Abstract
The pollen morphology of 37 species of Trichosanthes was examined, using light microscopy, and scanning and transmission electron microscopy. On the basis of the diverse exine ornamentation it is possible to distinguish five pollen types. With aperture characters, two of them can be subdivided into subtypes. Two types can be characterised with ultrastructural features as well. It appeared that several of the types (alt. subtypes) correspond very well to existing macromorphological groupings. The most deviating type, including only the monotypic section Trichosanthes (T. cucumerina), shows verrucate ornamentation, a thick granular infratectum and a thin, indistinctly delimited nexine. It is similar to that of the Madagascan genus Tricyclandra.
Trichosanthes (Cucurbitaceae) is a genus with c. 100 species distributed from eastern Asia to tropical Australia and Fiji (Yueh & Cheng Citation1980, Jeffrey Citation1980, Citation1990, Huang et al. Citation1997, Citation1998, Rugayah & De Wilde Citation1999). Gymnopetalum (Southeast Asia, 3 spp.) and the monotypic genus Tricyclandra (Madagascar), together with Trichosanthes constitute the subtribe Trichosanthinae of the tribe Trichosantheae of subfamily Cucurbitoideae (Jeffrey Citation1980, Citation1990). The other subtribes within this tribe are the Ampelosicyinae, with Ampelosicyos (Madagascar, 3 spp.) and Peponium (Africa, Madagascar, 20 spp.), the Herpetosperminae, with Biswarea (Himalaya; 2 spp.) and the monotypic genera Cephalopentandra (northeast Africa), Edgaria (Himalaya) and Herpetospermum (China, Himalaya), and the Hodgsoniinae, with the monotypic Hodgsonia (Indo‐Malaysia). The relationships of Trichosanthes with the other genera in the subtribe and tribe are not yet clear. The genus has been intensively revised in recent years. Rugayah & De Wilde (Citation1999) dealt with 39 species from Malaysia, of which 14 appeared to be undescribed. They found that seed characters are useful to characterise species and groups of species. De Wilde & Duyfjes (Citation2004) studied 21 species from Sabah, including four new species. Duyfjes & Pruesapan (in press) discovered seven new species among the 17 species occurring in Thailand. Only few Chinese collections could be involved in these revisions. The delimitation of a number of Trichosanthes species is still problematic, which is partly due to their dioecy (either male or female plant known). Also the subdivision of the genus is still a matter of debate. All authors distinguish a number of sections of varying contents, usually grouped into two subgenera: Cucumeroides and Trichosanthes (see Discussion).
The pollen of Trichosanthes was studied by Erdtman (Citation1952; 1 sp.), Ikuse (Citation1956; 2 spp.), Marticorena (Citation1963; 9 spp.), Keraudren (Citation1968; 1 sp.), Keraudren‐Aymonin et al. (Citation1969, Citation1984; 1 sp.), Huang (Citation1972; 3 spp.), Mandal & Chanda (Citation1981; 1 sp.), Beevy & Kuriachan (Citation1996; 5 spp.) and Khunwasi (Citation1998; 5 spp.). Marticorena (Citation1963) was the first to note a marked variation in size, shape, aperture characters and ornamentation. Jeffrey (Citation1964, Citation1990) used these characters and those of the other genera studied to support the classification of Trichosanthes as a member of the subtribe Trichosanthinae. Most extensive are the light and scanning electron microscopical studies of the pollen of mostly Chinese species by Yue & Zhang (Citation1986; 31 spp.) and Huang et al. (Citation1997; 31 spp.). The present study aims to complement the above treatments by examining a relatively large number of species from Southeast Asia and Malaysia. In total, the pollen of 37 Trichosanthes species were studied in order to provide additional data for a subdivision of the genus.
MATERIAL AND METHODS
In total, 69 samples (37 species) covering all sections and subsections of Trichosanthes distinguished by Jeffrey (Citation1980), Huang et al. (Citation1997), Rugayah & De Wilde (Citation1999) and/or De Wilde & Duyfjes (Citation2004) were investigated (see Specimens Examined, Discussion & ). Most samples were taken from collections preserved in the National Herbarium Nederland at Leiden (L), 13 samples come from the Kew herbarium (K), and two from Paris herbarium (P). All samples were prepared for light microscopy (LM) and scanning electron microscopy (SEM), and some of them (10 spp.) also for transmission electron microscopy (TEM; see Specimens Examined), according to the LM, SEM and TEM techniques described by Van der Ham (Citation1990). The pollen data are listed in . Ten pollen grains per sample was measured. The terminology used follows Punt et al. (Citation1994).
Table I. Selected characters of Trichosanthes pollen, including types, dimensions and morphology, with references to illustrations.
RESULTS
Trichosanthes ( – )
LM & SEM
Pollen grains isopolar, suboblate to prolate spheroidal. Amb (sub)circular. P=30.4 (50.9) 76.5 μm, E=36.6 (55.9) 74.5 μm. P/E=0.76–1.07.
