Abstract
Nanotechnology is a ground-breaking scientific innovation, but there are possible hazards to environment and human health. Therefore, there is a need to understand the toxic potential of nanoparticles. Cytotoxic and genotoxic effects of biogenic cocoa pod husk and cocoa bean silver nanoparticles (CPHE-AgNPs and CBE-AgNPs, respectively), and silver nitrate salts (Ags) were evaluated using the A. cepa root assay. Twenty onion bulbs were exposed to various concentrations (0.01, 0.10, 1.0, 10.0, and 100.0 μg ml–1) of AgNP and Ags solutions. Effects on cell division and chromosomes were observed at 24, 48 and 72 h, while root number and growth inhibition were evaluated at 72 h. Both biogenic AgNPs have potential to be cytotoxic with disturbances to mitotic phases. Mitotic index was less than one half the values of control for almost all concentrations throughout exposure periods. The highest concentration of both AgNPs induced complete cell arrest at both 48 and 72 h, except for Ags. Induction of chromosomal aberrations (chromosomal bridge, c-mitosis, vagrant chromosome, sticky chromosome) pointed to potential for genotoxicity. AgNPs demonstrated a clear mito-depressive effect culminating in growth inhibition of the A. cepa roots, except for 0.01 μg ml–1 of CPHE-AgNPs (p < 0.05). EC50 values showed that growth inhibition was in the order of CPHE-AgNPs>Ags>CBE-AgNPs. While indiscriminate usage of AgNPs might have an impact on the health status of exposed organisms, raising concerns, the cell arresting potential of both AgNPs can be explored in the control of growth of cancerous cells.
1. Introduction
The environments that surround most cities and urban centres are becoming less healthy due to the high level of pollution from anthropogenic activities that release highly toxic and hazardous materials into the ecosystem. High levels of industrialization have been accompanied by large amounts of complex and hazardous industrial wastes (Fathima et al. Citation2012; Yekeen et al. Citation2016; Koolivand et al. Citation2017) that are direct consequences of industrial activities and manufacturing processes (Lei et al. Citation2015). Large numbers of nanoparticle (NP)-based products are continually being released into the market. They have being adopted in an array of applications, including pharmaceutics, catalysts, cosmetics, food packaging, medical devices (i.e. bio-imaging), textiles, electronics, optics, fuel cells, biosensors, water treatment technology and in agriculture (Aitken et al. Citation2006; Handy et al. Citation2008; Benelli Citation2016). The introduction of nanomaterials either as wastes or as by-products of manufacturing processes may signify danger to the ecosystem, the adverse impact of which may not be immediately visible until many years later, especially on human life (Nowack and Bucheli Citation2007).
As the applications of nanotechnology are increasing, so also is the market for these materials, with associated increases in nanomaterials present in the environment and exposure of large numbers of people to NPs (Lux Report Citation2008). Metal NPs are the most widely used type of NPs, and of these, silver nanoparticles (AgNPs) are well known biocidal substances with antimicrobial activity in pharmaceuticals (Kim et al. Citation2009; Lara et al. Citation2010; Xiu et al. Citation2012; Lateef et al. Citation2016a; Oladipo et al. Citation2017a, Citation2017b), as well as hydrogen peroxide scavenging, thrombolytic (Lateef et al. Citation2017) and antiplatelet activity (Shrivastava et al. Citation2009), and use as paint additives (Lateef et al. Citation2016b). The more favoured method of synthesis of AgNPs is the green route because of its cost effectiveness, and eco-friendly and nontoxic nature compared to both physical and chemical methods (Geethalakshmi and Sarada Citation2012). Several biologically active molecules of plant (Mukunthan and Balaji Citation2012; Anwar et al. Citation2015; Dhand et al. Citation2016), insect (Lateef et al. Citation2016c; Lateef et al. Citation2016d) and microbial (Lateef et al. Citation2015a; Oladipo et al. Citation2017a, 2017b) origin have been employed in the green synthesis of AgNPs of desired size and morphology. These biomolecules in most cases serve as reducing and capping agents for the synthesized NPs, making them suitable for various biomedical applications (Arokiyaraj et al. Citation2014).
The wide application of AgNPs in consumer products may lead to increased bioavailability of NPs in the environment, so there is a need to evaluate the potential health implications on lifeforms in the ecosystem. It has been reported that fullerenes, carbon nanotubes and metal oxide NPs are potentially toxic to plants (Castiglione and Cremonini Citation2009; Castiglione et al. Citation2016) and animals (Hussain et al. Citation2005; Jia et al. Citation2005; Lam et al. Citation2006). Lee et al. (Citation2008) reported an increase in bioaccumulation of NPs at higher concentrations. In a similar investigation, it was observed that metal oxide NPs had higher phytotoxicity than free metal ions of the equivalent concentrations (Wu et al. Citation2012). Kumari et al. (Citation2009) inferred that AgNPs could penetrate plant systems, impairing stages of cell division, while a similar observation was made in a more recent work (Yekeen et al. Citation2017).
Green synthesis of AgNPs using cocoa pod husk and cocoa been extracts has been explored and their antimicrobial, larvicidal, anticoagulant and antioxidant activities have been demonstrated (Lateef et al. Citation2016a; Azeez et al. Citation2017). These biotechnological potentials attributed to cocoa (pod husk and beans) extract-mediated AgNPs and possibility of their incorporation into array of products especially pharmaceutics necessitated their safety evaluation, which was the focus of the present investigation. Therefore, this study was designed to evaluate the cytotoxic and genotoxic effects of cocoa-mediated AgNPs on Allium cepa roots.
2. Materials and methods
2.1. Collection and processing of cocoa pods and beans
Cocoa fruits were obtained from farmers in Ipetumodu, Osun State, Nigeria in fresh form and washed thoroughly in the laboratory to remove dirt and other extraneous substances. The pods were split open with the pod husk and beans separated. The pod husk (Lateef et al. Citation2016a) and cocoa bean (Azeez et al. Citation2017) were separately transformed into chips and then air dried for seven days at room temperature (30 ± 2°C). The pod husk chips as well as de-shelled cocoa bean were milled into powder with the aid of electric blender as described by Lateef et al. (Citation2015b, Citation2016b). The schematic processes of preparation are shown in Figure .
