Abstract
A pot scale trial investigated the agronomic performance of two organomineral fertilizers (OMF15—15:4:4 and OMF10—10:4:4) in comparison with urea and biosolids granules to establish ryegrass (Lolium perenne L.). Two soils of contrasting characteristics and nitrogen (N) application rates in the range of 0–300 kg ha−1 were used over a period of 3 years. Fertilizer effects were determined on: (1) dry matter yield (DMY) and crop responses, (2) nitrogen use efficiency (NUE), and (3) selected soil chemical properties. Ryegrass responded linearly (R2 ≥ 0.75; P < 0.001) to organomineral fertilizers (OMF) application increasing DMY by 2–27% compared with biosolids but to a lesser extent than urea (range: 17–55%). NUE was related to concentration of readily available N in the fertilizer: urea and OMF showed significantly (P < 0.05) greater N recoveries than biosolids. Total N in soil and soil organic matter showed increments (P < 0.05), which depended on the organic-N content in the fertilizer applied. Soil extractable P levels remained close to constant after 3 years of continuous OMF application but increased with biosolids and decreased with urea, respectively (P < 0.05). The application of biosolids changed soil P Index from 5 to 6; hence, there is a need to monitor soil P status. Both OMF10 and OMF15 formulations are suitable for application in ryegrass.
Introduction
The production of sewage sludge (biosolids) in England and Wales is approximately 1.6 million tonnes per year (DEFRA Citation2011). Presently, approximately two thirds of total sludge production is treated to standards suitable for application to farmland. The remaining amount is mainly disposed of through incineration and landfill (DEFRA Citation2011). Land application of biosolids is relatively less expensive compared with other disposal options, e.g., up to 30–40% less compared with incineration and landfill (Antille et al. Citation2013 with 2007 figures), and it is regarded as the best practicable environmental option in most circumstances (Edge Citation1999). Recycling biosolids to agriculture, however, presents some challenges that combine to restrain the agricultural route as well as the opportunities for increasing recycling targets in the longer term. Among these challenges are: variable chemical composition of biosolids (Sommers Citation1997), nutrient availability and concentration (Bowden and Hann Citation1997; O’Connor et al. Citation2004), relatively low nitrogen (N) to phosphorus (P) ratio (N:P ratio) which is often recognized as a significant factor affecting potential build-up of soil P (Hogan, McHugh, and Morton Citation2001; Antille et al. Citation2013), land-bank availability for recycling, farming practice, and soil P status (Moseley et al. Citation1998, Skinner and Todd Citation1998), and farmers’ perception of biosolids regarding its fertilizing value, ease of handling and spreading (Antille, Gallar-Redondo, and Godwin Citation2013; Antille et al. Citation2013c). Build-up and downward movement of heavy metals in soils amended with sludge (Torri and Corrêa Citation2012), and risk of transfer onto the food chain (Jones and Johnston Citation1989) are also of concern.
By contrast, a number of opportunities to increase recycling of biosolids are also recognized. These arise from significant increments recorded in recent years in the price of mineral fertilizers, which combined with relatively weak grain prices have resulted in reduced profit margins to farmers (Heffer and Prud’homme Citation2013). Land application of biosolids offers the scope for reduced fertilizer inputs (Hogan, McHugh, and Morton Citation2001), but it is essential that wastewater companies can deliver improved quality products to enable for more efficient use of nutrients in biosolids applied to soil for crop production. Improvements of biosolids quality will contribute to minimize environmental concerns (Davis Citation1996), and secure the agricultural route for recycling (Antille et al. Citation2013). Technology exists for the production of biosolids-derived organomineral fertilizers (OMF) which are obtained by coating biosolids granules with urea (46% N) and potash [60% potassium oxide (K2O)] to provide a balanced compound fertilizer (Antille et al. Citation2013c). This type of OMF are available in two formulations, namely OMF15 (15:4:4) and OMF10 (10:4:4), and their physical properties have been defined to enable application with conventional fertilizer spreading equipment (Antille, Gallar-Redondo, and Godwin Citation2013; Antille et al. Citation2013). Such product development required determining the agronomic efficiency of OMF and associated effects of their use on soil fertility and long-term crop productivity. Therefore, the objectives of this study were to: (1) investigate dry matter yield (DMY) and responses of ryegrass (Lolium perenne L.) to application of OMF under semi-controlled environmental conditions in a glasshouse, (2) determine the effects of OMF on nitrogen uptake and nitrogen use efficiency (NUE), and (3) examine the effects of continuous application of OMF on selected soil chemical properties with particular regards to soil N and soil extractable P. It was hypothesized that OMF, when applied at the rates used in this study, will not induce changes in soil extractable P and not compromise on yield of ryegrass. The experimental data collected from this study provided background dataset, which was used to formulate fertilizer recommendations for a new product, such as OMF, to establish grass crops and nutrient management practices at larger scales in England.
Materials and Methods
Description of the Experiment
The studies were conducted in a glasshouse facility at Cranfield University (Bedford, England) using two soils of contrasting characteristics described in King (Citation1969) as Cottenham series sandy loam (67% sand, 13% clay, 17% silt) and Holdenby series clay loam (46% sand, 25% clay, 29% silt). The experiment used four fertilizer materials as follows: OMF15 (15:4:4), OMF10 (10:4:4) (Antille et al. Citation2013), urea (46% N), and biosolids granules (4.5:5.5:0.2). The fertilizers were applied at field rates equivalent to 0 (unfertilized control), 150, and 300 kg ha−1 of N. The experiment used pots of 10 L capacity filled with 8 kg of air-dried soil previously ground to pass a 2 mm sieve (Cornforth and Sinclair Citation1997). During the preparation of pots, soil was mixed with corresponding fertilizer material to conform a layer of 50 mm beneath ryegrass seeds (Lolium perenne L.) to avoid their direct contact with fertilizer. Subsequently, soil in the pot was packed to replicate (bulk) densities corresponding to those found in the field from which soils were taken. Bulk densities as determined in the field were 1.34 and 1.22 g cm−3 for sandy loam and clay loam soils, respectively (Antille, Sakrabani, and Godwin Citation2014a). Ryegrass seeds were spread on the soil surface at a rate equivalent to 4 g m−2. The soil types and grass crop were selected because of their often occurrence in the northwest region of England (Ragg et al. Citation1984), which was the area of interest for this study and where a leading wastewater company is based.