Fig. 1. Trichosanthes pollen. A–H. Subtype 1.1a. A–C & F. T. cucumeroides (Oldman 286): (A) ±Polar view of 4‐porate grain (LM, middle focus); (B) Polar/equatorial view of 4‐porate grain (LM, middle focus); (C) Equatorial view (LM, upper focus); (F) Porate ectoaperture, showing margo, SEM. D, E & G, H. T. cucumeroides (Karasuwuri s.n.): (D) Polar view of 4‐porate grain, SEM; (E) Equatorial view, SEM; (G) TEM of exine, showing tectum (t), granular infratectum (i) and foot layer (f); (H) TEM of aperture, showing margo (m), costa (c) and endexine (e). I–K. Subtype 1.1b. T. pulleana : (I) Polar view, SEM; (J) Equatorial view, SEM; (K) Porate aperture (margo absent), SEM. Scale bars – 20 µm (A, B); 10 µm (C–E, I, J); 5 µm (F, K); 1 µm (G, H).
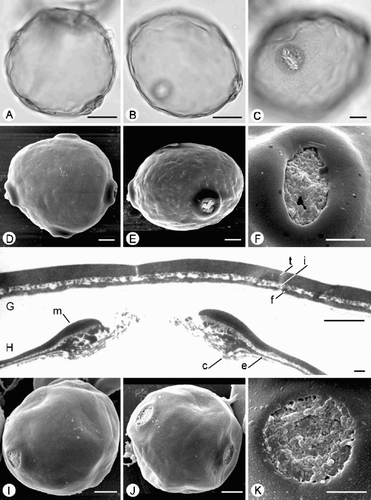
Fig. 2. Trichosanthes pollen. A–D. Subtype 1.2. A, B. T. rosthornii (Ford 81): (A) Polar view of 4‐colporate grain, SEM; (B) Equatorial view, SEM. C. T. multiloba (Ko‐isi‐wara s.n.): Finely perforate ornamentation, SEM. D. T. rosthornii (Teng 90473): TEM of exine, showing tectum (t), granular infratectum (i), foot layer (f) and endexine (e). E–H. Type 2. T. globosa : (E) Equatorial view, SEM; (F) Porate ectoaperture, SEM; (G) Perforate ornamentation, SEM; (H) TEM of exine, showing tectum (t), columellate/granular infratectum (i), foot layer (f) and endexine (e). Scale bars – 10 µm (A, B, E); 5 µm (F); 1 µm (C, D, G, H).
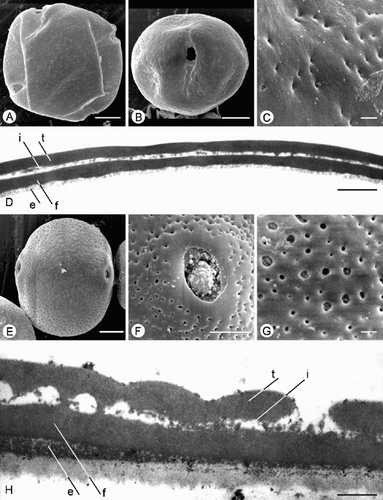
Fig. 3. Trichosanthes pollen. A–H. Type 3. T. phonsenae ; (A) ±Polar view (LM, upper/middle focus), (B) ±Polar view (LM, middle focus), (C) Equatorial view (LM, upper focus); (D) Polar view, SEM; (E) Colpate ectoaperture, SEM; (F) Microreticulate ornamentation, SEM; (G) TEM of exine, showing tectum (t), granular infratectum (i), foot layer (f) and endexine (e); (H) TEM of aperture, showing costa (c). Scale bars – 10 µm (A, B, D); 5 µm (C); 1 µm (E–H).
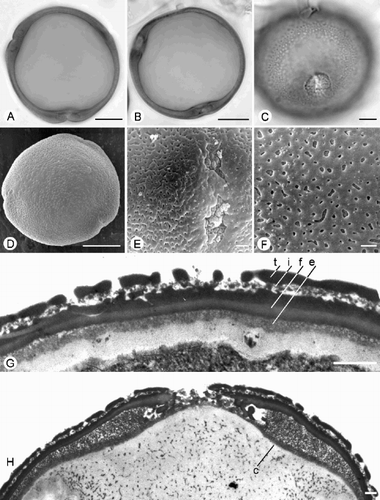
Fig. 4. Trichosanthes pollen. A–J. Subtype 4.1. A, F & J. T. pubera (Phonsena, De Wilde & Duyfjes 3926): (A) ±Polar view (LM, middle focus); (F) Reticulate ornamentation, SEM; (J) TEM of exine, showing tectum (t), granular infratectum (i), foot layer (f) and endexine (e). B, C. T. wallichiana: Equatorial views (LM, upper foci), showing costae and reticulate ornamentation. D. T. bracteata: Polar view, SEM. E. T. sepilokensis (SAN 143739): Equatorial view, SEM. G. T. pallida: Porate ectoaperture, SEM. H. T. wawrae (Cogniaux 57): Porate‐colpate aperture, SEM. I. T. species aff. laceribractea (Poilane 16778): Endoaperture with costa, SEM. Scale bars – 20 µm (C); 10 µm (A, B, D, E); 5 µm (H, I); 1 µm (F, G, J).