2.2. Preparation of cocoa pod husk extract (CPHE) and cocoa bean extract (CBE)
The methods of Lateef et al. (Citation2015b, Citation2016b) were adopted in preparation of cocoa pod husk extract (CPHE) and cocoa bean extract (CBE). For each extract 0.1 g of the powder was weighed and suspended separately in 10 ml of distilled water, and heated in water bath at 60°C for 1 h. The extract was filtered using Whatman no. 1 filter paper and then centrifuged at 4000 rpm. The final clear extracts for cocoa pod husk and cocoa beans were tagged CPHE and CBE respectively; these were stored at 4°C for further use.
2.3. Green synthesis of CPHE-AgNPs and CBE-AgNPs and characterization
The green synthesis was carried out using CPHE and CBE, to produce CPHE-AgNPs and CBE-AgNPs respectively. The previously reported protocol was used (Lateef et al. Citation2015b, Citation2016b) for the preparation of each of the AgNPs as follows: to 40 ml of 1 mM silver nitrate (AgNO3), 1 ml of the extract was added at room temperature (30 ± 2°C) and the reaction mixture was allowed to stand. A change in colour which stabilized after 10 min was observed, and the formation of CPHE-AgNPs and CBE-AgNPs was confirmed by measuring their absorbance spectra using a UV-visible spectrophotometer (Cecil, Cambridge, UK) operated at 200–900 nm. Fourier transform infrared (FTIR) spectroscopy was employed for the identification of the biomolecules that were involved in the green synthesis, while transmission electron microscopy (TEM) and energy dispersive X-ray (EDX) analyses were used for evaluation of particle size, morphology, and elemental composition of the CPHE-AgNPs and CBE-AgNPs (Lateef et al. Citation2015b, Citation2016b).
2.4. Allium cepa assay
Onion bulbs (380) of approximately equal size used for this assay were obtained from an open market in Ogbomoso, Oyo State, Nigeria and sundried for two weeks. For the purpose of the experiment, only high quality bulbs were selected for use. The modified protocols of Fiskesjo (Citation1985) and Rank and Nielsen (Citation1993), were adopted with slight modifications as previously reported (Yekeen et al. Citation2011; Yekeen et al. Citation2013a; Azeez et al. Citation2016; Yekeen et al. Citation2017). The outer scale and brownish bottom plate of the sundried Allium cepa were removed, leaving the ring of the root primordial intact. Twenty onion bulbs were placed directly on 50 ml capacity beaker and exposed to 0.01, 0.1, 1.0, 10.0 and 100.0 μg ml–1 of each of the CPHE-AgNPs, CBE-AgNPs and Ags prepared using distilled water as diluent and as the control. The experiment was performed in the dark at 30 ± 2°C with the liquid being replaced with freshly prepared concentration at every 24 h. Root tips from five onions from each of the AgNPs and Ags concentrations as well as the negative control (distilled water) were harvested at 24, 48 and 72 h with roots from each bulb fixed separately in ethanol–ethanoic acid (3:1 v/v) as recommended by Sharma and Sharma (Citation1980).
2.4.1. Microscopic evaluation
The fixed root tips were hydrolysed in 1 N HCl at 65°C for 3 min. Two root tips were squashed on each slide, and stained with aceto-orcein for 15 min. From each slide 1000 cells were scored for chromosomal aberrations (CA) using various aberrant templates as previously reported (Bakare et al. Citation2000; Yekeen and Adeboye, Citation2013; Azeez et al. Citation2016). A total of 5000 cells were scored per concentration in each treatment and the control. The mitotic index and mitotic inhibition were calculated from the score obtained for dividing cells (Lateef et al. Citation2007).
2.4.2. Macroscopic evaluation
The length of each root from five onion bulbs per concentration in each treatment and the control were measured at 72 h. The root EC50 values were obtained from the growth curves of percentage root length relative to control against various concentrations of the green-synthesized NPs (AgNPs) and Ags.
2.5. Statistical analysis
Data obtained from cell division and mitotic indices as well as root length of the treated A. cepa from different concentrations and control were compared using analysis of variance (ANOVA) on SPSS software version 13 (IBM Corporation, New York, USA). Duncan’s post hoc test was conducted at 0.05 level of significance. Box plot and EC50 of root inhibition graphs were plotted using Prism 6 software. The mitotic index, mitotic inhibition and percentage of chromosome aberration observed were calculated as previously described (Yekeen et al. Citation2017) i.e.:
3. Results
3.1. Green synthesis of biogenic AgNPs and characterization
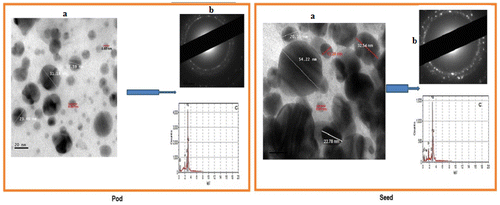
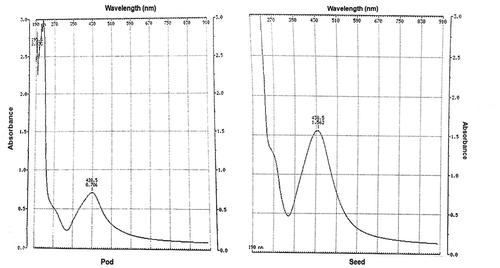
Evidence of CPHE and CBE in production of CPHE-AgNPs and CBE-AgNPs respectively was observed via colour changes within 10 min, resulting in brown coloration in both cases (Figure ). Further confirmation of the biogenic synthesis of CPHE-AgNPs and CBE-AgNPs was via UV-vis spectrophotometer with readings occurring at wavelength of 428.5 nm and 438.5 nm respectively (Figure ). The FTIR spectrum for CPHE-AgNPs revealed strong peaks at 3294.42, and 1635.64 cm−1, while CBE-AgNPs revealed strong peaks at 3275.13, and 1635.54 cm−1.
Figure 2. UV-vis absorption spectra of biogenic synthesized AgNPs using extracts of cocoa pod husk and seed.
The TEM (Figure (a)) revealed that CPHE-AgNPs and CBE-AgNPs were fairly spherical in shape, well dispersed within the organic matrix with sizes ranging from 4 to 32 nm and 8.96 to 54.22 nm respectively. The poly-dispersed nature of the particles was supported by the broadness of UV-vis absorption data, which hover around 350–510 nm for CBE-AgNPs and CPHE-AgNPs. A ring-like selected area electron diffraction (SAED) associated with the face-centred cubic crystalline structure of silver was displayed for both AgNPs (Figure (b)). The EDX patterns (Figure (c)) revealed high presence of silver in both CPHE-AgNPs and CBE-AgNPs colloidal solution: 95% and 93%, respectively. Both synthesized AgNPs maintained high stability without any form of aggregation or deterioration or formation of sediment over a long period of more than six months.