The experiment commenced on 27 April 2007 and it was conducted over a period of 3 years, referred to in the text as year one (Y1), two (Y2), and three (Y3), respectively. Germination was recorded on 7 May 2007. Water (pH = 7.03) was supplied by means of a drip irrigation system controlled by a timer and leaching was avoided at all times. In Y2 and Y3 of the experiment, the fertilizers were surface-applied. The experiment used a completely randomized design and all treatments, including controls, were setup in triplicate (n = 3).
Crop and Soil Measurements and Analyses
A total of three cuts were performed annually throughout the main growing season ().
Table 1 Timing of fertilizer application and corresponding date of harvest
The grass was cut at 20 mm above the soil surface (Cordovil, Cabral, and Coutinho Citation2007) and the harvested plant material was subsequently oven-dried at 60 °C for 48 h (MAFF 1986, Method No.: 1) for determination of DMY. A sub-sample of the oven-dried herbage was taken for determination of N in plant material from which N uptake (U) and NUE were derived. Nitrogen uptake corresponds to the cumulative value of three cuts conducted each year. NUE was calculated with Equation (1) (Baligar, Fageria, and He Citation2001):
where NUE is nitrogen use efficiency (kg kg−1), UF and UF=0 are nitrogen uptake (kg ha−1) of fertilizer treatment and control (zero-fertilizer), respectively, and NRate is the corresponding nitrogen application rate for the treatment (kg ha−1).
The soils were sampled prior to start of experiment (baseline level) and routinely thereafter following standard operating procedures. Soil sampling was performed to the full available depth of the pot (200 mm) by extracting three sub-samples. The following soil analyses were conducted: soil pH (MAFF Citation1986, Method No.: 32), soil organic matter (SOM) (MAFF Citation1986, Method No.: 56), soil mineral Nitrogen (SMN) (MAFF Citation1986, Method No.: 53), soil extractable P (BS7755 Section 3.6 Citation1995), soil exchangeable potassium (K) (MAFF Citation1986, Method No.: 63), total carbon (C) (BS7755 Section 3.8 Citation1995), and total N (BS13654-2 Citation2001). Soil P and K Indexes are based on DEFRA (Citation2010).
Statistical Analyses
Statistical analyses were undertaken using GenStat Release 14.1 (Citation2011) and included analysis of variance (ANOVA) and least significant differences (LSD) to compare means using a 5% probability level (P < 0.05). Repeated measurement of analysis of ANOVA was used to compare annual DMY data as well as DMY corresponding to individual cuts both within- and between-years. The same technique was applied to data corresponding to measured soil chemical properties. Grass responses to application of nitrogen were investigated using generalized linear models. Regression analyses were undertaken for annual (cumulative) DMY, which was the primary focus of this work.
Results
Initial Soil Analyses
shows results of soil chemical analyses conducted prior to the start of the experiment, which corresponded to the baseline level.
Table 2 Soil analyses conducted prior to the start of the experiment in the glasshouse. The standard deviation (SD) is shown as ± the mean value, except when n = 1. Soil P and K Indexes are based on DEFRA (Citation2010)
Dry Matter Yield
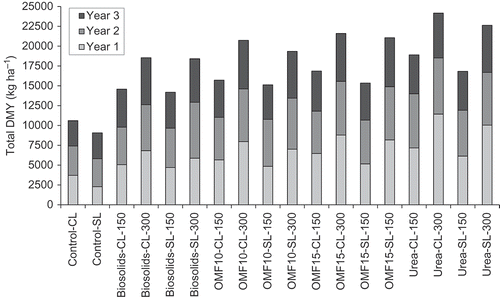
shows total DMY as recorded in years one (Y1) to three (Y3) of the experiment for both controls and treatments. In Y2 and Y3, residual effects of N applied as fertilizer in the previous year was determined at cut one, prior to fertilizer application in the corresponding year, by comparing DMY levels of fertilizer-treated grass. These comparisons showed no differences (P > 0.05) in DMY between treatments, which suggested that residual N effects on DMY associated with fertilizer type were negligible.
Figure 1. Dry matter yield (DMY) of ryegrass corresponding to controls and treatments as recorded in years one to three of the experiment. CL is clay loam and SL is sandy loam followed by corresponding N application rate in kg per ha. LSD (5% level) = 734.6 kg ha−1, P = 0.19, n = 3.
The responses of grass to application of fertilizer were linear (P-values < 0.001) for the range of N application rates investigated, and data showed acceptable fits to linear models (R2 ≥ 0.75). For every additional unit of N added, DMY showed increments (kg DM per kg of additional N) which were in the range of 7–12 kg kg−1 for biosolids, 8–16 kg kg−1 for OMF10, 8–20 kg kg−1 for OMF15, and 6.5–26 kg kg−1 for urea, depending on soil type and year. These responses, and differences between treatments, were smaller in Y2 and Y3 compared with Y1 possibly due to surface-application of fertilizer in those years. Overall, responses to OMF-N were about 10% higher in sandy loam compared with clay loam soil, as denoted by the slope of regression lines obtained. Enhanced response in sandy loam soil was expected given its relatively lower fertility status compared with clay loam soil (). There were significant differences (P-values < 0.001) in DMY with respect to fertilizer type, N application rate and the interaction fertilizer type × N rate. In Y1, on average across both N application rates, OMF10 and OMF15 increased DMY by about 13% and 28%, respectively, compared with biosolids whereas urea recorded an increase of about 55%. DMY increased with concentration of N in fertilizer applied, particularly, with readily available N fraction in the material, which explains significant interaction fertilizer type × N rate.
