Abstract
The potential genotoxic influences of fipronil insecticide were analyzed by studying mitotic index and phases, chromosomal abnormalities, and micronucleus percentage on the somatic cells of Allium cepa L. The roots were treated with 1, 2.5, 5 and 10 ppm of fipronil insecticide within 6, 12 and 24 h. Mitotic index was clearly diminished by fipronil in each treatment group in comparison with control. The percentages of mitotic stages have been significantly affected. Fipronil markedly enhanced the abnormality cell percentage in almost all of the concentrations and treatment times compared to the control. Chromosomal abnormalities were recorded as disturbed prophase, sticky, chromatid bridges, c-mitosis, and laggards. The micronucleus was found at interphase and its frequency was calculated at each concentration and in the control. Generally, micronucleus formation augmented with increasing concentration of fipronil as compared to control. Consequently, the genotoxic potency of fipronil insecticide with different assessments was evaluated by using the somatic chromosomes of A. cepa, and the use of a defined non-toxic dose was suggested.
Introduction
Pesticides are used in agricultural areas to avoid losses resulting from pests (Karaismailoglu et al. Citation2013; Karaismailoglu Citation2015). Pesticides and products contaminated with them permanently damage living systems with the risk of mutagenicity and teratogenicity (Bag Citation2000; Gupta Citation2004; Liman et al. Citation2011; Karaismailoglu Citation2015; Kuchy et al. Citation2015). The genotoxic potential of the different pesticides and environmental pollutants have been widely assessed with various plant and animal bioassays (Grant et al. Citation1960; Sta et al. Citation2012; Karaismailoglu Citation2014a; Kuchy et al. Citation2015). Pesticides can be highly sensitive complexes, which can link covalent bonds with nucleophilic midpoints of cellular molecules such as DNA (Crosby Citation1982). The use of pesticides may cause adverse effects on human health (Ji et al. Citation2001).
Fipronil is a selective phenylpyrazole insecticide, used in Turkey to control pests such as Agriotes sp. in corn and sunflower agricultural areas at 2.5–5 ppm concentrations ([MARA] Ministry of Agricultural & Rural Affairs (Turkey) Citation2009; EPA Citation2011). Fipronil is a highly active molecule and has a detrimental effect on the nervous system of invertebrates, blocking the uptake of ion in cells and causing hyperexcitation and cell death (Rhone-Poulene Citation1995; Çelik et al. Citation2014). Some previous studies showed that fipronil leads to production of reactive oxygen species in cells, which can cause enlarged lipid peroxidation and oxidative stress in living organisms (Möhler et al. Citation2004; Ki et al. Citation2012; Stefani Margarido et al. Citation2013; Gripp et al. Citation2017). Investigations in rats indicate that fipronil is rapidly metabolized, and is harmful to tissue (Hugnet et al. Citation1999). In addition, it can lead to hyperactivity, convulsions and death in fish (Beggel et al. Citation2012). It is lethal for crustaceans and insects (Yildirim & Agar Citation2016). Several studies including various toxicological analyses have indicated that fipronil insecticide has genotoxic and mutagenic effects (Tisch et al. Citation2007; Patricia et al. Citation2012; Qureshi et al. Citation2016; Yildirim & Agar Citation2016; Gripp et al. Citation2017).
Some plant assays can be used to study the genotoxic effects of chemicals in living systems (Singh et al. Citation2008), e.g. by investigating mitotic index and phase, chromosomal abnormalities, and micronucleus percentage in the somatic cells. The most widely used plants are Allium cepa (Turkoglu Citation2009; Karaismailoglu Citation2015; Verma et al. Citation2016; de Souza et al. Citation2017), Tradescantia pallida (de Souza et al. Citation2017), Vicia faba (Khadra et al. Citation2012; Yildirim & Agar Citation2016) and Helianthus annuus (Karaismailoglu et al. Citation2013). In particular, A. cepa is one of the most widely used plant materials in genotoxicity experiments, because this plant is very susceptible and dependable in comparison with the other plant systems (Grant Citation1994; Karaismailoglu Citation2015). Thus, it has been accepted as an indicator organism for bio-monitoring of environmental pollutants (Liu et al. Citation1992; Ateeq et al. Citation2002; Kuchy et al. Citation2015).