3.2. Cytogenotoxic effects of biogenic AgNPs on Allium cepa cells
Our study on exposure of A. cepa root cells to different concentrations of CPHE-AgNPs, CBE-AgNPs and Ags for 24 h revealed a dose-dependent decrease in cumulative dividing cells, with the value obtained for the control higher than all concentrations of the treatments. However, the values of dividing cells obtained for each concentration in Ags were higher than the corresponding concentrations in both AgNPs. The values obtained for CBE-AgNPs for each concentration were also higher than the values for the corresponding concentrations in CPHE-AgNPs, except at 100 μg ml–1 where the values were the same (48). At 24 h the mitotic index was found to be less than half of the control at all concentrations of CPHE-AgNPs, CBE-AgNPs (except at 0.01 μg ml–1) and only at 10 and 100 μg ml–1 of Ags (Table ).
Table 1. Cytogenotoxic effects of cocoa extract mediated silver nanoparticles on Allium cepa cells at 24 h exposure.
Variations were observed in the mitotic phase index for all treatments and the control, with prophase more dominant. However, the per cent proportion of prophase as well as other stages of division was more in biogenic AgNPs compared to Ags and the control, while reduction was observed at their anaphase and telophase in the treatment groups compared to the control. CA were observed in different proportions for each of the concentrations in the treatment groups except the control. The highest frequency of CA observed in 1.0 μg ml–1 of CBE-AgNPs (0.34%) was found to be higher than aberrant cells observed at all concentrations of Ags (Table ). Aberrant cells across treatments showed chromosome bridges, c-mitosis, vagrant chromosomes, sticky chromosomes and chromosome fragmentation (Figure ).
Figure 4. Mitotic stages and chromosomal aberrations observed for A. cepa root cells treated with biogenic synthesized AgNPs and Ags.
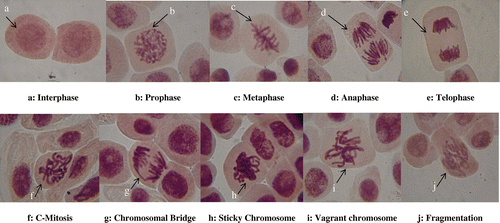
Table showed the cytogenotoxic effects of cocoa extract mediated AgNPs and Ags on Allium cepa cells at 48 h exposure. Cumulative dividing cells revealed dose-dependent reduction at 48 h for all treatment groups and were found lower than the value recorded for the control, as similarly observed for 24 h. However, no dividing cell was observed at 100 μg ml–1 in both CPHE-AgNPs and CBE-AgNPs, unlike at 24 h. Mitotic index values for all concentrations of CPHE-AgNPs and CBE-AgNPs were less than half of the control, except at 0.01 μg ml–1 for both AgNPs, while a similar observation was noticed only at 100 μg ml–1 of Ags. A dose-dependent mitotic inhibition was observed for the three treatment groups relative to the control. The mitotic phase index showed higher proportions of prophase and metaphase at all concentrations of CPHE-AgNPs and CBE-AgNPs compared to the control, while reduced values were observed for anaphase and telophase. Five types of CA were observed at different concentrations of each treatment group in different proportions with the highest aberration per cell observed at 1.0 μg ml–1 of CPHE-AgNPs (Table ; Figure ).
Table 2. Cytogenotoxic effects of cocoa extract mediated silver nanoparticles on Allium cepa cells at 48 h exposure.
Cytogenotoxic evaluation of the treatment groups at 72 h followed the trend observed in other exposure periods especially at 48 h but with fewer dividing cells across the concentrations in each treatment group (Table ). A dose-dependent value was recorded for dividing cells, mitotic index and mitotic inhibition. The mitotic index obtained at all concentrations of biogenic AgNPs was less than half of the value obtained for the control, except 0.01 of CBE-AgNPs. A similar trend was observed for Ags only at 1.0, 10.0 and 100.0 μg ml–1. Cell arrest as observed at 100 μg ml–1 of 48 h CPHE-AgNPs and CBE-AgNPs was also observed at the same concentration at 72 h.
Table 3. Cytogenotoxic effects of cocoa extracts mediated silver nanoparticles on Allium cepa cells at 72 h exposure.
The per cent proportion of different mitotic stages at 72h still reflected prophase to be more than the other stages but not as high as observed at 24 and 48 h in all treatments. The phase index showed that prophase values at all concentrations were higher than those of the control, while reduction was observed for anaphase and telophase. More CA was observed in A. cepa treated with biogenic AgNPs compared to the Ags, while none was observed at the control (Table ; Figure ). The prophase index and metaphase index in this study were found to increase, while reduction was experienced in the anaphase index and telophase index for all exposure periods except for a few concentrations at 72 h for metaphase index only. CA induced by the two green synthesized AgNPs were similar to those observed for Ags, except for the addition of fragmentation in Ags.
3.3. AgNPs influence on root number and growth inhibition
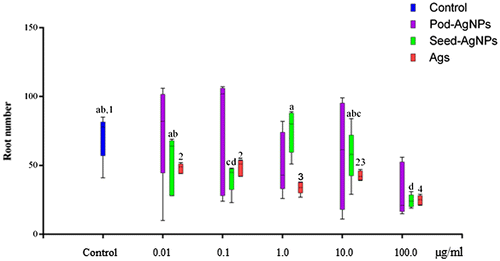
After 72 h of exposure, the root number harvested showed variations across the concentrations of each treatment group (CPHE-AgNPs, CBE-AgNPs and Ags) and the control (Figure ). The root numbers obtained at 0.01, 0.1 and 10.0 μg ml–1 of CPHE-AgNPs were more than that of the control. However, there was no significant difference in the root number of A. cepa exposed to different concentrations of CPHE-AgNPs compared to the value obtained for the control. Similar to CPHE-AgNPs, there was variation in number of root in CBE-AgNPs treated A. cepa, with only the value obtained at 1.0 μg ml–1 found to be higher but not significantly different from the value obtained for the control. Significant reduction in root number was however observed at 0.1 and 100 μg ml–1 CBE-AgNPs compared to the control. The root numbers obtained at all tested concentration of Ags were found to be significantly lower than the value obtained for the control (p < 0.05). The root number obtained for Ags at all concentrations were equally lower in number compared to their corresponding concentrations in the CPHE-AgNPs and CBE-AgNPs (Figure ).
Figure 5. Variations in root numbers of A. cepa treated with cocoa pod and seed extract mediated AgNPs.