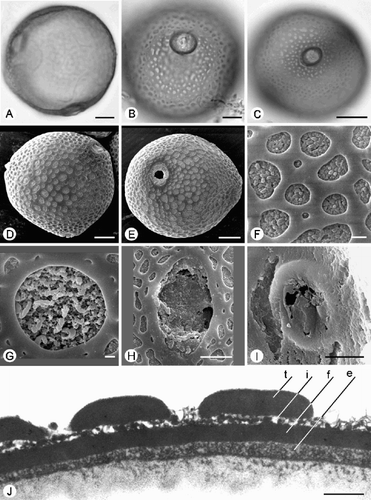
Fig. 5. Trichosanthes pollen. A–G. Subtype 4.2. A, D & E. T. villosa : (A) Polar view, SEM; (D) TEM of exine, showing tectum (t), granular infratectum (i), foot layer (f) and endexine (e); (E) TEM of aperture, showing costa (c). B. T. kerrii: Equatorial view, SEM. C. T. ishigakiensis; reticulate ornamentation, SEM. F, G. T. truncata (Maxwell 93‐946): (F) TEM of exine, showing tectum (t), rather thick granular infratectum (i), foot layer (f) and endexine (e); (G) TEM of aperture, showing costa (c). Scale bars – 10 µm (A); 5 µm (B); 1 µm (C–G).
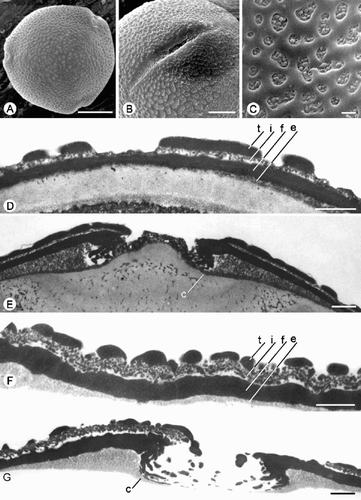
Fig. 6. Trichosanthes pollen. A–H. Type 5. A–F. T. cucumerina (De Wilde & Duyfjes 21804): (A, B) ±Polar views (LM, middle foci); (C) Equatorial view (LM, upper focus); (D) Polar view, SEM; (E) Porate aperture, showing margo, SEM; (F) Verrucate ornamentation, SEM. G–H. T. cucumerina (Kostermans 18027): (G) TEM of exine, showing tectum (t), thick granular infratectum (i), foot layer (f) and endexine (e); (H) TEM of aperture edge, showing margo (m). Scale bars – 20 µm (B, C); 10 µm (A, D); 5 µm (E); 1 µm (F–H).
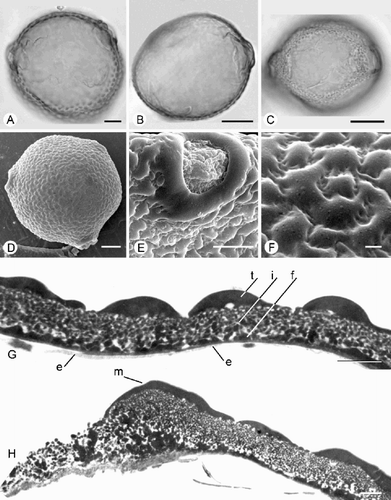
Aperture system 3(–4)‐colporate or 3(–4)‐porate. Ectoapertures (short) colpi, 5.5 – 34×1.5 – 8 μm, or pori, 4 – 16×3 – 10 μm, which may be coincident with the endoapertures; margo (slightly thickened sexine around ectoaperture) sometimes present (, ). Endoapertures pori, 2 – 16×2 – 12.5 μm, sometimes indistinct; costae 1 – 4 μm wide () or indistinct.
Exine 1 – 2(–4) μm thick. Sexine thicker, as thick as, or thinner than nexine. Ornamentation psilate to finely perforate, perforate, microreticulate, reticulate or verrucate.
TEM
Exine 0.6 – 2 μm thick. Sexine thicker, as thick as or thinner than nexine. Nexine 0.2 – 1.2 μm thick. Endexine between apertures inconspicuous (, ) or up to about half of the nexine thickness (), spongy, near apertures thickening (costae) and sometimes somewhat lamellate (, ). Foot layer relatively thick and well‐delimited towards the infratectum, sometimes thin and indistinctly delimited (T. cucumerina, ), thickening towards the apertures (costae; , , , ). Infratectum relatively thin (c. 0.1 μm) and loosely, irregularly granular () to relatively thick (0.7 μm) and densely, regularly granular (T. cucumerina, ; T. truncata, ), not or slightly thickening near the apertures (margo); sometimes columella‐like structures are present as well (e.g. T. globosa, ). Tectum 0.6 – 2 μm thick, sometimes thickening near the apertures (margo; ).