The target of this investigation was to determine the potential genotoxic effects of the insecticide fipronil, by investigating mitotic index and phases, chromosomal abnormalities, and micronucleus percentage in the somatic cells of Allium cepa L.
Materials and methods
Onions (2n = 16) were obtained from a commercial market. Fipronil [(RS)-5-amino-1-(2,6-dichloro-a,a,a-trifluoro-p-toly)-4-trifluoro methylsulfinylpyrazole-3-carbonitrile; molecular weight: 437.2 g/mol CAS No: 20068–37-3] (EPA Citation2011) was obtained from a retail outlet and utilized as stock solution for experimental tests.
Bulbs were treated with only distilled water until reaching 1 cm in length. After, they were treated with 1, 2.5, 5, and 10 ppm fipronil concentrations, at 6, 12 and 24 h application periods. Five bulbs were used for each treatment and 25 roots were evaluated per bulb. Tests were made at 22°C in the dark in order to minimize fluctuations in cell division. In preparation of the application concentration were based on the used dosage of fipronil in agriculture, and its multiples ([MARA] Ministry of Agricultural & Rural Affairs (Turkey) Citation2009). Distilled water was used as a control. Root tips were fixed in ethanol:glacial acetic acid (3:1) and stored at 4°C overnight. Afterwards, they were hydrolyzed in 5 N HCl at 25°C for 30 min and dyed by Schiff’s reagent for 2 h. Five slides were determined randomly from each treatment group for the genotoxic analysis and mitotic index (MI), micronucleus (MN) in interphase, and chromosome aberrations such as disturbed prophase, chromatid bridge, stickiness, c-mitosis, and laggards were investigated in the dividing cells (Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014a, Citation2014b).
The obtained data are analyzed using analysis of variance (ANOVA) with the SPSS Inc. (Citation2008) computer program and a significance level of p < 0.05. Dunnett’s multiple range test was used for the definition of statistical importance among the monitored differences. The outcomes of statistical analyses are given in Tables .
Table 1. Effects of the applied fipronil concentrations and control on mitotic cell division in A. cepa.
Table 2. Chromosomal abnormalities in the root tips of A. cepa exposed to concentrations of fipronil and control.
Table 3. The effects of fipronil insecticide on the micronucleus assay.
Results
The influence of fipronil insecticide on the MI and the frequency of mitotic phases are presented in Table . Generally, all concentrations of fipronil markedly decreased the MI of A. cepa somatic chromosomes compared to the control (p < 0.05). Exceptionally, the MI values between the application groups and the control group is statistically insignificant in 1 ppm fipronil applications at all treatment periods. There were clear distinctions in MI of A. cepa root tips treating concentrations of fipronil when compared to the control (p < 0.05) (Table ). As a result of 10 ppm fipronil application, MI substantially declined at each application period (Table ). When mitotic phase frequencies were compared with the control in application periods, there were statistically significant results as well (p < 0.05). Almost all of the treatments significantly influenced the mitotic phase frequencies (Table ). As the percentage of anaphase-telophase rise, the frequencies of metaphase and prophase decreased in most of the analyzed cells. A significant increase in the frequencies of abnormalities in phases with increasing fipronil concentrations was determined (Table ).
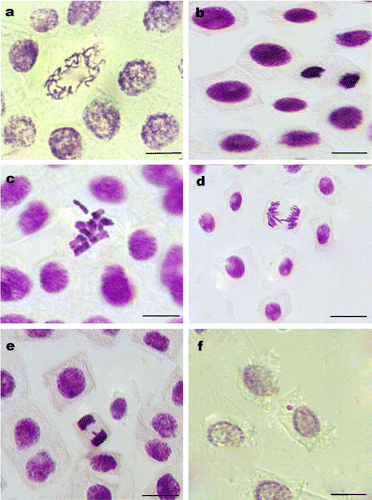
Chromosome abnormalities were studied in mitotic phases and their types and percentages are demonstrated in Table and Figure . The used fipronil concentrations caused five types of abnormalities: disturbed prophase, c-mitosis, sticky, laggards, and chromatid bridges.