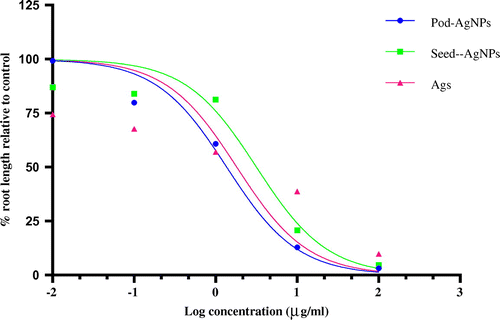
Evaluation of the root length of the CPHE-AgNPs, CBE-AgNPs and Ags relative to the control showed a dose-dependent significant reduction in each of the treatments (Table ). All root length values for each of the concentrations in each of the biogenic AgNPs treatment groups as well as Ags were significantly lower than the value obtained for the control, except at 0.01 μg ml–1 of CPHE-AgNPs. Variations were also observed in percentage root length relative to control (PRLRC) with the lowest value obtained at the highest concentration for each of the treatment groups. The EC50 values obtained from PRLRC against concentrations for CPHE-AgNPs, Ags, CPHE-AgNPs were 1.369, 1.819 and 3.17 respectively (Figure ). The order of growth inhibition was revealed as CPHE-AgNPs>Ags>CBE-AgNPs.
Table 4. Toxic effects of cocoa extract mediated green synthesized AgNPs on A. cepa root growth.
4. Discussion
4.1 Synthesis and characterization
Evidence of colour changes resulting in brown coloration in both biogenic AgNPs cases indicates the formation of AgNPs. Previous reports showed colloidal biogenic synthesized AgNPs solutions exhibited shades of colours from yellowish through brown to dark brown (Shaligram et al. Citation2009; Lateef et al. Citation2015a, Citation2015b, Citation2015c; Lateef et al. Citation2016b, Citation2016d), suggesting that the different macromolecules present in the extracts played catalytic and stabilization roles in the formation of the particles. The production of AgNPs was further confirmed via UV-vis spectrophotometer readings at wavelengths of 428.5 nm and 438.5 nm. The peaks as revealed by FTIR suggest the involvement of protein and phenolic compounds as capping and stabilizing agents. In both AgNPs, the higher of the two prominent peaks is typical of N–H bond of amines while the lower peak is typical of the C=C stretch of alkenes or C=O stretch of amides (Shankar et al. Citation2014). Cocoa pod and beans have been reported to contain various organic compounds which are biomolecules known to be very rich in the identified chemical bonds (Karim et al. Citation2014; Lateef et al. Citation2016a; Azeez et al. Citation2017). A report has shown CBE as the reducing and stabilizing agent in the synthesis of anisotropic gold NPs (Fazal et al. Citation2014).
The sizes of 4–32 nm and 8.96–54.22 nm for CPHE-AgNPs and CBE-AgNPs respectively are in line with other reports on AgNPs (Zaki et al. Citation2011; Lateef et al. Citation2015a, Citation2015b, Citation2015c; Lateef et al. Citation2016d). The poly-dispersed nature of the particles is supported by the broadness of UV-vis absorption data, which hover around 350–510 nm for CBE-AgNPs and CPHE-AgNPs. The EDX patterns as obtained revealed high presence of silver in both CPHE-AgNPs and CBE-AgNPs colloidal solution (Shameli et al. Citation2011; Lateef et al. Citation2015b, Citation2015c, Citation2016a, 2016b): 95% and 93% respectively. The reduction of silver ions was due to the excitation of surface plasmon resonance of the AgNPs (Mulvaney Citation1996). SAED reflected a ring-like pattern associated with the face-centred cubic crystalline structure of silver as previously reported by Shankar et al. (Citation2014). The ability of both biogenic AgNPs not to aggregate or form sediment over a period of more than six months showed stability. A similar observation has been reported for biosynthesized AgNPs from different parts of Cola nitida fruits (Yekeen et al. Citation2017). This makes the CHPE-AgNPs and CBE-AgNPs stable for the repeated usage over the periods of evaluation.
4.2. Cytogenotoxic effects of biogenic AgNPs on Allium cepa cells
The Allium cepa assay has been used in evaluation of different materials for the purpose of environmental monitoring and has been found to be effective, reproducible and reliable (Fiskesjo Citation1985; Rank and Nielsen Citation1993). Our study on exposure of A. cepa root cells to different concentrations of CPHE-AgNPs, CBE-AgNPs and Ags for 24 h revealed a dose-dependent decrease in cumulative dividing cells, with the value obtained for the control highest compared to all concentrations of the treatments. It was evident from the results that both AgNPs impacted negatively on the cell division and mitotic index of treated A. cepa at different concentrations in each of the treatment groups. CPHE-AgNPs and CBE-AgNPs exhibited cytotoxicity through dose-dependent reduction in cell division coupled with reduction of mitotic index to less than one half of the value obtained for the control in almost all concentrations at different exposure periods. Reports have shown that reduction of mitotic index is an indicator of cytotoxicity in A. cepa assay (Asita and Matebesi Citation2010; Yekeen and Adeboye Citation2013; Yekeen et al. Citation2017). This could be due to a slower progression of cells from S (DNA synthesis) phase to M (mitosis) phase of the cell cycle as a result of exposure to AgNPs.
The reduction in mitotic index may be caused by the effect of the AgNPs or test chemicals on the microtubule; a similar observation has been reported for NP-treated V. faba (Patlolla et al. Citation2012). The higher number of dividing cells obtained from root grown in Ags at all exposure periods compared to those of the two AgNPs indicates it was less cytotoxic than the two AgNPs. This can be further corroborated by the complete cell arrest observed at 48 and 72 h of 100 μg ml–1 of both AgNPs, which was not observed for the Ags. The present findings confirm that CPHE-AgNPs and CBE-AgNPs have the potential to cause a mito-depressive effect on the exposed A. cepa roots. This occurrence may be attributed to reduction in cell activity which could be due to changes in duration of mitotic cycle. Inhibition of mitosis has been attributed to an increase in S phase duration (Kumari et al. Citation2009). It might also be due to reduction of mitotic index resulting from the inhibition of DNA synthesis and blocking of G2 phase of the cell cycle, thus preventing the cell from entering mitosis (Sudhakar et al. Citation2001). The reduction of MI value lower than the control suggests the level of cytotoxicity that the substance inflicted on meristematic cells (Akinboro and Bakare Citation2007).
