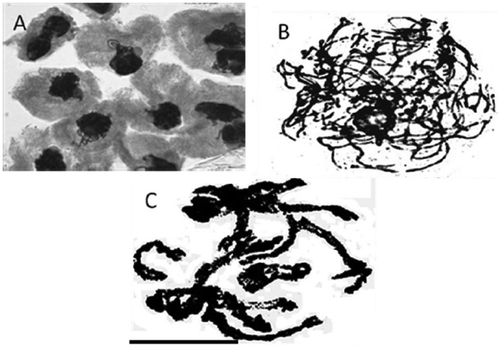
Figure 3. Representative meiotic cells in Anchusa species. (A) cytomictic cells of A. italica var. italica (Evin population); (B) extra chromosome in prophase I in A. arvensis subsp. orientalis; (C) micronucleus in A. italica var. kurdica (Kilak population); (D) anaphase bridge in A. italica var. italica (Darband population); (E) sticky chromosomes (telophase II) in A. italica var. kurdica (Kilak population); (F) micronucleus (telophase II) in A. strigosa; (G) tripolar cell; (H) metaphase I in A. italica var. italica (Vanak population) (Scale bar=20 μm).
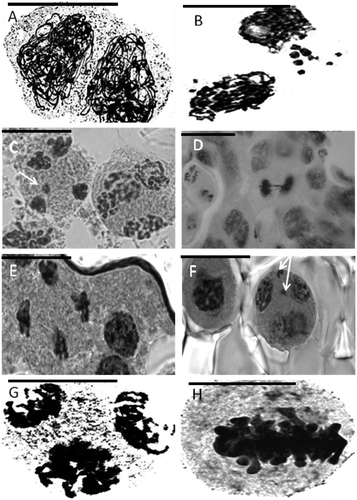
Sticky chromosomes
Sticky chromosomes were observed from early stages of prophase and continued to the final stages of meiosis in most of the species studied. The number of chromosomes involved in stickiness varied from two to many, often forming a complete clumping of the chromosomes. Stickiness varied among different meiocytes and the species studied. Such a phenomenon has also been reported in Avena species (Baptista-Giacomelli et al. Citation2000; Sheidai et al. Citation2003). Genetic and environmental factors (Nirmala and Rao Citation1996), as well as genotype environment interactions (Baptista-Giacomelli et al. Citation2000), have been implicated as reasons for chromosome stickiness in different plant species, and this may be true for the studied Anchusa species as well.
Cytomixis
Chromatin/chromosome migration occurred in different directions from early prophase to telophase-II in almost all Anchusa species studied (Figure (A)). Several metaphase/diakinesis cells in these species possessed extra or missing chromosomes showing aneuploid condition (Figure (H)). Such aneuploid cells form aneuploid gametes/pollen grains, and several meiocytes with extra chromosomes were observed. The other result of cytomixis is the formation of cells with missing chromatin material, which leads to formation of abnormal tetrads, and infertile pollen grains.
Migration of chromatin material among the adjacent meiocytes occurs through cytoplasmic connections originating from the pre-existing system of plasmodesmata formed within the tissues of the anther. The plasmodesmata become completely obstructed by the deposition of callose, but in some cases they still persist during meiosis and increase in size, forming conspicuous inter-meiocytic connections or cytomictic channels that permit the transfer of chromosomes. Chromosome migration may also occur through cell wall dissolution among the neighboring meiocytes and forming syncyte (Falistocco et al. Citation1995).
Cytomixis is not considered to be of great evolutionary importance, but it may lead to production of aneuploid plants (Sheidai et al. Citation1993), or result in the production of unreduced gametes, as reported in several grass species (Falistocco et al. Citation1995).