Figure 1. Chromosomal abnormalities induced with fipronil in root meristem cells of A. cepa: (a) disturbed prophase; (b) stickiness; (c) c-mitosis; (d) chromatid bridge; (e) laggards; (f) micronucleus. Scale bars = 10 μm.
The outcomes of the micronucleus test in A. cepa root tips exposed to control group and concentrations of fipronil are given in Table and Figure . The percentage of micronucleus induction was found to be dependent on the application dose and determine to be significantly distinct in all treatments in comparison with the control (p < 0.05), with the exception of the 1 ppm treatment. It was clearly higher at 10 ppm than the other applications of fipronil in all the treatments (Table ). However, at 6, 12 and 24 h, applications of 2.5 and 5 ppm concentrations of fipronil markedly increased the MN frequency.
Discussion
Random or uncontrolled use of chemical agents causes environmental pollution and negative effects on living systems. Therefore, evaluation of the effects of fipronil insecticide and its impact on mitotic index and chromosomes provide useful information regarding the effects of these mostly treated toxic materials (Karaismailoglu Citation2015).
Mitotic index can be used as a biological signal of cell increment, which calculates the ratio of cells in various mitotic phases (Yuet Ping et al. Citation2012). The impacts of different concentrations of fipronil on MI in A. cepa somatic cells are given in Table . Mitotic effect decreased clearly with increased fipronil solutions at each application periods, as compared with the controls (p < 0.05) (Table ). Furthermore, at concentrations of 1 ppm fipronil, MI was not significantly different to the control at almost all the treatment periods (p < 0.05) (Table ). The most genotoxic dose of fipronil is 10 ppm; this has a more mitodepressive effect than 1, 2.5 and 5 ppm applications at all application periods.
If the MI rate declines below 22% of the control, this status induces lethal impacts on living systems. Reductions under 50% are of sublethal impact and are referred to as the genotoxicity limit value (Panda & Sahu Citation1985; Karaismailoglu et al. Citation2013; Karaismailoglu Citation2014b, Citation2015). In this investigation, sublethal impact was determined at 10 ppm as compared to control in 6, 12 and 24 h treatments, and sublethal impacts values were found as 24.25, 37.22 and 41.04%, respectively. These results are compatible with outcomes obtained from prior works, which include toxic effects of various pesticides (Liman et al. Citation2011; Karaismailoglu Citation2013, Citation2014a).
The mitodepressive effects of fipronil insecticide can negatively affect the mitotic cycle and hence prevent cells entering prophase and stop the mitosis cycle in interphase (Badr Citation1986; Kuchy et al. Citation2015). This reduction in MI owing to environmental substances may be due to a block on DNA/protein synthesis of the living organisms (Elghamery et al. Citation2000). de Oliveira et al. (Citation2012) stated that concentrations of fipronil may cause DNA damage on mice.
The reductions in MI may be in the consequence of cells not advancing from the synthesis stage to the mitosis phase of the cell cycle (Patlolla et al. Citation2012), or because of blocked DNA synthesis (Sudhakar et al. Citation2001; Karaismailoglu Citation2014b, Citation2015) after fipronil insecticide applications. Besides, notable reductions in the number of mitosis with the effect of some metallic insecticides have been shown in the some toxicity tests used A. cepa (Akdeniz and Özmen Citation2011; de Souza et al. Citation2017). Pyrethroid pesticides in other works were found to have genotoxic potency in rodent bone marrow (Agarwal et al. Citation1994), in peripheral lymphocyte cultures (Surralles et al. Citation1990) and in aquatic organisms (Campana et al. Citation1999).