Accumulation of silver compounds within the cells might also result in generation of reactive oxygen species which could decrease mitotic index. Similar accumulation of TiO2 and Al2O3 within the cells of A. cepa has been reported to result in the generation of reactive oxygen species and decreased MI values (Castiglione et al. Citation2014; Pakrashi et al. Citation2014; Rajeshwari et al. Citation2015). AgNPs have also been reported to induce a cytotoxic effect in a different biological model, A. cepa inclusive (Sobieh et al. Citation2016). Complete cell arrest, which indicates cessation of cell division as observed for the 100 μg ml–1 at 48 and 72 h for the two NPs used, further corroborates their cytotoxicity. However, the potential of the CPHE-AgNPs and CBE-AgNPs to induce complete cell arrest may be explored in growth inhibition in cancer treatment.
Prokhorova et al. (Citation2008) reported that increasing frequency of prophases is associated with the violation of the chromosomal supramolecular structure. The situation when the frequency of metaphases increases while that of anaphases and telophases decreases can be associated with the action of AgNPs on the achromatic spindle. In this case chromosomal segregation cannot occur, which may result in the appearance of genomic mutations. An observation similar to ours has been reported for chitosan-capped AgNPs evaluated for cytotoxicity using the A. cepa assay (Pesnya Citation2013). Reduction in mitotic activity can also be due to impaired nucleoprotein synthesis and reduced level of ATP to provide energy for spindle elongation, microtubule dynamics and chromosomal movement (Majewska et al. Citation2003). Several studies have reported differences in mitotic and alteration indices according to NP concentration and the duration of exposure (Kumari et al. Citation2009; Braroo et al. Citation2014).
CA are changes in chromosome structure resulting from a break or exchange of chromosomal material. The aberrations induced by the two green synthesized AgNPs were similar to those observed for Ags except for the addition of fragmentation in Ags. Induction of these CA (sticky chromosomes, chromosome bridges, c-mitosis and vagrant chromosomes) shows that the NPs have potential to be genotoxic. The processes of formation of these aberrations have been explained by various authors (Patil and Bhat Citation1992; Pesnya Citation2013; Yekeen and Adeboye Citation2013; Yekeen et al. Citation2017). Induction of CA as observed for biogenic AgNPs in this study using A. cepa might be due to the interference of chemicals during DNA repair; a similar observation was reported in Vicia faba exposed to AgNPs (Patlolla et al. Citation2012). Induction of CA can affect the vigour, fertility, yield or competitive ability of the exposed plants (Kara et al. Citation1994)
4.3. AgNPs influence on root number and growth inhibition
Studies on the A. cepa assay revealed that any genotoxic effects manifested in a test sample are likely to result in inhibition of root growth (Fiskesjo, Citation1997; Yekeen et al. Citation2011). Increased root number observed at 0.01, 0.1 and 10.0 μg ml–1 of CPHE-AgNPs and 1.0 μg ml–1 of CBE-AgNPs compared to the control suggests their potential to induce root sprouting. The results of the macroscopic evaluation in this study manifested various degree of inhibition in the two AgNPs and Ags treated onion bulb roots. The trend of reduction observed in cell division and mitotic index was similar to what was obtained for the root growth in each of the treatment groups, indicating growth inhibition at the various concentrations used. The EC50 values revealed their root growth inhibitory effect as CPHE-AgNPs>Ags>CBE-AgNPs. With the reduced number of dividing cells observed in CBE-AgNPs compared to Ags, one would expect that the growth inhibition order between them to be in reverse order. This occurrence may mean that the numerous numbers of cell division in Ags does not translate to cell elongation compared to lower number of cells in CBE-AgNPs. EC50 has been reported as an efficient means of ranking toxicity resulting from the inhibitory effects of test substances on A. cepa root as reported from various studies: on chemicals (Nielsen and Rank 1994), wastewater sludge (Rank and Neilsen Citation1998) and plant extracts (Oyeyemi and Bakare Citation2013; Yekeen et al. Citation2013b).
As also revealed in this study, many other engineered NPs, including AgNPs with different chemical properties, have been shown to be cytotoxic, genotoxic and mito-depressive both to plant and mammalian cells (Kumari et al. Citation2009; Foldbjerg et al. Citation2011). Our result is however the first to report such occurrences in AgNPs from cocoa pod husk and cocoa bean/seed. Some reports have shown that AgNPs are capable of entering the nucleus, and can directly or indirectly interact with nuclear material, resulting in alterations of DNA integrity (Kruszewski et al. Citation2011; Asare et al. Citation2012). AgNP is believed to induce DNA damage through oxidative stress (Reeves et al. Citation2008). Kim et al. (Citation2011) attributed the possible mechanisms to include direct generation of ROS from the surface of the particles, from soluble compounds such as transition metals, and via altered function of mitochondria or NADPH oxidase.
5. Conclusion
Our results indicate that biogenic AgNPs produced from cocoa pod (CPHE-AgNPs) and seed (CBE-AgNPs) have the potential to inhibit mitotic activity in plant tissue and disturb proportions of mitotic phases, which may result in mitotic abnormalities and root growth inhibition. CPHE-AgNPs and CBE-AgNPs exhibited dose- and time-dependent cytotoxic effects on A. cepa compared to the control. Both AgNPs reduced dividing cells and mitotic stages especially at 48 and 72 h compared to Ags and the control. Phase index was found to be higher than that of the control for prophase and metaphase, while reduction in values was recorded for anaphase and telophase for the period of evaluation. Complete cell arrest was observed for both AgNPs at their highest concentration at both 48 and 72 h. Both AgNPs demonstrated a clear mito-depressive effect culminating in growth inhibition of the A. cepa root. Our data showed that the response of Allium roots was concentration-dependent for the growth inhibition exhibited by CPHE-AgNPs, CBE-AgNPs and Ags, with the effect more pronounced in CPHE-AgNPs. The NPs also induced various CA, an indication of their potential for genotoxicity. While indiscriminate usage of the AgNPs might have impact on health status of exposed organisms, which calls for concern, the cell arresting potential of both AgNPs can be explored in the control of cancerous cells.
Geolocation information
The research was conducted in Ogbomoso, Oyo State, West Africa, Nigeria.
Acknowledgements
We acknowledge the effort of Ojo S. A. of the Department of Pure and Applied Biology, LAUTECH, Ogbomoso in some of the laboratory experiments.