In Y2, there were significant differences in DMY between control and treatments which was also observed in Y3 (P < 0.001). In Y2, there were fertilizer type and N application rate effects on DMY (P < 0.05). The fertilizer type effect was observed in both soils (P < 0.05) and it was due to urea applied on clay loam soil at 150 kg ha−1 of N, which largely outperformed the other fertilizers materials applied at the same N rate (). OMF10 and OMF15 increased DMY by about 8% on average compared with biosolids granules whereas the increment recorded for urea was 17%.
In Y3, there were no effects of soil type, fertilizer type, or interaction soil type × fertilizer type (P-values > 0.05), but there was an effect of the N application rate (P < 0.05). Overall, comparisons between-years showed significant differences in DMY as a result of fertilizer type (P < 0.05). Over the 3 years period, there was a decline in DMY across all treatments (). Differences in DMY observed between-treatments became progressively smaller from the start of the experiment. In Y3, all fertilizer treatments exhibited similar DMY levels; mean values across the entire experiment ranged between 5178 and 5483 kg ha−1 (LSD 5% level = 367 kg ha−1). DMY levels recorded in biosolids-treated pots were generally lower but more sustained over the years compared with other treatments (range of 5613 kg ha−1 in Y1 to 5178 kg ha−1 in Y3). The interaction time × soil type was significant (P < 0.001), but the effect was largely due to high DMY levels recorded in Y1 across all fertilizer treatments in the clay loam soil (), that were associated with soil disturbance during the setup of the experiment and enhanced release of SMN ().
Nitrogen Uptake
Cumulative N uptake, as recorded in Y1 and Y2 of the experiment, is shown in . There were significant differences in N uptake between controls and treatments, fertilizer types, and N application rates (P-values < 0.05). In Y1 there was an effect fertilizer type × N rate (P < 0.05) which was not observed in Y2 (P > 0.05). Nitrogen uptake was influenced by concentration of available N in fertilizer, i.e., urea-treated grass yielded consistently higher N uptakes compared with other fertilizer treatments (). For OMF10 and OMF15, N uptakes in Y1 were on average approximately 20% and 40% higher compared with biosolids but about 25–35% lower than urea, respectively. Overall, N uptake was between 12% and 15% higher in OMF15 compared with OMF10. Regression analyses showed that N uptake was significantly correlated (P < 0.001, R2 ≥ 0.98) with N application rate, which was observed in all fertilizer treatments. For OMF10 and OMF15, for every additional unit of N applied, there were increments in N uptake of 0.4–0.5 kg kg−1 of N, compared to 0.8 and 0.3 kg kg−1 of N for urea and biosolids granules, respectively.
Figure 2. Nitrogen uptake by ryegrass corresponding to control and treatments as recorded in year one (top) and two (bottom) of the experiment, respectively. CL is clay loam and SL is sandy loam followed by corresponding N application rate in kg ha−1. LSD (5% level) = 17.8 kg ha−1, P < 0.05, n = 3.
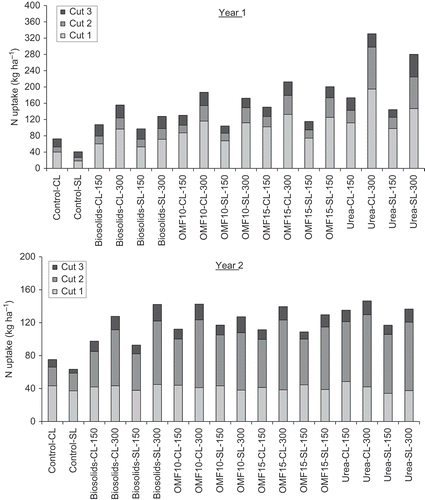
In Y2, OMF10 and OMF15 showed intermediate levels of N uptake (range: 110–135 kg ha−1) between biosolids (range: 97–128 kg ha−1) and urea (range: 126–140 kg ha−1). There were significant relationships (R2 ≥ 0.81; P < 0.001) between N uptake and N rate, but the slopes denoted lower increments (range: 0.20–0.25 kg kg−1 of N) compared with Y1. This suggested possible losses of N by volatilization of ammonia (NH3) in urea- and OMF-treated soils as a result of surface-application of fertilizers in Y2. The differences (P < 0.05) in N uptake recorded between-cuts indicated that N was mostly taken up between fertilizer application and subsequent cut. For biosolids, N recorded between the second and third cuts represented a relatively larger proportion of the total N uptake compared with other fertilizer treatments (). Therefore, N release from biosolids was sustained, at a lower rate, over a longer period of time, and it progressed further into the autumn compared with OMF and urea. On average, N uptake recorded in the third cut (% of total N uptake) represented about 19% for biosolids, 15% for OMF10, and 13% for OMF15 and urea. In Y2, N uptake recorded for all treatments up to cut one, i.e., prior to fertilizer application, were comparable (range: 36–43 kg ha−1), which confirmed no fertilizer type effect on DMY as a result of residual fertilizer N.