On the basis of the diverse ornamentation (SEM) it is possible to distinguish five pollen types. With aperture characters (LM, SEM), type 1 and type 4 can be subdivided into subtypes. Two types can be characterised with TEM as well: type 2 (perforate; T. globosa) has a granular infratectum with columella‐like structures and type 5 (verrucate; T. cucumerina) has thick densely granular infratectum and a thin, indistinctly delimited nexine. A main subdivision based on the distinction between porate (length/width of ectoaperture < 2) and colporate (length/width of ectoaprture > 2) is less satisfying, because these states are continuous. Moreover, such a subdivision would give less resolution.
Type 1 – psilate to finely perforate ( , )
Pollen grains suboblate to prolate spheroidal. P=34.1 (49.4) 61 μm; E=40.3 (55.6) 71.1 μm. P/E=0.80 – 1.01.
Aperture system 3(–4)‐aperturate. Ectoapertures pori, 5 – 16×3 – 13 μm, or colpi, 5.5 – 25×2 – 8 μm; margo distinct or indistinct. Endoapertures pori, 3.5 – 14×3 – 12.5 μm, or indistinct; costae 1 – 4 μm wide or indistinct.
Ornamentation more or less psilate to finely perforate; perforations up to 1 μm; muri 0.2 – 1.9 μm wide.
Subtype 1.1a – porate, margo distinct ()
Pollen grains suboblate. P=48 (52.5) 60 μm; E=57.5 (63.6) 71.1 μm. P/E=0.80 – 0.84.
Aperture system 3 – 4‐aperturate. Ectoapertures pori, 5 – 13×3 – 10 μm, sometimes short colpi, 7.5 – 16×3 – 8 μm; margo distinct, except in T. cucumeroides (Furuse 43876). Endoapertures indistinct, sometimes pori, 4 – 5×3 – 5 μm; costae usually indistinct (more or less distinct in T. mucronata).
Species included: T. cucumeroides, T. mucronata, T. ovigera.
Subtype 1.1b – porate, margo indistinct ()
Pollen grains oblate spheroidal to prolate spheroidal. P=45 (53.4) 61 μm; E=49.1 (55.6) 60.5 μm. P/E=0.91 – 1.01.
Aperture system 3(–4)‐aperturate. Ectoapertures pori, 6 – 16×4 – 13 μm, sometimes short colpi, 5.5 – 10×c. 2 μm; margo indistinct. Endoapertures pori, 5 – 14×3 – 12.5 μm; costae 1.5 – 4 μm wide.
Species included: T. coriacea, T. edulis, T. pulleana, T. schlechteri.
Subtype 1.2 – colporate ()
Pollen grains suboblate. P=34.1 (36.7) 39.3 μm; E=40.3 (43.7) 47.1 μm. P/E=0.83 – 0.85.
Aperture system 3 – 4‐aperturate. Ectoapertures colpi, 8 – 25×2 – 7 μm; margo indistinct. Endoapertures pori, 3.5 – 10×3 – 10 μm; costae 1 – 1.5 μm wide.
Species included: T. multiloba, T. rosthornii.
Type 2 – perforate ( )
Pollen grains prolate spheroidal. P=64.8 μm; E=60.6 μm. P/E=1.07.
Aperture system 3 – 4‐aperturate. Ectoapertures short colpi, 9 – 11×4 – 5 μm, sometimes pori, 5 – 8×4 – 6 μm; margo indistinct. Endoapertures pori, 4 – 6×4 – 6 μm; costae 1 – 2 μm wide.
Ornamentation perforate; perforations up to 1 μm; muri 0.5 – 2.0 μm wide.
Species included: T. globosa.
Type 3 – microreticulate ( )
Pollen grains suboblate to oblate spheroidal. P=30.4 (32) 34.3 μm; E=36.6 (38.1) 39.7 μm. P/E=0.76 – 0.89.
Aperture system 3(–4)‐aperturate. Ectoapertures colpi, 7 – 34×1.5 – 3 μm; margo indistinct. Endoapertures pori, 5 – 10×4 – 7 μm; costae 1 – 4 μm wide, sometimes indistinct.
Ornamentation microreticulate.
Species included: T. auriculata, T. fusca, T. phonsenae, T. postarii.
Type 4 – reticulate ( )
Pollen grains oblate spheroidal to prolate spheroidal. P=36.9 (59.9) 76.5 μm; E=38.9 (61) 74.5 μm. P/E=0.89 – 1.06.