The influences of the test solutions on stages of the cell cycle in A. cepa somatic cells are given in Table . As the stage frequencies are compared with the control in all application periods, there are statistically important results (p < 0.05). As can be seen Table , the rates of the mitotic stages were clearly impacted in all treatments (p < 0.05). As the rates of prophase and metaphase diminished, ana-telophase was enhanced in the examined somatic cells. The influences on mitotic stages of fipronil solutions might be connected to obstacles in prophase or retention in the mitotic stages in answer to mitotic stress (Liman et al. Citation2011). In addition, applied doses of fipronil generally caused a significant increment in the percentages of abnormalities in mitotic stages in onion somatic cells (p < 0.05). Similar outcomes have been found in previous works, which identified negative influences of some pesticides on cell cycles (Kaymak & Goc Rasgele Citation2009; Karaismailoglu Citation2014a, Citation2014b, Citation2015, Citation2016, Citation2017).
Chromosome abnormalities may be used to study genotoxic influences of pesticides (Caritá & Marin-Morales Citation2008). The impacts of fipronil solutions and the control applications on mitotic anomalies and rates of total anomalies in onion somatic cells are presented in Table . Chromosomal abnormalities were recorded as disturbed prophase, c-mitosis, stickiness, chromatid bridges and laggards, and demonstrated in Figure . The most frequently seen type of chromosomal anomaly was sticky chromosomes, which can result from deterioration in DNA (Mercykutty & Stephen Citation1980). Also, c-mitosis may occur with preventing spindle formation like colchicines effect (Badr Citation1983). Another common anomaly is chromatid bridges, which form with breaks and fusion of chromatins (Shehab & Adam Citation1983). Disturbed prophase forms as a result of chromatin erosion. Stickiness, chromatid bridges and disturbed prophase are genotoxic effects and irreversible (Karaismailoğlu Citation2015), and they may cause cellular death. However, laggards are formed by the failure of spindle fiber structure, and indicate a low genotoxic impact and can be recycled (Fiskesjö Citation1985). Increased concentrations of fipronil induced increased rates of total anomalies. With the 10 ppm treatment for each application period, genotoxicity was more than expected. This study showed that fipronil induces cell inhibition, and chromosomal, numerical, and structural abnormalities, ranging from chromosome destruction to the disorder of the mitotic spindle. These outcomes are in agreement with prior studies (Türkoğlu Citation2012; Yuet Ping et al. Citation2012; Karaismailoglu Citation2015).
Micronucleus assays have a key role in evaluation of the toxicity influences of pesticides (Gebel et al. Citation1997; Karaismailoglu Citation2013, Citation2014a, Citation2014b). MN formation and its frequencies in treated groups are presented in Table and Figure . The MN rate clearly increased with enhanced fipronil concentrations in comparison with the control groups, with the exception of 1 ppm at all treatment periods (p < 0.05). The MN rate was clearly higher at the highest fipronil dose (10 ppm) than at others. MN forms with fractures of the microtubules and results in impairments at ploidy situations (like poly or aneu-ploidy) owing to breaks in chromosomes (Konuk et al. Citation2007; Karaismailoglu Citation2015). MN may also occur due to single-strands breaks in DNA by fipronil insecticide (Qureshi et al. Citation2016)
Consequently, exposure to fipronil because of reduced frequency of mitotic index, leading to the formation of chromosomal aberrations and micronuclei. While this insecticide reduces loss of product and enhances output in agricultural fields, it is not practical to hold it back fully. This paper finds that if fipronil is utilized in concentrations below 2.5 ppm, the genotoxic effects on A. cepa would be decreased.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Agarwal DK, Chauhan LKS, Gupta SK, Sundararaman V. 1994. Cytogenetic effects of deltamethrin on rat bone marrow. Mutat Res. 311:133–138. 10.1016/0027-5107(94)90081-7
- Akdeniz D, Özmen A. 2011. Antimitotic effects of the biopesticide oxymatrine. Caryologia. 64:117–120. 10.1080/00087114.2011.10589771
- Ateeq B, Abul Farah MA, Niamat Ali M, Ahmad W. 2002. Clastogenicity of pentachlorophenol, 2,4-D and butachor evaluated by Allium root tip test. Mutat Res. 514:105–113. 10.1016/S1383-5718(01)00327-8
- Badr A. 1983. Mitodepressive and chromotoxic activities of two herbicides in A. cepa. Cytologıa. 48(3):451–457. 10.1508/cytologia.48.451
- Badr A. 1986. Effect of the s-triazine herbicide terbutryn on mitosis chromosomes and nucleic acids in root tips of Vicia faba. Cytologıa. 51:571–577. 10.1508/cytologia.51.571
- Bag D. 2000. Pesticides and health risks. Econ Polit Wkly. 6:20–21.