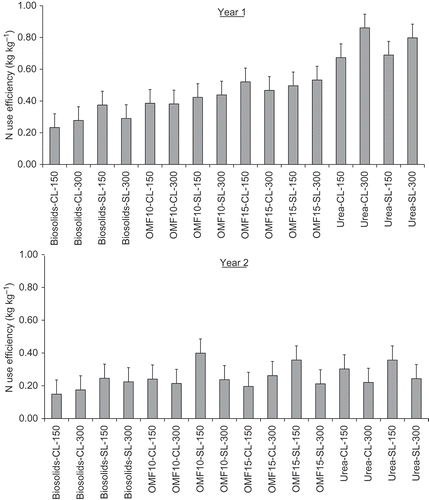
Nitrogen Use Efficiency
shows NUE calculations recorded in Y1 and Y2, respectively. Overall, there was a fertilizer type effect that was observed in both soil types (P < 0.05). Nitrogen recoveries in OMF10- and OMF15-treated grass were in the range of 0.33–0.37 kg kg−1 compared to 0.25 and 0.53 kg kg−1 obtained with biosolids granules and urea, respectively (LSD 5% level = 0.03 kg kg−1). Overall, NUE recorded in Y1 (≈0.5 kg kg−1) were greater than Y2 (≈0.25 kg kg−1), which is explained by surface-application of fertilizer and possible losses by volatilization of NH3 in the second year of the experiment. NUE decreased to a larger extent when concentration of readily available in fertilizer (as urea-N) or N application rate was higher. For example, urea-treated grass showed a reduction in NUE from about 0.75 kg kg−1 in Y1 to 0.30 kg kg−1 in Y2 whereas in biosolids-treated grass NUE decreased from about 0.30 kg kg−1 in Y1 to 0.20 in kg kg−1 in Y2. In OMF-treated grass, NUE decreased from about 0.45 to 0.25 kg kg−1 in Y1 and Y2, respectively. Soil incorporation of fertilizer materials in Y1 reduced N loses in urea-containing fertilizers and enhanced mineralization of organic-N in OMF and biosolids granules.
Soil Chemical Properties
Nitrogen in Soil
SMN measurements recorded consistently low values in both soils (≤5 mg kg−1), except for the year of establishment (), which explains higher DMY levels (P < 0.05) in Y1 compared with Y2 and Y3. There was no fertilizer type effect (P > 0.05) on SMN levels, which denotes rapid uptake of N available for all fertilizer treatments under the prevailing experimental conditions. Clay loam soil exhibited higher SMN levels than sandy loam soil throughout the experiment (P < 0.001), which explains differences in DMY between both soil types.
Table 3 Mean soil extractable P and corresponding soil P Indexes (DEFRA Citation2010) recorded in year one, prior to the start of the experiment, and at the end of year three. Different letters indicate values that are significantly different at a 95% confidence interval
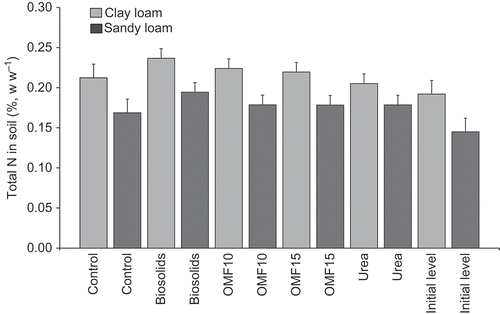
As shown in , there was a fertilizer type effect on total N in soil, which was observed in both soil types (P-values < 0.05). The interaction fertilizer type × N rate was significant (P = 0.02) which was due to differences in total N in soil between biosolids- and urea-treated grass, particularly, at 300 kg ha−1 of N. OMF10 and OMF15 yielded intermediate levels of total N in soil (≈0.20%, w w−1) compared with biosolids and urea, but differences between the two OMF formulations were not significant. Total N in soil increased over the 3 years period (P < 0.05), which was influenced by fertilizer type (P < 0.05), particularly, when the organic-N content in the fertilizer applied was higher (). Surface-application of fertilizers in Y2 and Y3 favored build-up of total N in biosolids- and OMF-treated soils.
Soil Extractable Phosphorus
shows soil extractable P as recorded for control and treatments. Differences in soil extractable P between control and treatments were not significant (P > 0.05). There was a fertilizer type effect (P < 0.001) because of differences recorded between biosolids- and urea-treated soils which showed an increase and decrease in soil extractable P levels, respectively, relative to controls (). The effect of biosolids on soil extractable P suggests a change in soil P Index from 5 (initial level) to 6 at the end of the experiment (). In Y3, unfertilized control soils exhibited an increase in soil extractable P of about 3% compared with initial levels which was not significant, however, it suggests a change in soil P Index from 5 to 6 ().
Figure 5. Soil extractable P corresponding to control and treatments as recorded in year three of the experiment, and initial levels prior to fertilizer application in year one. CL is clay loam and SL is sandy loam followed by corresponding fertilizer application rate as kg of N per ha. Error bars show the LSD at 5% level, P < 0.05, n = 3.
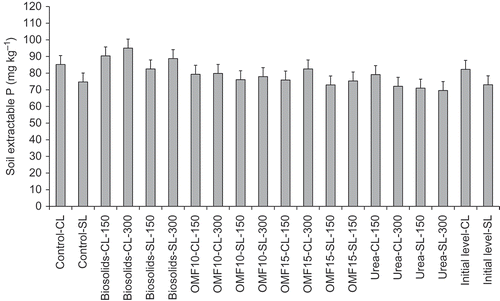
Analyses of total P in harvested plant material conducted after the first cut in Y1 indicated lower P uptake (P < 0.001) in controls compared with treatments, i.e., 0.9 versus 1.6 kg ha−1 of P, respectively. There was also a significant effect of fertilizer type and fertilizer application rate on P uptake (P-values < 0.05). Phosphorus uptake increased with fertilizer application rate and the amount of readily available N in the fertilizer. Biosolids-treated grass showed relatively lower rates of P uptake compared with OMF and urea, i.e., 1.4 versus 1.7 kg ha−1 of P, respectively (LSD 5% level = 0.16 kg ha−1). These results suggest a nutrient interaction N × P which enhanced uptake, effect that had been demonstrated in earlier studies (e.g., Mouat and Nes Citation1983; Ebdon, Petrovic, and White Citation1999). The relative increase in soil extractable P observed in control soils compared to initial levels is explained by reduced P uptake that resulted from lack of soil available N in the absence of N fertilization. Reduced P uptake enables replenishing the concentration of P in soil solution and readily available soil pools, hence, the relatively higher values of extractable P detected in soil analyses for the unfertilized controls. shows that OMF10, OMF15, and urea did not induce changes in soil P Index compared with controls, but analytical values were significantly lower than those recorded for biosolids. The decrease in soil extractable P observed with urea was not sufficient to modify soil P Index in that treatment.