Aperture system 3(–4)‐aperturate. Ectoapertures pori, 5 – 16×3.7 – 12 μm, or (short) colpi, 6 – 22×1.9 – 7 μm; margo indistinct. Endoapertures pori, 2 – 16×2 – 12 μm, sometimes indistinct; costae 1 – 4 μm wide.
Ornamentation reticulate, sometimes reticulate‐perforate or microreticulate; lumina circular to elliptic, or irregular in shape, (0.2–)1 – 5 μm, with crowded infratectal granules; muri (0.25–)0.5 – 3 μm wide.
Subtype 4.1 – porate ()
Pollen grains oblate spheroidal to prolate spheroidal. P=54.2 (65.6) 76.5 μm; E=53.6 (66) 74.5 μm. P/E=0.92 – 1.06.
Aperture system 3(–4)‐aperturate. Ectoapertures mostly pori, 5 – 16×3.7 – 12 μm, sometimes indistinct or short colpi, 6 – 17×1.9 – 7 μm. Endoapertures pori, 2 – 16×2 – 12 μm, sometimes indistinct; costae 1 – 4 μm wide.
Species included: T. borneensis, T. bracteata, T. dunniana, T. ellipsoidea, T. elmeri, T. kostermansii, T. sp. aff. laceribractea, T. pallida, T. pubera, T. quinquangulata, T. refracta, T. sepilokensis, T. siamensis, T. subrosea, T. tricuspidata, T. wallichiana, T. wawrae.
Subtype 4.2 – colporate ()
Pollen grains oblate spheroidal. P=36.9 (40.8) 45.4 μm; E=38.9 (44.2) 50 μm. P/E=0.89 – 0.98.
Aperture system 3(–4)‐aperturate. Ectoapertures colpi, 8 – 22×2 – 6 μm. Endoapertures pori, 4 – 8×2.5 – 7.5 μm; costae 1 – 1.5 μm wide.
Species included: T. ishigakiensis, T. kerrii, T. sericeifolia, T. truncata, T. villosa.
Type 5 – verrucate ( )
Pollen grains oblate spheroidal. P=57.8 μm; E=67.1 μm. P/E=0.89.
Aperture system 3‐aperturate. Ectoapertures pori, 5 – 14×2.5 – 10 μm; margo distinct. Endoapertures indistinct; costae indistinct.
Ornamentation verrucate; verrucae mostly irregularly elongate, 1 – 4 μm, connected by short muri that are separated by small perforations.
Species included: T. cucumerina.
DISCUSSION
Yue & Zhang (Citation1986) described and illustrated the pollen of T. multiloba as rugulate; here it is psilate to finely perforate (). Huang et al. (Citation1997) reported T. ishigakiensis to have coarsely reticulate pollen, while it shows reticulate‐perforate pollen in our study (). Trichosanthes ovigera pollen was found to be finely rugulate, with distinct costae, but here it is psilate to finely perforate, with indistinct costae. The largest deviation is in the description of the pollen of T. villosa by Huang et al. (Citation1997). He denoted it as psilate, with indistinct colpi. In the present study, however, it appeared to be reticulate (), with distinct colpi. Unfortunately, the identity of the material studied by Huang et al. (Citation1997; Yue 82‐084, Yunnan) could not be checked.
Intra‐ and intertribal relationships
The subdivision of the Cucurbitaceae into the subfamilies Zanonioideae and Cucurbitoideae is well‐supported by pollen morphology (Marticorena Citation1963, Jeffrey Citation1964, Citation1990; Khunwasi Citation1998). Pollen of the Zanonioideae is uniform: 3‐colpor(oid)ate, usually medium‐sized (up to 40 μm) and striate, sometimes large (up to 52 μm: Alsomitra, Bolbostemma, Gerrardanthus) and/or perforate or reticulate (Alyoshina Citation1971, Van der Ham Citation1999). Pollen of the Cucurbitoideae is more diverse: usually larger than 40 μm, with various aperture and ornamentation types. According to macromorphology, Trichosanthes is clearly a Cucurbitoideae, and also its pollen, being 30.4 (50.9) 76.5×36.6 (55.9) 74.5 μm, 3(–4)‐colporate or 3(–4)‐porate and not striate, conforms to this subfamily.