- Beggel S, Werner I, Connon RE, Geist JP. 2012. Impacts of the phenylpyrazole insecticide fipronil on larval fish: Time-series gene transcription responses in fathead minnow (Pimephales promelas) following short-term exposure. Sci Total Environ. 426:160–165. 10.1016/j.scitotenv.2012.04.005
- Campana MA, Panzeri AM, Moreno VJ, Dulout FN. 1999. Genotoxic evaluation of the pyrethroid lambda-cyhalothrin using the micronucleus test in erytrocytes of fish Cheirodon interruptus. Mut Res. 438:155–161. 10.1016/S1383-5718(98)00167-3
- Caritá R, Marin-Morales MA. 2008. Induction of chromosomeaberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere. 72(5):722–725. 10.1016/j.chemosphere.2008.03.056
- Çelik A, Ekinci SY, Güler G, Yildirim S. 2014. In Vitro genotoxicity of fipronil sister chromatid exchange, cytokinesis block micronucleus test, and comet assay. DNA Cell Biol. 33(3):148–154. 10.1089/dna.2013.2158
- Crosby DG. 1982. Pesticides as environmental mutagens. In: Fleck RA, Hollander A, editors. Genetic toxicology: an agricultural perspective. London: Plenum Press, New York; p. 201–218.
- de Oliveira PR, Bechara GH, Denardi SE, Oliveira RJ, Mathias MI. 2012. Genotoxic and mutagenic effects of fipronil on mice. Exp Toxicol Pathol. 64(6):569–573. 10.1016/j.etp.2010.11.015
- de Souza RP, de Souza CP, Bueno OC, Fontanetti CS. 2017. Genotoxicity evaluation of two metallic-insecticides using Allium cepa and Tradescantia pallida: a new alternative against leaf-cutting ants. Chemosphere. 168:1093–1099. 10.1016/j.chemosphere.2016.10.098
- Elghamery AA, Elnahas AI, Mansour MM. 2000. The action of atrazine herbicide as an inhibitor of cell division on chromosomes and nucleic acids content in root meristems of Allium cepa and Vicia faba. Cytologia. 55:209–215.
- EPA 2011. Fipronil Summary Document Registration Review. Initial Docket June 201, EPA-HQ-OPP-2011-0448.
- Fiskesjö G. 1985. The Allium test as a standard in environmental monitoring. Hereditas. 112(1):99–112.
- Gebel T, Kevekordes S, Pav K, Edenharder R, Dunkelberg H. 1997. In vivo genotoxicity of selected herbicides in the mouse bone-marrow micronucleus test. Arch Toxicol. 71(3):193–197. 10.1007/s002040050375
- Grant WF. 1994. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat Res. 310(2):175–185. 10.1016/0027-5107(94)90112-0
- Grant WL, Lee HG, Harney PM. 1960. Cytogenetic effects of malic hydrazide treatment of tomato seed. Call J Gellet Cytol. 2:162–174.
- Gripp HS, Freitas JS, Almeida EA, Bisinoti MC, Moreira AB. 2017. Biochemical effects of fipronil and its metabolites on lipid peroxidation and enzymatic antioxidant defense in tadpoles (Eupemphix nattereri: Leiuperidae). Ecotoxicol Environ Saf. 136:173–179. 10.1016/j.ecoenv.2016.10.027
- Gupta P. 2004. Pesticide exposure-Indian scene. J Technol. 198:118–119.
- Hugnet C, Cadore JL, Bourdoiseau G. 1999. Use of fipronil spray (0.25%) for the treatment of Damalinia equi. Prat Vet Equine. 31:65–68.
- Ji BT, Silverman DT, Stewart PA. 2001. Occupational exposure to pesticides and pancreatic cancer. Am J Ind Med. 39:92–99. 10.1002/(ISSN)1097-0274
- Karaismailoglu MC. 2013. Deltamethrin ve quizalofop-p-etil pestisitlerinin Helianthus annuus L. (Ayçiçeği) kök ucu hücreleri üzerine mutajenik etkilerinin araştırılması [MSc Thesis]. Trabzon: Karadeniz Technical University.