References
- Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M. 2006. Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med. 56:300–306.10.1093/occmed/kql051
- Akinboro A, Bakare AA. 2007. Cytotoxic and genotoxic effects of aqueous extracts of five medicinal plants on Allium cepa Linn. J Ethnopharmacol. 112(3):470–475.10.1016/j.jep.2007.04.014
- Anwar MF, Yadav D, Kapoor S, Chander J, Samim M. 2015. Comparison of antibacterial activity of Ag nanoparticles synthesized from leaf extract of Parthenium hystrophorus L. in aqueous media and Gentamicin sulphate: in-vitro. Drug Dev Ind Pharm. 41:43–50.10.3109/03639045.2013.845840
- Arokiyaraj S, Arasu MV, Vincent S, Prakash NU, Choi SH, Oh Y, Choi KC, Kim KH. 2014. Rapid green synthesis of silver nanoparticles from Chrysanthemum indicum L. and its antimicrobial and cytotoxic effects: an in vitro study. Inter J Nanomedicine. 9:379–388.10.2147/IJN
- Asare N, Instanes C, Sandberg WJ, Refsnes M, Schwarze P, Kruszewski M, Brunborg G. 2012. Cytotoxic and genotoxic effects of silver nanoparticles in testicular cells.ToxicolIn Vitro. 291(1 - 3): 65-72.
- Asita AO, Matebesi LP. 2010. Genotoxicity of hormoban and seven other pesticides to onion root tip meristematic cells. Afr J Biotechnol. 9(27):4225–4232.
- Azeez MA, Yekeen TA, Adedeji AO, Bello OS. 2016. Proximate and phytochemical constituents of four medicinal plants and their cytogenotoxic effects using Allium cepa assay. J Agroaliment Proc Technol. 22(3):132–141.
- Azeez MA, Lateef A, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Gueguim-Kana EB, Beukes LS. 2017. Biomedical applications of cocoa bean extract-mediated silver nanoparticles as antimicrobial, larvicidal and anticoagulant agents. J Clust Sci. 28(1):149–164.10.1007/s10876-016-1055-2
- Bakare AA, Mosuro AA, Osibanjo O. 2000. Effects of simulated leachates on chromosomes and mitosis of Allium cepa (L). J Environ Biol. 21:263–271.
- Benelli G. 2016. Plant-mediated biosynthesis of nanoparticles as an emerging tool against mosquitoes of medical and veterinary importance: a review. Parasitol Res. 115:23–34.10.1007/s00436-015-4800-9
- Braroo K, Sharma AK, Thakur M, Kasu YA, Singh K, Bhori M. 2014. Colloidal silver nanoparticles from Ocimum sanctum: synthesis, separation and their implications on pathogenic microorganisms, human keratinocyte cells, and Allium cepa root tips. J Colloid Sci Biotechnol. 3:1–8.
- Castiglione MR, Cremonini R. 2009. Nanoparticles and higher plants. Caryologia. 62(2):161–165.
- Castiglione MR, Giorgetti L, Cremonini R, Bottega S, Spanò C. 2014. Impact of TiO2 nanoparticles on Vicia narbonensis L.: potential toxicity effects. Protoplasma. 251:1471.10.1007/s00709-014-0649-5
- Castiglione MR, Giorgetti L, Bellani L, Muccifora S, Bottega S, Spanò C. 2016. Root responses to different types of TiO2 nanoparticles and bulk counterpart in plant model system Vicia faba L. Environ Exper Bot. 130(2016):11–21.10.1016/j.envexpbot.2016.05.002
- Dhand V, Soumya L, Bharadwaj S, Chakra S, Bhatt D, Sreedhar B. 2016. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater Sci Eng C. 58:36–43.10.1016/j.msec.2015.08.018
- Fathima N, Rao R, Nair Bu. 2012. Tannery solid waste to treat toxic liquid wastes: a new holistic paradigm. Environ Engineering Sci. 29:363–372.10.1089/ees.2010.0445
- Fazal S, Jayasree A, Sasidharan S, Koyakutty M, Nair SV, Menon D. 2014. Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl Mater. 6:8080–8089.10.1021/am500302t
- Fiskesjo G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 102:99–112.
- Fiskesjo G. 1997. Allium test for screening chemical evaluation of cytological parameters. In: Wang W, Gorsuch JW, Hughes JS, editors. Plants for environmental studies. New York, NY: CRC Lewis Publishers; p. 307-333.10.1201/9781420048711
- Foldbjerg R, Dang DA, Autrup H. 2011. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 85(7):743–750.10.1007/s00204-010-0545-5
- Geethalakshmi R, Sarada DV. 2012. Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Inter J Nanomedicine. 7:5375–5384.10.2147/IJN
- Handy RD, von der Kammer F, Lead JR, Hassellöv M, Owen R, Crane M. 2008. The ecotoxicology and chemistry of manufactured nanoparticles. Ecotoxicology. 17:287–314.10.1007/s10646-008-0199-8
- Hussain SM, Hess KL, Gearhart JM, Geiss KT, Schlager JJ. 2005. In vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol in vitro: An inter J Published in Assoc with BIBRA. 19(7):975–983.10.1016/j.tiv.2005.06.034
- Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, Zhao Y, Guo X. 2005. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 39(5):1378.10.1021/es048729 l
- Kara M, Þanda MA, Ateş A. 1994. Cytogenetic effects of the insecticide cypermethin on the root meristems of Allium cepa L. Tr J of Biology. 18:323–331.
- Karim AA, Azlan A, Ismail A, Hashim P, Gani SSA, Zainudin BH, Abdullah NA. 2014. Phenolic composition, antioxidant, anti-wrinkles and tyrosinase inhibitory activities of cocoa pod extract. BMC complementary and Alternative Med. 14:381. https://doi.org/https://link.springer.com/content/pdf/10.1186%2F1472-6882-14-381.pdf.
- Kim K, Sung WS, Suh BK, Moon S, Choi J, Kim JG, Lee DG. 2009. Antifungal activity and mode of action of silver nano-particles on Candida albicans. Bio Metals. 22(2):235–242.10.1007/s10534-008-9159-2
- Kim HR, Kim MJ, Lee SY, Oh SM, Chung KH. 2011. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cell. Mut Res. 726(2):113–122.