Within the tribe Trichosantheae, four subtribes are distinguished: Ampelosicyinae, Herpetosperminae, Hodgsoniinae and Trichosanthinae (Jeffrey Citation1990). Sufficient pollen data for comparison of all genera in these subtribes are provided by Alyoshina (Citation1971; Cephalopentandra), Keraudren (Citation1968; Tricyclandra), Keraudren‐Aymonin et al. (Citation1969, Citation1984; Tricyclandra) and Khunwasi (Citation1998). It appears that pollen in each of the subtribes Ampelosicyinae, Herpetosperminae and Hodgsoniinae clearly differs from that of the Trichosanthinae by their ornamentation (); striate (‐reticulate), baculate/verrucate or coarsely reticulate pollen does not occur in the Trichosanthinae. Hodgsonia pollen is also distinct due to its very large size and relatively long and wide ectoapertures. Pollen of Trichosanthes is more or less similar to that of the other two genera in subtribe Trichosanthinae: Gymnopetalum and Tricyclandra (see below). Because Jeffrey (Citation1964, Citation1990) also used pollen characters to support his classification of the Cucurbitaceae, we cannot use his classification to provide additional support for a close relationship between these three genera. Preliminary results from a molecular analysis (Kocyan et al. Citation2004; Trichosantheae included: Cephalopentandra, Gymnopetalum, Peponium and Trichosanthes) show the tribe Trichosantheae and subtribe Trichosanthinae to be polyphyletic. Trichosanthes is grouped together with Luffa of the monotypic subtribe Luffinae (tribe Benincaseae; Jeffrey Citation1990) and with all members studied of the American tribe Sicyeae (10 out of 18 genera included). Luffa is at the base of the clade, followed by two Trichosanthes species (T. kirilowii and T. cucumeroides), which are sister to a clade in which a third Trichosanthes species (T. rubriflos) is sister to the Sicyeae genera. When we include the pollen data onto this new molecular classification, there is good correlation between the two data sets: pollen of Luffa is 3‐colporate and perforate, while that of the Sicyeae is 4 – 14‐colp(or)ate and either perforate‐(reticulate) or echinate (Khunwasi Citation1998). Trichosanthes pollen is usually 3‐(col)porate and psilate to finely perforate, perforate or (micro)reticulate, sometimes 4‐(col)porate, rarely 5‐porate (Yue & Zhang Citation1986), and rugulate (Huang et al. Citation1997) or verrucate. Within the multiaperturate Sicyeae clade, the genera Microsechium, Sechium, Sechiopsis and Sicyos form an exclusive subclade characterised by pollen with echinate ornamentation. Luffa occurs both in the Old and the New World, Trichosanthes only in the Old World and the Sicyeae only in the New World. Kocyan et al. (Citation2004) already noticed the Old World origin of the largely American Luffa‐Trichosanthes‐Sicyeae clade.
Table II. Selected pollen characters of the genera in the tribe Trichosantheae (Jeffrey Citation1990).
The monotypic genus Tricyclandra (Madagascar; subtribe Trichosanthinae) was not included in the molecular study by Kocyan et al. (Citation2004). Its pollen (Keraudren Citation1968, Keraudren‐Aymonin et al. Citation1969, Citation1984) is similar to that of the verrucate Trichosanthes type 5 (T. cucumerina, ). Macromorphologically, Tricyclandra and T. cucumerina appear to be closely related (W. J. J. O. de Wilde 2004: Pers. comm.). It would be very interesting to include Tricyclandra in the molecular tree and also to study its exine structure using TEM.
Infrageneric relationships
There has been a lot of rearranging of the taxa in subgenera and sections within Trichosanthes. Yueh & Cheng (Citation1974, Citation1980) have subdivided Trichosanthes on the basis of the male bract, fruit pulp and seed characters into subgenus Cucumeroides [with two sections (1974): Cucumeroides and Tetragonosperma, or with one section (1980): Cucumeroides] and subgenus Trichosanthes (with five sections: Foliobracteola, Involucraria, Pedatae, Trichosanthes and Truncata). Jeffrey (Citation1980) listed five sections (Cucumeroides, Foliobracteola, Involucraria, Trichosanthes, Truncata), without mentioning any subgenera (). He considered Tetragonosperma to be a synonym of Cucumeroides and placed section Pedatae as a subsection in Involucraria, proposing subsection Bracteatae for the other species in this section. On account of pollen morphology, Huang et al. (Citation1997) then subdivided section Foliobracteola into the subsections Foliobracteola and Villosa (). Huang et al. (Citation1998) revised the genus again, using macromorphological, cytological, pollen morphological and anatomical characters and reinstalled the subdivision into the two subgenera: Cucumeroides, with two sections (Cucumeroides, Tetragonosperma), and Trichosanthes, with three sections (Involucraria, Foliobracteola, Trichosanthes). Later, Rugayah & De Wilde (Citation1999) used the male bract, fruit pulp and seed characters to distinguish five sections (no subgenera) in Malaysia: Cucumeroides (subsections Cucumeroides and Tetragonosperma), Foliobracteola, Involucraria (subsections Involucraria and Pedatae), Trichosanthes, and Edulis, a new section from New Guinea (). In Sabah, five sections are distinguished (De Wilde & Duyfjes Citation2004): Cucumeroides, Foliobracteola, Involucraria and Trichosanthes, which are more or less the same groups as those of Rugayah & De Wilde (Citation1999), and the new section Asterosperma (). One reason for the problem in subdividing Trichosanthes is due to the lack of a recent monographic revision. So far, the sections are largely based on regional studies, which make their naturalness questionable (see also Duyfjes & Pruesapan: in press). For this reason it is also difficult to compare pollen morphology with macromorphology. The best one can manage under the present circumstances is to compare our mainly southeast Asian and Malaysian pollen data with the subdivision by Rugayah & De Wilde (Citation1999), which includes the Trichosanthes species from Southeast Asia and Malaysia ().