Soil pH and Soil Organic Matter
Soil pH increased (P < 0.001) from 6.6 in Y1 to 7.2 in Y3, on average across all treatments. Urea hydrolysis is expressed as . The
is likely to increase soil pH, solubility of organic matter, and desorption of anions such as orthophosphates held on exchange sites (Shand et al. Citation2002). There was an effect (P < 0.05) of N application rate on soil pH. The pots fertilized with a field equivalent rate of 300 kg ha−1 of N showed a slightly lower value (pH = 6.8) compared with those treated with 150 kg ha−1 of N (pH = 6.9). This difference is small but reflects a potential acidifying effect of urea-based fertilizers applied at high rates (Mahler and McDole Citation1987). The acidification of urea occurs because of nitrification process (
) releases protons, which reduced soil pH (Shand et al. Citation2002).
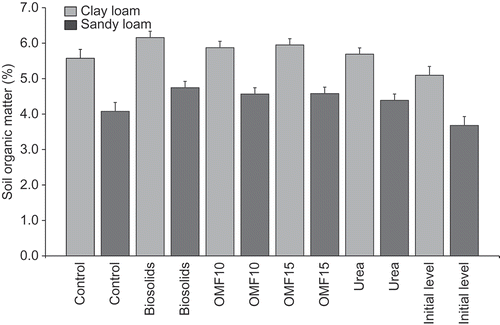
Changes recorded in SOM are summarized in . Overall, there was a mean increase (P < 0.05) in SOM from 4.4% in Y1 to 5.2% in Y3. SOM levels were affected by N rate and fertilizer type (P-values < 0.001). SOM increased by approximately 3.2% and 6% in treatments fertilized with 150 and 300 kg ha−1 of N, respectively, at the end of the 3 years’ period, compared with initial levels. Application of biosolids increased SOM levels by about 7% on average compared with controls, and they were approximately 2.3% and 4.8% higher with urea and OMF, respectively. An increase in SOM between initial levels and control treatments can be attributed to accumulation of roots in pots. When SOM analyses were conducted using loss-on-ignition (MAFF Citation1986), samples were ground and would have included both soil and root material that may have contributed to the increase in SOM recorded in control soils. A more qualitative determination of SOM (e.g., Kogel-Knabner Citation1997) would be beneficial to distinguish between recent and older organic C fractions in soil.
Discussion
Fertilizer Application Effects on Crop
Yield-to-nitrogen response curves showed acceptable fits to linear models (R2 ≥ 0.75, P < 0.05) in the range of N application rates investigated. On average, OMF10 and OMF15 increased DMY by about 8% and 14%, respectively, compared with biosolids over the 3 years period. Responses encountered with OMF10, OMF15, and urea in Y1 (range of 14–26 kg DM kg−1 N) were within the range (14–30 kg DM kg−1 N) obtained by Morrison, Jackson, and Sparrow (Citation1980) with mineral N fertilizers but exceeded those reported by McFeely and MacCarthy (Citation1981), and O’Donovan et al. (Citation2004) (range: 5–17 kg DM kg−1 N). Nitrogen responses were related to concentration of readily available N in the fertilizer; biosolids showed consistently lower responses compared with other treatments over the 3 years period (range: 10–12 kg DM kg−1 of N). In Y2 and Y3, responses were of similar magnitude for all treatments (range: 6.5–12 kg DM kg−1), which was attributed to surface-application of fertilizer; except for biosolids where responses were lower but more sustained throughout the experiment. The timing of fertilizer application influenced yield-to-nitrogen responses. In Y2 and Y3, fertilizer application was relatively late () compared with the characteristic pattern of seasonal rate of growth (i.e., peak growth) of grass crops (Orr et al. Citation1988). To some extent, this explains the overall decline in responses and DMY observed in Y2 and Y3 compared with Y1.
Relatively high temperatures recorded on the date of fertilizer application in Y3 (24.3 °C) supports the possibility of N losses by volatilization of NH3. For urea-containing fertilizers, these are enhanced by increasing N application rate or temperature (range: 10–30 °C) (Sainz-Rozas et al. Citation1997). These conditions impaired N uptake and the subsequent translation into DMY, hence, the similarity observed in the slope of the response curves. By contrast, warm conditions in the glasshouse enhanced mineralization of organic-N in biosolids, which contributed to sustain N uptake and DMY levels with this treatment. As for biosolids, this process also occurred with OMF but to a lesser extent due to smaller proportion of organic-N in their composition.
In Y1, N uptake showed increments in the range of 0.30–0.82 kg kg−1 of N, and between 0.20 and 0.25 kg kg−1 of N in Y2 on average across all treatments, which denotes lower fertilizer use efficiency as a result of surface-application. Low N supply to grass crops compromises growth rate, tiller density, and biomass production, and reduces N concentration in plant (Wilman and Mohamed Citation1980; Delagarde, Peyraud, and Delaby Citation1997). Nitrogen in harvested plant material recorded with OMF and urea applied at 300 kg ha−1 of N were relatively lower (range: 2.2–2.8%) than a critical leaf concentration for deficiency of N of 3.2% by weight (Smith, Cornforth, and Henderson Citation1985). This threshold value is defined as N concentration in the leaves below which reductions in maximum yield of 10% or greater may occur due to insufficient supply of that nutrient (Smith, Cornforth, and Henderson Citation1985). However, nitrogen in harvested plant material with OMF and urea were within the range required by high-producing dairy cattle (Aavola and Kärner Citation2008).