- Koolivand A, Mazandaranizadeh H, Binavapoor M, Mohammadtaheri A, Saeedi R. 2017. Hazardous and industrial waste composition and associated management activities in Caspian industrial park. Iran Environ Nanotechnol, Monitoring Management. 7:9–14.10.1016/j.enmm.2016.12.001
- Kruszewski M, Brzoska K, Brunborg G, Asare N, Dobrzynska M, Duzinska M, Fjellsbo LM, Georgantzopoulou A, Gromadzka-Ostrowska J, Gutleb AC. 2011. Toxicity of silver nanomaterials in higher eukaryotes. Adv Mol Toxicol. 5:179–218.10.1016/B978-0-444-53864-2.00005-0
- Kumari M, Mukherjee A, Chandrasekaran N. 2009. Genotoxicity of silver nanoparticles in Allium cepa. Sci Total Environ. 407(19):5243–5246.10.1016/j.scitotenv.2009.06.024
- Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. 2006. A review of carbon nanotube toxicity and assessment of potential and environmental health risks. Crit Rev Toxicol. 36:189–217.10.1080/10408440600570233
- Lara HH, Ayala-Nuñez NV, Ixtepan-Turrent L, Rodriguez-Padilla C. 2010. Mode of antiviral action of silver nanoparticles against HIV-I. J Nanobiotechnology. 8:1. https://www.honeycolony.com/wpcontent/uploads/2015/06/Mode_of_Antiviral_Action_of_Silver_Nanoparticles_Against_HIV-1.pdf.10.1186/1477-3155-8-1
- Lateef A, Yekeen TA, Ufoma P. 2007. Bacteriology and genotoxicity of some pharmacaeutical wastewater in Nigeria. Int J Environ and Health. 1(4):551–562.10.1504/IJENVH.2007.018572
- Lateef A, Ojo SA, Akinwale AS, Azeez L, Gueguim-Kana EB, Beukes LS. 2015a. Biogenic synthesis of silver nanoparticles using cell-free extract of Bacillus safensis LAU 13: antimicrobial, free radical scavenging and larvicidal activities. Biologia. 70:1295–1306.
- Lateef A, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Ajetomobi FE, Gueguim-Kana EB, Beukes LS. 2015b. Cola nitida-mediated biogenic synthesis of silver nanoparticles using seed and seed shell extracts and evaluation of antibacterial activities. BioNanoSci. 5(4):196–205.10.1007/s12668-015-0181-x
- Lateef A, Adelere IA, Gueguim-Kana EB, Asafa TB, Beukes LS. 2015c. Green synthesis of silver nanoparticles using keratinase obtained from a strain of Bacillus safensis LAU 13. Int Nano Lett. 5:29–35.10.1007/s40089-014-0133-4
- Lateef A, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Azeez L, Ojo SA, Gueguim-Kana EB, Beukes LS. 2016a. Cocoa pod husk extract-mediated biosynthesis of silver nanoparticles: its antimicrobial, antioxidant and larvicidal activities. J Nanostruct Chem. 6(2):159–169.10.1007/s40097-016-0191-4
- Lateef A, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Azeez L, Ajibade SE, Ojo SA, Gueguim-Kana EB, Beukes LS. 2016b. Biogenic synthesis of silver nanoparticles using pod extract of Cola nitida: antibacterial, antioxidant activities and application as additive in paint. J Taibah Univ. 10(4):551–562.10.1016/j.jtusci.2015.10.010
- Lateef A, Akande MA, Ojo SA, Folarin BI, Gueguim-Kana EB, Beukes LS. 2016c. Paper wasp nest-mediated biosynthesis of silver nanoparticles for antimicrobial, catalytic, anticoagulant, and thrombolytic applications. 3 Biotech. 6:140. https://link.springer.com/article/10.1007/s13205-016-0459-x.
- Lateef A, Ojo SA, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Oladipo IC, Gueguim-Kana EB, Beukes LS. 2016d. Cobweb as novel biomaterial for the green and ecofriendly synthesis of silver nanoparticles. Appl Nanosci. 6(6):863–874.10.1007/s13204-015-0492-9
- Lateef A, Ojo SA, Elegbede JA, Azeez MA, Yekeen TA, Akinboro A. 2017. Evaluation of some biosynthesized silver nanoparticles for biomedical applications: hydrogen peroxide scavenging, anticoagulant and thrombolytic activities. J Clust Sci. 28(3):1379–1392.10.1007/s10876-016-1146-0
- Lee W, An Y, Yoon H, Kweon H. 2008. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants Mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem. 27(9):1915–1921.10.1897/07-481.1
- Lei L, Wang S, Lin Y, Liu W, Chi T. 2015. A covering model application on Chinese industrial hazardous waste management based on integer program method. Ecological Indices. 51:237–243.10.1016/j.ecolind.2014.05.001
- Lux Report. 2008. Nanomaterials state of the market: stealth success, broad impact. Available from the website: https://portal.luxresearchinc.com/research/document/3735.
- Majewska A, Wolska E, Śliwińska E, Furmanowa M, Urbańska N, Pietrosiuk A, Zobel A, Kuraś M. 2003. Antimitotic effect, G2/M accumulation, chromosomal and ultrastructure changes in meristematic cells of Allium cepa L. root tips treated with the extract from Rhadiola rosea roots. Caryologia. 56:337–351.10.1080/00087114.2003.10589343
- Mukunthan KS, Balaji S. 2012. Silver nanoparticles shoot up from the root of Daucus carrota (L.). Int J Green Nanotechnol. 4:54–61.10.1080/19430892.2012.654745
- Mulvaney P. 1996. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 12:788–800. doi:10.1021/la9502711.
- Nielsen MH, Rank J. 1994. Screening of toxicity and genotoxicity in wastewater using the Allium test. Hereditas. 121:249–254.
- Nowack B, Bucheli TD. 2007. Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut. 150:5–22.
- Nowack B, Bucheli TD. 2007. Occurrence, behavior and effects of nanoparticles in the environment. Environ Poll. 150:5–22.10.1016/j.envpol.2007.06.006
- Oladipo IC, Lateef A, Elegbede JA, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Gueguim-Kana EB, Beukes LS, Oluyide TO, Atanda OR. 2017a. Enterococcus species for the one-pot biofabrication of gold nanoparticles: characterization and nanobiotechnological applications. J Photochem Photobiol, B: Biology. 173:250–257.10.1016/j.jphotobiol.2017.06.003
- Oladipo IC, Lateef A, Azeez MA, Asafa TB, Yekeen TA, Akinboro A, Akinwale AS, Gueguim-Kana EB, Beukes LS. 2017b. Green synthesis and antimicrobial activities of silver nanoparticles using cell free-extracts of Enterococcus species. Not Sci Biol. 9(2):196–203.