Statistical analyses
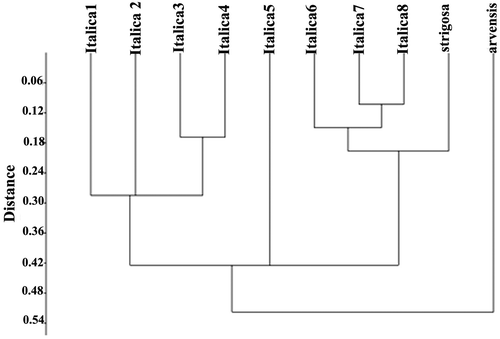
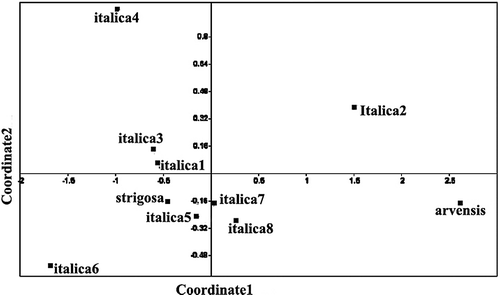
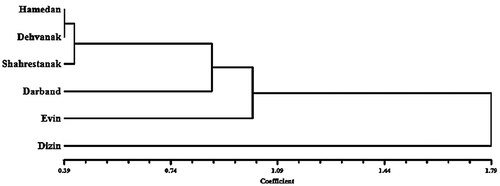
UPGMA cluster analysis and PCA of the A. italica var. italica populations and of all species of Anchusa (performed separately) were done. In UPGMA Euclidean distance clustering dendrogram and PCA ordination (Figures and ), the n = 16 species (A. italica and A. strigosa) appear to be very similar and are placed very close to each other in a single cluster, while A. arvensis subsp. orientalis n = 8 appears to be more distant.
The UPGMA dendrogram for the A. italica var. italica populations (Figure ) shows two major clusters. The Dizin population is located a long way the first major cluster and forms a single cluster. Dizin was in higher geographic region with colder weather conditions, and most 2n pollen grains (about 1/5%) were observed in this population. These factors are suggested as the cause of this separation.
PCA (Figure ) of A. italica var. italica populations supports the cluster analysis showing separation of Dizin population from the others.
Unreduced pollen grain formation
The occurrence of large pollen grains (possibly 2n ones) were observed along normal ones in all studied Anchusa species (Figure ). The large pollen grains comprised about 1% of pollen grains in these populations. The mean diameter of normal (reduced) pollen grains ranged from 19.97 μm to 71.12 μm, while the mean diameter of unreduced pollen grains ranged from 41.56 μm to 139.73 μm. 2n pollen production is indicated by presence of large pollen grains (Bretagnolle and Thomson Citation1995).
Discussion
The present study showed the meiotic chromosome number of Anchusa species. Selvi et al. Citation2004 also pointed out the chromosome counts of some other taxa of Boraginaceae (Boragineae) on the phylogenetic tree to clarify the species relationship. As a final conclusion it should be mentioned that the chromosome number supported previous studies. The meiotic analysis revealed that in Anchusa species there were variations in the prophase sub-stages of meiosis-I, abnormal tetrads and also infertile pollen grains. Unreduced gamete formation is of evolutionary importance as it can lead to the production of plants with higher ploidy levels.
Unreduced gametes produce individuals with higher ploidy level by sexual polyploidization (Villeux Citation1985), as the major factor in formation of naturally occurring polyploids. Unreduced gametes are produced by different cytological mechanisms (Bretagnolle and Thomson Citation1995). The occurrence of multi-polar cells and cytomixis and irregularities in anaphase segregation of the chromosomes might be considered as the possible mechanisms of unreduced pollen grain formation in the studied Anchusa species. So far, this is the first report on the occurrence of unreduced pollen grains in Anchusa species.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Iran National Science Foundation [grant number 95s45031].
References
- Akcin TA, Ulu S, Akcin A. 2010. Morphological, anatomical and numerical studies on some Anchusa L. (Boraginaceae) taxa from Turkey. Pak J Bot. 42(4):2231–2247.
- Baptista-Giacomelli FR, Pagliarini MS, Almeida JL. 2000. Meiotic behavior in several Brazilian oat cultivars (Avena sativa L.). Cytologia. 65:371–378.10.1508/cytologia.65.371
- Bretagnolle F, Thompson JD. 1995. Gametes with the somatic chromosome number: mechanisms of their formation and role in the evolution of autopolyploid plants. New Phytol. 129(1):1–22.10.1111/nph.1995.129.issue-1
- Chater AO. 1972. Anchusa L. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europaea. Vol. 3. Cambridge: University Press; p. 106.
- Constantinidis T, Kamari G. 1995. Reports (401-414). In: Kamari G, Felber F, Garbari F, editors. Mediterranean chromosome number reports, 5, Fl. Medit. Vol. 5; p. 265–278.