NUE calculations indicated large differences between-years with an overall decrease in all treatments in Y2 (). This is explained by late timing of N application relative to maximum rate of growth of ryegrass (Orr et al. Citation1988), and combined effects of high temperatures around the time of fertilizer application with surface-application of fertilizers leading to losses of N by volatilization of NH3. The latter is recognized to be the main reason for inefficiency of urea-based fertilizers compared with other straight N fertilizers such as ammonium nitrate (Watson et al. Citation1990). Urea-based fertilizers can have similar efficiencies to ammonium nitrate when applications are conducted in spring but can be lower in summer (Watson et al. Citation1990). In Y1, NUE recorded in urea- and OMF-treated grass were within the range reported in the literature (e.g., Whitehead, Jones, and Barnes Citation1978; Morrison, Jackson, and Sparrow Citation1980; Antille, Sakrabani, and Godwin Citation2013), but exceeded those obtained in Y2 which were similar to values obtained by Williams, Rowarth, and Tregurtha (Citation2000).
Fertilizer Application Effects on Soil
The relatively larger build-up of total N in soil and SOM in sandy loam compared with clay loam soil contributed to narrow differences in DMY observed in Y1 between-soil types through enhanced release of SMN.
Soil extractable P in OMF-treated grass remained close to constant but showed increases with biosolids (). Application of OMF10 and OMF15 did not induce changes in soil P Index over the 3 years period, which therefore confirms the suitability of the product formulations for application in grass crops, even in soils with satisfactory soil P status. In urea-treated soils, soil P Index did not change but there was a significant decrease in soil extractable P compared with baseline levels (). After 3 years, there was an increase of 14 mg L−1 in soil extractable P with application of biosolids (), which can have implications for an increase in soil P Index. This result was largely due to reduced P uptake in biosolids-treated grass that resulted from reduced N availability in the material, and low N:P ratio of biosolids, which leads to application of P in excess of crop requirement (Hogan, McHugh, and Morton Citation2001; Antille, Gallar-Redondo, and Godwin Citation2013; Antille et al. Citation2013; Antille, Sakrabani, and Godwin Citation2014a).
The increase in extractable P levels exhibited in soils amended with biosolids occurred despite of typically low phytoavailability of biosolids-P (Römer and Samie Citation2001; O’Connor et al. Citation2004). Soil incubation studies (Antille, Sakrabani, and Godwin Citation2014b) confirmed that availability of OMF-P is typically lower than 10% of total P applied as fertilizer. However, limited N availability in biosolids-treated soil reduced P uptake in that treatment, which replenished P in soil solution and readily available soil pools (Johnston and Syers Citation2006), i.e., the two fractions detected in routine soil extractions (e.g., BS7755 Section 3.6 Citation1995), and yielded relatively higher analytical values than OMF and urea. Results obtained with biosolids also suggest slow release of P from OMF and biosolids, which may be sustained for several years following soil application. These results support findings reported in earlier studies (e.g., Kelling et al. Citation1977; Morgan Citation1997) which showed that P from applied fertilizer, including biosolids, can be utilized by crops in subsequent years following application. Results agree with Johnston and Syers (Citation2006) who discarded earlier views that sustained that a proportion of P applied with fertilizers can be irreversibly fixed in soil. Under these experimental conditions, it was demonstrated that application of OMF at 300 kg ha−1 of N replenishes but does not necessarily exceed P off-take by the grass crop.
The increase in soil extractable P in control soils was marginal compared to baseline levels but suggested a change in soil P Index (). This occurred as a result of reduced P uptake in the controls, which is confirmed by analyses of total P in harvested plant material (authors’ own data). The reduction in soil extractable P in urea-treated pots provided an indication of the rate of decline of soil P when this nutrient is not included as part of the fertilization strategy, and it confirms a positive interaction N × P on DMY. Evidence from long-term experiments (Johnston Citation1997) showed that continuous omission of P and K fertilization is likely to result in loss of crop yield and quality when reserves of these nutrients decrease below critical levels (DEFRA Citation2010). At low (available) P and K status, NUE in grass crops is significantly affected, which is both economically and environmentally undesirable (Johnston et al. Citation2001; Johnston and Poulton Citation2009; Dawson Citation2011). The increase in soil extractable P observed with biosolids indicates the need to monitor P status in soils receiving biosolids routinely so that P off-take is not exceeded. Build-up of soil P can increase concentration of dissolved and particulate P in runoff, and increase the risk of P transfer from soil to water when soil P exceeds a critical value of 20% saturation (Withers et al. Citation2009).
Conclusions
The OMF10 and OMF15 formulations are suitable for application in ryegrass. DMY and yield-to-nitrogen responses with OMF were comparable to urea, and within the range of values reported in the literature for straight N fertilizers. The conversion of biosolids into balanced OMF significantly improves its agronomic and environmental performances. Application of OMF increased DMY and NUE by about 2–27%, and by 19–29%, respectively, compared with biosolids. The overall efficiency of the N applied as OMF is greater when the fertilizer is applied in early spring, which: (1) allows for improved mineralization of the organic-N fraction in OMF and (2) reduces the risk of NH3 volatilization toward the summer from the fraction carrying urea, particularly, when the fertilizer is applied on the surface.
Application of OMF at field rates which do not exceed those used in this study should not induce significant changes in soil extractable P levels, hence, soil P Index is likely to remain close to constant. Therefore, application of OMF at these rates will replenish, approximately, P off-take by grass crops thereby maintaining soil P levels overtime, which confirms the hypothesis formulated prior to this study.
Funding
This research received funding from the European Union Seventh Framework Programme (FP7-ENV.2010.3.1.1-2 ENV) under grant agreement no.: 265269 (http://www.end-o-sludg.eu/). The article represents the opinion of the authors and does not necessarily represent the view of the European Union or United Utilities Group PLC. The authors are grateful to United Utilities Group PLC, The Engineering and Physical Science Research Council, and Cranfield University for financial and operational support. Contributions from Dr S.F. Tyrrel and P. Bellamy are appreciated.
Additional information
Funding
References
- Aavola, R., and M. Kärner. 2008. Nitrogen uptake at various fertilization levels and cutting frequencies of Lolium species. Agronomy Research 6 (1):5–14.