- Karaismailoglu MC. 2014a. Investigation of the cytotoxic and genotoxic effects of Artemisia annua methanol extract with the allium test. Ekoloji. 23(91):64–74. 10.5053/ekoloji
- Karaismailoglu MC. 2014b. Evaluation of potential genotoxic effect of trifluralin in Helianthus annuus L. (sunflower). Caryologia. 67(3):216–221. 10.1080/0144235X.2014.974348
- Karaismailoglu MC. 2015. Investigation of the potential toxic effects of prometryne herbicide on Allium cepa root tip cells with mitotic activity, chromosome aberration, micronucleus frequency, nuclear DNA amount and comet assay. Caryologia. 68(4):323–329. 10.1080/00087114.2015.1109927
- Karaismailoglu MC. 2016. The evaluation of the genotoxic and cytotoxic effects of pyriproxyfen insecticide on Allium cepa somatic chromosomes with mitotic activity, chromosome abnormality, and micronucleus frequency. Turk J Life Sci. 1(2):065–069.
- Karaismailoglu MC. 2017. Investigation of the antimitotic and antimutagenic effects of methanolic extracts of Pyracantha coccinea. Turk J Life Sci. 2(1):110–116.
- Karaismailoglu MC, Inceer H, Hayırlıoglu-Ayaz S. 2013. Effects of Quizalofop-p-Ethyl Herbicide on the somatic chromosomes of Helianthus annuus (Sunflower). Ekoloji. 22(89):49–56.10.5053/ekoloji
- Kaymak F, Goc Rasgele P. 2009. Genotoxic effects of raxil on root tips and anthers of Allium cepa L. Caryologia. 62(1):1–9.
- Khadra A, Pinelli E, Lacroix MZ, Bousquet-Melou A, Hamdi H, Merlina G, Guiresse M, Hafidi M. 2012. Assessment of the genotoxicity of quinolone and fluoroquinolones contaminated soil with the Vicia faba micronucleus test. Ecotoxicol Environ Safe. 76(2):187–192. 10.1016/j.ecoenv.2011.10.012
- Ki YW, Lee JE, Park JH, Shin IC, Koh HC. 2012. Reactive oxygen species and mitogen-activated protein kinase induce apoptotic death of SH-SY5Y cells in response to fipronil. Toxicol Lett. 211:18–28. 10.1016/j.toxlet.2012.02.022
- Konuk M, Liman R, Cigerci IH. 2007. Determination of genotoxic effect of Boron on Allium cepa root meristematic cells. Pak J Bot. 39(1):73–79.
- Kuchy AH, Wani AA, Kamili AN. 2015. Cytogenetic effects of three commercially formulated pesticides on somatic and germ cells of Allium cepa. Environ Sci Pollut Res. 23:895–906.
- Liman R, Ciğerci İH, Akyıl D, Eren Y, Konuk M. 2011. Determination of genotoxicity of fenaminosulf by Allium and comet tests. Pesticide Biochem Physiol. 99(1):61–64. 10.1016/j.pestbp.2010.10.006
- Liu D, Jiang W, Li M. 1992. Effects of trivalent and hexavalent chromium on root growth and cell division of Allium cepa. Hereditas. 117:23–29.
- [MARA] Ministry of Agricultural and Rural Affairs (Turkey). 2009. General directorate of protection and control; plant protection products. Ankara: Ministry of Agricultural and Rural Affairs of Republic of Turkey.
- Mercykutty VC, Stephen J. 1980. Adriamycin induced genetic toxicity as demonstrated by Allium cepa test. Cytologıa. 45(4):769–777. 10.1508/cytologia.45.769
- Möhler H, Fritschy JM, Crestani F, Hensch T, Rudolph U. 2004. Specific GABA(A) circuits in brain development and therapy. Biochem Pharmacol. 68:1685–1690. 10.1016/j.bcp.2004.07.025
- Panda BB, Sahu UK. 1985. Induction of abnormal spindle function and cytokinesis inhibition in mitotic cells of Allium cepa by the organophosphorus insecticide fensulfothion. Cytobios. 42(167/168):147–155.