- D’Amato G, Trojani Z. 1985. Giemsa banding and karyotype in three species of Anchusa (Boraginaceae). Caryologia. 38:13–21.10.1080/00087114.1985.10797725
- D’Amato G. 1989. C-band patterns and karyotype morphology in some Anchusa species. Caryologia. 42:267–274.10.1080/00087114.1989.10796974
- Diaz Lifante Z, Luque T, Santa BC. 1992. Chromosome numbers of plants collected during Iter Mediterraneum II in Israel. Bocconea. 3:229–250.
- Falistocco E, Falcinelli M, Veronesi F. 1995. Karyotype and C-banding pattern of mitotic chromosomes in alfalfa Medicago sativa L. Plant Breed. 114:451–453.10.1111/pbr.1995.114.issue-5
- Ghaffari SM. 1996. Chromosome studies in some species of Boraginaceae from Iran. Iran J Bot. 7(1):81–93.
- Greuter W, Burdet HM, Long L. 1984. Med checklist 1: pteridophyta (ed. 2), gymnospermae, dicotyledones (acanthaceae- cneoraceae). Geneve: Conservatoire et Jardin Botanique.
- Guşuleac M. 1927. Die europaischen Arten der Gattung Anchusa Linn. Bul Fac Sti Cernauti. 1:73–123.
- John CK, Somani VJ, Thengane RJ. 1989. Some interesting features of male meiosis in Chlorophytum tuberosum Baker. Curr Sci. 58:254–255.
- Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ. 1999. Plant systematics. Sunderland: A Phylogenetic Approach. Sinauer associates, Inc..
- Khatamsaz M. 2002. Anchusa. In: Khatamsaz M, editor. Flora of Iran. Vol. 39. Tehran: Research Institute of Forests and Rangelands; p. 191–214.
- Lövkvist B, Hultgård UM. 1999. Chromosome numbers in south Swedish vascular plants. Opera Bot. 137:1–42.
- Luque T. 1980. Numeros cromosomicos de algunas Boraginaceas de Portugal. Bol Soc Brot, ser 2, 53: 663–670.
- Markova M, Goranova V. 1995. Mediterranean chromosome number reports 5 (435–473). Fl Medit. 5:289–317.
- Nirmala A, Rao PN. 1996. Genetics of chromosome numerical mosaism in higher plants. Nucleus. 39:151–175.
- Rees H, Dale PJ. 1974. Chiasma and variability in Lolium and Festuca populations. Chromosoma. 47:335–351.10.1007/BF00328866
- Riedl H. 1967. Anchusa L. In: Rechinger KH, editor. Flora Iranica. vol 48. Graz: Akademische Druck-u. Verlagsanstalt; p. 232–239.
- Selvi F, Bigazzi M. 1998. Anchusa L. and allied genera (Boraginaceae) in Italy. Plant Biosyst. 132(2):113–142.10.1080/11263504.1998.10654198
- Selvi F, Bigazzi M. 2003. Revision of genus Anchusa (Boraginaceae-Boragineae) in Greece. Bot J Linean Soc. 142:431–454.10.1046/j.1095-8339.2003.00206.x
- Selvi F, Papini A, Hilger HH, Bigazzi M, Nardi E. 2004. The phylogenetic relationships of Cynoglottis (Boraginaceae-Boragineae) inferred from ITS, 5.8S and trnL sequence variation. Plant Syst Evol. 246(3–4):195–209.
- Sheidai M, Inamdar AC. 1991. Variation in the meiotic chromosomes in Asparagus L. Biovigyanam. 17:45–47.
- Sheidai M, Koobaz P, Termeh F, Zehzad B. 2002. Phenetic studies in Avena species and population of Iran. J Sci Is Rep Ira. 13(1):19–28.
- Sheidai M, Noormohamadi Zahra, Mirabdolbaghi-Kashani N, Ahmadi M. 2003. Cytogenetic study of some rapeseed (Brassica napus L.) cultivars and their hybrids. Caryologia. 56:387–397.10.1080/00087114.2003.10589349
- Sheidai M, Riahi H, Hakim M. 1993. Cytomixis in Asparagus L. Nucleus. 36:59–62.
- Villeux R. 1985. Diploid and polyploid gametes in crop plants: mechanisms of formation and utilization in plant breeding. In: Janick J, editor. Plant Breed Rev. vol 3. Wesport, Connecticut: Avi Publishing Co.; p. 442.
- Zargari A. 1989. Medicinal plants, (in Persian). Vol. 3. Tehran: Tehran University.