- Antille, D. L., L. Gallar-Redondo, and R. J. Godwin. 2013. Determining the particle size range of organomineral fertilizers based on the spreading characteristics of the material. ASABE 5:4251–4268. doi:10.13031/aim.20131620197.
- Antille, D. L., R. Sakrabani, and R. J. Godwin. 2013. Field-scale evaluation of biosolids-derived organomineral fertilizers applied to ryegrass (Lolium perenne L.) in England. Applied and Environmental Soil Science, Vol. 2013, Article ID 960629, 9 pp., http://dx.doi.org/10.1155/2013/960629
- Antille, D. L., R. Sakrabani, and R. J. Godwin. 2014a. Nitrogen release characteristics from biosolids-derived organomineral fertilizers. Communications in Soil Science and Plant Analysis 45 (12):1687–1698.
- Antille, D. L., R. Sakrabani, and R. J. Godwin. 2014b. Phosphorus release characteristics from biosolids-derived organomineral fertilizers. Communications in Soil Science and Plant Analysis doi:10.1080/00103624.2014.912300.
- Antille, D. L., R. Sakrabani, S. F. Tyrrel, M. S. Le, and R. J. Godwin. 2013. Characterisation of organomineral fertilizers derived from nutrient-enriched biosolids granules. Applied and Environmental Soil Science, Vol. 2013, Article ID 694597, 11 pp., http://dx.doi.org/10.1155/2013/694597
- Baligar, V. C., N. K. Fageria, and Z. L. He. 2001. Nutrient use efficiency in plants. Communications in Soil Science and Plant Analysis 32 (7–8):921–950.
- Bowden, W., and M. J. Hann. 1997. The availability of nitrogen following digested sludge incorporation in arable land. Nutrient Cycling in Agroecosystems 47 (2):167–172.
- British Standard 7755 Section 3.6. 1995. Determination of phosphorus. Spectrometric determination of phosphorus soluble in sodium hydrogen carbonate solution. Equivalent to ISO 11263:1994. London, UK: The British Standards Institution.
- British Standard 7755 Section 3.8. 1995. Soil quality. Chemical methods. Determination of organic and total carbon after dry combustion (elementary analysis). Equivalent to ISO 10694:1995. London, UK: The British Standards Institution.
- British Standard EN 13654-2. 2001. Soil improvers and growing media. Determination of nitrogen (Dumas method). Equivalent to ISO 5725:1994. London, UK The British Standards Institution.
- Cordovil, C. M. D. S., F. Cabral, and J. Coutinho. 2007. Potential mineralization of nitrogen from organic wastes to ryegrass and wheat crops. Bioresource Technology 98 (17):3265–3268.
- Cornforth, I. S., and A. G. Sinclair. 1997. Two pot techniques for controlled-environment cabinets. New Zealand Journal of Experimental Agriculture 5 (2):189–192.
- Davis, R. D. 1996. The impact of EU and UK environmental pressures on the future of sludge treatment and disposal. Water and Environment Journal 10 (1):65–69.
- Dawson, C. J. 2011. Phosphate and potash – reconsidering their importance and use. Journal of the Royal Agricultural Society of England 172:1–9.
- Department for Environment, Food and Rural Affairs. 2010. Fertilizer manual. Reference Book 209. 8th ed. London, UK: The Stationery Office.
- Department for Environment, Food, and Rural Affairs. 2011. Government review of waste policy in England. PB13540. Accessed 8 October 2013. https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/69401/pb11354-waste-policy-review110614.pdf
- Delagarde, R., J. L. Peyraud, and L. Delaby. 1997. The effect of nitrogen fertilization level and protein supplementation on herbage intake, feeding behaviour and digestion in grazing dairy cows. Animal Feed Science and Technology 66 (1–4):165–180.
- Ebdon, J. S., A. M. Petrovic, and R. A. White. 1999. Interaction of nitrogen, phosphorus, and potassium on evapotranspiration rate and growth of Kentucky bluegrass. Crop Science 39 (1):209–218.
- Edge, D. 1999. Perspectives for nutrient removal from sewage sludge and implications for sludge strategy. Environmental Technology 20 (7):759–763.
- GenStat Release 14.1 (14th Edition). 2011. Hemel Hempstead, VSN International, UK.
- Heffer, P., and M. Prud’homme. 2013. Fertilizer outlook 2013-2017. In Proceedings of the 81st International Fertilizer Industry Association Conference, Chicago, USA, 20–22 May 2013. Paper No.: A/13/78, 8 pp. www.fertilizer.org
- Hogan, F., M. McHugh, and S. Morton. 2001. Phosphorus availability for beneficial use in biosolids products. Environmental Technology 22 (11):1347–1353.
- Johnston, A. E. 1997. The value of long-term field experiments in agricultural, ecological and environmental research. Advances in Agronomy 59:291–333.
- Johnston, A. E., and J. K. Syers. 2006. Changes in understanding the behaviour of soil and fertilizer phosphorus: implications for their efficient use in agriculture. Proc. No.: 589. York, UK: The International Fertilizer Society.
- Johnston, A. E., and P. R. Poulton. 2009. Nitrogen in agriculture: an overview and definitions of nitrogen use efficiency. Proc. No.: 651. York, UK: The International Fertilizer Society.
- Johnston, A. E., P. R. Poulton, D. J. Dawson, and M. J. Crawley. 2001. Inputs of nutrients and lime for the maintenance of fertility of grasslands soils. Proc. No.: 486. York, UK: The International Fertilizer Society.
- Jones, K. C., and A. E. Johnston. 1989. Cadmium in cereal grain and herbage from long-term experimental plots at Rothamsted, UK. Environmental Pollution 57 (3):199–216.
- Kelling, K. A., L. M. Walsh, D. R. Keeney, J. A. Ryan, and A. E. Paterson. 1977. A field study of the agricultural use of sewage sludge: II. Effect on soil N and P. Journal of Environmental Quality 6 (4):345–352.