- Oyeyemi IT, Bakare AA. 2013. Genotoxic and anti-genotoxic effect of aqueous extracts of Spondias mombin L., Nymphea lotus L. and Luffa cylindrica L. on Allium cepa root tip cells. Caryologia: Inter J Cytol Cytosystem Cytogene. 66(4):360–367.10.1080/00087114.2013.857829
- Pakrashi S, Jain N, Dalai S, Jayakumar J, Chandrasekaran PT, Raichur AM, Chandrasekaran N, Mukherjee A. 2014. In Vivo genotoxicity assessment of Titanium dioxide nanoparticles by Allium cepa root tip assay at high exposure concentrations. PLOS ONE. 9(2):e87789. doi:10.1371/journal.pone.0087789.
- Patil BC, Bhat GI. 1992. A comparative study of MH and EMS in the induction ofchoromosomal aberrations on lateral root meristem in Clitoria aternata L. Cytologia. 57:259–264.10.1508/cytologia.57.259
- Patlolla AK, Berry A, May L, Tchounwou PB. 2012. Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. Int J Environ Res Public Health. 9:1649–1662.10.3390/ijerph9051649
- Pesnya DS. 2013 Cytogenetic effects of chitosan-capped silver nanoparticles in the Allium cepa test. Caryologia: Int J Cytol Cytosystematics Cytogenetics, 66 (3): 275–281.10.1080/00087114.2013.852342
- Prokhorova IM, Kovaleva MI, Fomicheva AN, Babanazarova OV. 2008. Spatial and temporal dynamics of mutagenic activity of water in lake Nero. Inland Water Biol. 1(3):1–25.
- Rajeshwari A, Kavitha S, Alex SA, Kumar D, Mukherjee A, Chandrasekaran N, Mukherjee A. 2015. Cytotoxicity of aluminum oxide nanoparticles on Allium cepa root tip—effects of oxidative stress generation and bio-uptake. Environ Sci Pollut Res. 22:11057–11066.10.1007/s11356-015-4355-4
- Rank J, Neilsen MH. 1998. Genotoxicity testing of wastewater sludge using the Allium cepa anaphase-telophase chromosome aberration assay. Mut Res. 418:113–119.10.1016/S1383-5718(98)00118-1
- Rank J, Nielsen MH. 1993. A modified Allium test as a tool in the screening of the genotoxicity of complex mixtures. Hereditas. 18:49–53.
- Reeves JF, Davies FS, Dodd NJF, Jha AN. 2008. Hydroxyl radicals (OH) are associated with titanium dioxide (TiO2) nanoparticle-induced cytotoxicity and oxidative DNA damage in fish cells. Mutat Res. 640(1-2):113–122.10.1016/j.mrfmmm.2007.12.010
- Shaligram NS, Bule M, Bhambure R, Singhal RS, Singh SK, Szakacs G, Pandey A. 2009. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 44:939–943.10.1016/j.procbio.2009.04.009
- Shameli K, Ahmad MB, Zargar M, Yunus W, Ibrahim WMZ, Sha-banzadeh NA, Ghaffari-Moghadam PM. 2011. Synthesis and characterization of silver/montmorillonite/chitosan bionanocomposites by chemical reduction method and their antibacterial activity. Int J Nanomed. 6:271–284.10.2147/IJN
- Shankar S, Jaiswal L, Aparna RSL, Prasad RGSV. 2014. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater Lett. 137:75–78.10.1016/j.matlet.2014.08.122
- Sharma AK, Sharma A. 1980. Chromosomes techniques theory and practice. 3rd ed. London: Butterworth.
- Shrivastava S, Bera T, Singh SK, Singh G, Ramachandrarao P, Dash D. 2009. Characterization of antiplatelet properties of silver nanoparticles. ACS Nano. 3(6):1357–1364.10.1021/nn900277t
- Sobieh SS, Rushdy AA, Yakob NAN 2016. In vitro and in vivo genotoxicity and molecular response of silver nanoparticles on different biological model systems. Caryologia: Int J Cytol Cytosystematics Cytogenetics 69(2):1–15.
- Sudhakar R, Gowda KNN, Venu G. 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 66:235–239.10.1508/cytologia.66.235
- Wu SG, Huang L, Head J, Chen D, Kong I, Tang YJ. 2012. Phytotoxicity of metal oxide nanoparticles is related to both dissolved metal ions and adsorption of particles on seed surfaces. Pet Environ Biotechnol. 3:1–6.
- Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ 2012. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 12(8):4271–4275.10.1021/nl301934w
- Yekeen TA, Adeboye MK. 2013. Cytogenotoxic effects of cypermethrin, deltamethrin, lambdacyhalothrin and endosulfan pesticides on Allium cepa root cells. Afri J Biotechnol. 12(41):6000–6006.10.5897/AJB
- Yekeen TA, Adetiba OA, Azeez MA, Falodun MA, Akintaro SI, Yekeen TA. 2011. Studies on the proximate analysis of Solanum aethiopicum, Lactuca taraxacifolia and Talinum triangulare and potential cytotoxic effects of their aqueous extract using Allium cepa assay. Anal of Biol Res. 2(5):696–706.
- Yekeen TA, Ayandele AA, Akinboro A, Onipede AY, Alawode R. 2013a. Assessment of toxicity and microbial attributes of synthetic Epoxy Resin and Acetaminophen effluents. Pollu Res. 32(4):9–16.
- Yekeen TA, Akintaro OI, Akinboro A, Azeez MA. 2013b. Evaluation of cytogenotoxic and nutrient composition of three commonly consumed vegetables in south west Nigeria. Afri J Food SciNutrit Develop. 13(2):7452–7466.
- Yekeen TA, Xu X, Zhang Y, Wu Y, Kim S, Reponen T, Dietrich KN, Ho S, Chen A, Huo X. 2016. Assessment of health risk of trace metal pollution in surface soil and road dust from e-waste recycling area in China. Environ Sci Pollut Res. 23(17):17511–17524. doi:10.1007/s11356-016-6896-6.
- Yekeen TA, Azeez MA, Akinboro A, Lateef A, Asafa TB, Oladipo IC, Oladokun SO, Ajibola AA. 2017. Safety evaluation of green synthesized Cola nitida pod, seed and seed shell extracts-mediated silver nanoparticles (AgNPs) using an Allium cepa assay. J Taibah Univ Sci. doi:10.1016/j.jtusci.2017.06.005.
- Zaki S, El-Kady MF, Abd-El-Haleem D. 2011. Biosynthesis and structural characterization of silver nanoparticles from bacterial isolates. Mater Res Bull. 46:1571–1576.10.1016/j.materresbull.2011.06.025