Table III. Comparison of pollen types within selected subdivisions of Trichosanthes.
Our subdivision of Trichosanthes is the first classification based on pollen data only. Although two other extensive pollen revisions exist (Yue & Zhang Citation1986, Huang et al. Citation1997) merely give pollen descriptions of existing groupings (sections, subsections). Our classification, which divides Trichosanthes into five types, is based on ornamentation characters (). Several subtypes are distinguished on the basis of aperture characters (colporate vs. porate, margo distinct vs. indistinct). These types (subtypes) correspond very well to some of the macromorphological groupings of Rugayah & De Wilde (Citation1999), which is explained below (see also ).
Pollen subtype 1.1a (psilate to finely perforate, porate, margo distinct; () corresponds to section Cucumeroides. There is no difference between the pollen of subsection Cucumeroides (T. cucumeroides, T. ovigera) and that of section Tetragonosperma (T. mucronata). Section Cucumeroides represents the former subgenus Cucumeroides.
Pollen subtype 1.1b (psilate to finely perforate, porate, margo indistinct; ) includes all species of Section Edulis (T. edulis, T. pulleana, T. schlechteri), as well as T. coriacea of section Foliobracteola.
Pollen subtype 1.2 (psilate to finely perforate, colporate; ) includes T. multiloba (Japan) and T. rosthornii (China), which are not in Rugayah & De Wilde (Citation1999). However, they are both listed by Jeffrey (Citation1980) in section Foliobracteola. Trichosanthes ishigakiensis was regarded as a synonym of T. rosthornii, but these two species belong to different pollen types: 4 (reticulate) and 1, respectively, although both are in the colporate subtypes (4.2 and 1.2).
Pollen type 2 (perforate; ) includes only T. globosa, which is placed in section Involucraria, together with species that have pollen type 4.1 (reticulate, porate).
Pollen type 3 (microreticulate; ) includes T. phonsenae, T. postarii, T. fusca and T. auriculata. De Wilde & Duyfjes (Citation2004) placed the latter three species in section Asterosperma. Trichosanthes phonsenae may be grouped together with T. kerrii and T. villosa (Duyfjes & Pruesapan: in press), which belong to pollen subtype 4.2 (reticulate, colporate), but unfortunately its seeds are still unknown.
Pollen subtype 4.1 (reticulate, porate; ) corresponds to section Involucraria (T. borneensis, T. ellipsoidea, T. elmeri, T. globosa, T. pubera, T. quinquangulata, T. refracta, T. sepilokensis, T. tricuspidata, T. wallichiana, T. wawrae) in Rugayah & De Wilde (Citation1999), except that T. globosa has pollen type 2; there is no difference between the pollen of subsection Involucraria and that of subsection Pedatae (T. elmeri, T. wawrae). Four other species in our subtype 4.1 (T. bracteata, T. dunniana, T. sp. aff. laceribractea, T. subrosea) are included in section Involucraria in Jeffrey (Citation1980) and/or Huang et al. (Citation1997). Duyfjes & Pruesapan (in press) refrained from a subdivision of Trichosanthes species in Thailand, but according B. E. E. Duyfjes (2004: pers. comm.), T. kostermansii, T. pallida and T. siamensis (pollen subtype 4.1) may very well belong to section Involucraria.
Pollen subtype 4.2 (reticulate, colporate; ) includes T. ishigakiensis, T. kerrii, T. sericeifolia, T. truncata and T. villosa. Only T. villosa occurs in Rugayah & De Wilde (Citation1999), where it was placed in section Foliobracteola. However, in Jeffrey (Citation1980) they are in three sections: Foliobracteola (T. ishigakiensis, as synonym of T. rosthornii; see above: pollen subtype 1.2), Involucraria (T. villosa) and Truncata (T. kerrii, T. sericeifolia, T. truncata). The pollen of T. truncata resembles that of T. cucumerina (type 5) in its relatively thick granular infratectum, but they differ significantly in their ornamentation and nexine thickness.
Pollen type 5 (verrucate; ) includes only T. cucumerina, which belongs to the monotypic section Trichosanthes (see also Jeffrey Citation1980, Huang et al. Citation1997). It represents the most deviating pollen type within the genus, being characterised by verrucate ornamentation, a thick, granular infratectum and a thin nexine. Pollen morphologically and macromorphologically, T. cucumerina seems to be related to the monotypic genus Tricyclandra from Madagascar (see above: Intra‐ and intertribal relationships).