- King, D. W. 1969. Soils of the Luton and Bedford Districts: a reconnaissance survey. Harpenden, UK: The Soil Survey of England and Wales
- Kogel-Knabner, I. 1997. 13C and 15N NMR spectroscopy as a tool in soil organic matter studies. Geoderma 80 (3–4):243–270.
- Mahler, R. L., and R. E. McDole. 1987. Effects of soil pH on crop yield in northern Idaho. Agronomy Journal 79 (4):751–755.
- McFeely, P., and D. MacCarthy. 1981. Effect of time on initial spring grazing and nitrogen use on pasture production. Irish Journal of Agricultural Research 20 (2–3):137–146.
- Ministry of Agriculture, Fisheries and Food. 1986. The analysis of agricultural materials. Reference Book 427. 3rd ed. London: The Stationery Office.
- Morgan, M. A. 1997. The behaviour of soil and fertilizer phosphorus. Chapter 6. In Phosphorus loss from soil to water, ed. H. Tunney, O. T. Carton, P. C. Brookes, and A. E. Johnston. London, 137–149. UK:CAB International.
- Morrison, J., M. V. Jackson, and P. E. Sparrow. 1980. The response of perennial ryegrass to fertilizer nitrogen in relation to climate and soil. Technical Report No.: 27, Joint ADAS/GRI Grassland Manuring Trial GM20. Berkshire, UK: Grassland Research Institute.
- Moseley, P. J., T. H. Misselbrook, B. F. Pain, R. Earl, and R. J. Godwin. 1998. The effect of injector tine design on odour and ammonia emissions following injection of bio-solids into arable cropping. Journal of Agricultural Engineering Research 71 (4):385–394.
- Mouat, M. C. H., and P. Nes. 1983. Effect of the interaction of nitrogen and phosphorus on the growth of ryegrass. New Zealand Journal of Agricultural Research 26 (3):333–336.
- O’Connor, G. A., D. Sarkar, S. R. Brinton, H. A. Elliot, and F. G. Martin. 2004. Phytoavailability of biosolids phosphorus. Journal of Environmental Quality 33 (2):703–712.
- O’Donovan, M., L. Delaby, G. Stakelum, and P. Dillon. 2004. Effect of autumn/spring nitrogen application date and level on dry matter production and nitrogen efficiency in perennial ryegrass swards. Irish Journal of Agricultural and Food Research 43 (1):31–41.
- Orr, R. J., A. J. Parsons, T. T. Treacher, and P. D. Penning. 1988. Seasonal patterns of grass production under cutting or continuous stocking managements. Grass and Forage Science 43 (2):199–207.
- Ragg, J. M., G. R. Beard, H. George, F. W. Heaven, J. M. Hollis, R. J. A. Jones, R. C. Palmer, M. J. Reeve, J. D. Robson, and W. A. Whitfield. 1984. Soils and their use in Midland and Western England. Bulletin No.: 12. Harpenden, UK: The Soil Survey of England and Wales.
- Römer, W., and I. F. Samie. 2001. Influence of iron content in sewage sludges on parameters of phosphate availability in arable soils. Journal of Plant Nutrition and Soil Science 164 (3):321–328.
- Sainz-Rozas, H., H. E. Echeverría, G. A. Studdert, and F. H. Andrade. 1997. Volatilización de amoníaco desde urea aplicada al cultivo de maíz bajo siembra directa. Ciencia del Suelo (Argentina) 15 (1):12–16.
- Shand, C. A., B. L. William, L. A. Dawson, S. Smith, and M. E. Young. 2002. Sheep urine affects soil solution nutrient composition and roots: differences between field and sward box soils, and the effects of synthetic and natural sheep urine. Soil Biology and Biochemistry 34 (2):163–171.
- Skinner, R. J., and A. D. Todd. 1998. Twenty-five years of monitoring pH and nutrient status of soils in England and Wales. Soil Use and Management 14 (3):162–169.
- Smith, G. S., I. S. Cornforth, and H. V. Henderson. 1985. Critical leaf concentrations for deficiencies of nitrogen, potassium, phosphorus, sulphur, and magnesium in perennial ryegrass. New Phytologist 101 (3):393–409.
- Sommers, L. E. 1997. Chemical composition of sewage sludges and analysis of their potential use as a fertilizers. Journal of Environmental Quality 6 (2):225–232.
- Torri, S. I., and R. S. Corrêa. 2012. Downward movement of potentially toxic elements in biosolids amended soils. Applied and Environmental Soil Science, Vol. 2012, Article ID 145724, 7 pp., doi:10.1155/2012/145724.
- Watson, C. J., R. J. Stevens, M. K. Garret, and C. H. McMurray. 1990. Efficiency and future potential of urea for temperate grassland. Fertilizer Research 26 (1–3):341–357.
- Whitehead, D. C., L. H. P. Jones, and R. J. Barnes. 1978. The influence of fertilizer N plus K on N, S, and other mineral elements in perennial ryegrass at a range of sites. Journal of the Science of Food and Agriculture 29 (1):1–11.
- Williams, P. H., J. S. Rowarth, and R. J. Tregurtha. 2000. Recovery of 15N-labelled fertilizer by a perennial ryegrass seed crop and a subsequent wheat crop. Nutrient Cycling in Agroecosystems 56 (2):117–123.
- Wilman, D., and A. A. Mohamed. 1980. Response to nitrogen application and interval between harvests in five grasses – dry matter yield, nitrogen content and yield, number and weight of tillers, and proportion of crop fractions. Fertilizer Research 1 (4):245–263.
- Withers, P. J. A., H. Hartikainen, E. Barberis, N. J. Flynn, and G. P. Warren. 2009. The effect of soil phosphorus on particulate phosphorus in land runoff. European Journal of Soil Science 60 (6):994–1004.