- Patlolla AK, Berry A, May LB, Tchounwou PB. 2012. Genotoxicity of silver nanoparticles in Vicia faba: a pilot study on the environmental monitoring of nanoparticles. Int J Environ Res Public Health. 9(12):1649–1662. 10.3390/ijerph9051649
- Patricia RO, Gervasio HB, Sandra ED. 2012. Genotoxic and mutagenic effects of fipronil on mice. Exp Toxicol Pathol. 64:569–573.
- Qureshi IZ, Bibi A, Shahid S, Ghazanfar M. 2016. Exposure to sub-acute doses of fipronil and buprofezin in combination or alone induces biochemical, hematological, histopathological, and genotoxic damage in common carp (Cyprinus carpio L.). Aquat Toxicol. 179:103–114. 10.1016/j.aquatox.2016.08.012
- Rhoˆne-Poulenc. 1995. Fipronil worldwide technical bulletin. Lyon: Rhoˆne-Poulenc Agrochimie.
- Shehab AS, Adam ZM. 1983. Cytological effects of medicinal plants in Qatar III. Mitotic effect of water extract of Anastatica hierochuntico L. on Allium cepa. Cytologıa. 48:343–348. 10.1508/cytologia.48.343
- Singh P, Srivastava AK, Singh AK. 2008. Cell cycle stage specific application of cypermethrin and carbendazim to assess the genotoxicity in somatic cells of Hordeum vulgare L. Bull Environ Contam Toxicol. 81(3):258–261.
- SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago, IL: SPSS Inc.
- Sta C, Ledoigt G, Ferjani E, Goupil P. 2012. Exposure of Vicia faba to sulcotrione pesticide induced genotoxicity. Pestic Biochem Physiol. 103:9–14. 10.1016/j.pestbp.2012.02.002
- Stefani Margarido TC, Felício AA, de Cerqueira Rossa-Feres D, Alves de Almeida EA. 2013. Biochemical biomarkers in Scinax fuscovarius tadpoles exposed to a commercial formulation of the pesticide fipronil. Mar Environ Res. 91:61–67. 10.1016/j.marenvres.2013.02.001
- Sudhakar R, Gowda KN, Venu G. 2001. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologıa. 66(3):235–239. 10.1508/cytologia.66.235
- Surralles J, Carboneil E, Puig M, Xamena N, Creus A, Marcos R. 1990. Induction of mitotic micronuclei by fenvalerate in cultured human lymphocytes. Toxicol Lett. 54:151–155. 10.1016/0378-4274(90)90178-O
- Tisch M, Faulde M, Maier H. 2007. Genotoxic effects of insecticides in current use on mucosal epithelial cells from human tonsil tissue. Hals-Nasen-Ohrenheilkunde. 55:15–22.
- Turkoglu S. 2009. Genotoxic effects of mono-, di-, and trisodium phosphate on mitotic activity DNA content, and nuclear volume in Allium cepa L. Caryologia. 62(3):171–179.
- Türkoğlu Şifa. 2012. Determination of genotoxic effects of chlorfenvinphos and fenbuconazole in Allium cepa root cells by mitotic activity, chromosome aberration, DNA content, and comet assay. Pesticide Biochem Physiol. 103(3):224–230. 10.1016/j.pestbp.2012.06.001
- Verma S, Arora K, Srivastava A. 2016. Monitoring of genotoxic risks of nitrogen fertilizers by Allium cepa L. mitosis bioassay. Caryologia. 69:343–343.
- Yildirim N, Agar G. 2016. Determination of genotoxic effects of fipronil in Vicia faba using random amplified polymorphic DNA analysis. Toxicol Ind Health. 32(8):1450–1455. 10.1177/0748233714564416
- Yuet Ping KY, Darah I, Yusuf UK, Yeng C, Sasidharan S. 2012. Genotoxicity of Euphorbia hirta: an Allium cepa assay. Molecules. 17(12):7782–7791. 10.3390/molecules17077782