CONCLUSIONS
With regard to the subdivision of Trichosanthes by Rugayah & De Wilde (Citation1999), we can conclude the following. The sections Cucumeroides (subtype 1.1a) and Trichosanthes (type 5) can be well characterised by pollen characters. Section Cucumeroides represents the former subgenus Cucumeroides. Species of section Edulis have a single pollen subtype (subtype 1.1b), but this is also present in one species (T. coriacea) of section Foliobracteola. All species of the large section Involucraria except one (T. globosa, type 2) have a single pollen subtype (subtype 4.1). Section Foliobracteola cannot be recognised, according to pollen morphology. Instead, three pollen types/subtypes (1.1b, 3, 4.2) can be found in the circumscription of this medium‐sized section by Rugayah & De Wilde (Citation1999). The subsections of the sections Cucumeroides and Involucraria cannot be characterised using pollen. Section Trichosanthes, characterised by verrucate pollen (type 5), may be closely related to the genus Tricyclandra. Section Asterosperma (De Wilde & Duyfjes Citation2004) is well‐defined by pollen type 3.
On generic and tribal level there is good correlation between pollen morphological data and the new molecular classification by Kocyan et al. (Citation2004), in which Trichosanthes is placed between the 3‐colporate, perforate Luffa and the 4 – 14‐colp(or)ate, perforate‐(reticulate) or echinate Sicyeae.
ACKNOWLEDGEMENTS
We are very grateful to Brigitta Duyfjes and Willem de Wilde (Nationaal Herbarium Nederland) for taxonomic help, Bertie Joan van Heuven, Ben Kieft and Wim Star (Nationaal Herbarium Nederland) for technical assistance, the curators of the herbaria in Kew (K) and Paris (P) for making important material available, and two anonymous reviewers for their valuable comments on the manuscript. Thanks are also due to the ASEAN Regional Centre for Biodiversity Conservation (ARCBC), which funded the research by the first author at the Leiden branch of the Nationaal Herbarium Nederland.
SPECIMENS EXAMINED
(TEM – sample examined using transmission electron microscopy)
Trichosanthes species; Location. Collection; Herb.; * (TEM)
T. auriculata Rugayah; Indonesia: Borneo. Endert 3014; L;
T. auriculata Rugayah; Indonesia: Borneo. Church et al. 702; L;
T. borneensis Cogn.; Indonesia: Sumatra. Biesmeijer 3356; L;
T. borneensis Cogn.; Indonesia: Borneo. Mirmanto EM (98) 29; L;
T. bracteata (Lam.) Voigt; Japan. Furuse 4660; K;
T. coriacea Blume; Indonesia: Sumatra. Korthals s.n. (L 0130316); L;
T. coriacea Blume; Indonesia: Sumatra. Korthals s.n. (L 0130314); L;
T. cucumerina L.; Indonesia: Java. De Wilde & Duyfjes 21804; L;
T. cucumerina L.; Thailand. Maxwell 91‐911; L;
T. cucumerina L.; Indonesia: Sumbawa. Kostermans 18027; L; *
T. cucumeroides Maxim.; Japan. Furuse 43876; K;
T. cucumeroides Maxim.; Japan. Oldman 286; K;
T. cucumeroides Maxim.; Japan. Karasuwuri s.n.; L; *
T. dunniana Lév.; India. Hooker & Thomson s.n.; K;
T. edulis Rugayah; Indonesia: New Guinea. NGF 3993; L;
T. edulis Rugayah var. edulis; Indonesia: New Guinea. Kalkman 4401; L;
T. ellipsoidea Merr.; Malaysia: Sarawak. S 34116; L;
T. elmeri Merr.; Malaysia: Sabah. SAN 113625; L;
T. elmeri Merr.; Indonesia: Sumatra. De Wilde & Duyfjes 19677; L;
T. fusca De Wilde & Duyfjes; Indonesia: Kalimantan Timur; Kato. Okamoto & Walujo B‐11279; L;
T. globosa Blume; Malaysia: Sabah. SAN 144003; L; *
T. ishigakiensis E.H. Walk.; Japan. Furuse 3577; K;
T. kerrii Craib; Vietnam: Tonkin. Petelot 8415; P;
T. kostermansii Duyfjes & Pruesapan; Thailand. Kostermans 743; L;
T. mucronata Rugayah; Malaysia: Sabah. SAN 139467; L;
T. multiloba Miq.; Japan. Ko‐isi‐wara s.n. (1863); K;
T. ovigera Blume; Thailand. De Wilde & Duyfjes 22138; L;
T. ovigera Blume; Thailand. Maxwell 89‐1462; L;
T. ovigera Blume; China. Ford 510; K;
T. pallida Duyfjes & Pruesapan; Thailand. Geesink & Phengkhlai 6183; L;
T. phonsenae Duyfjes & Pruesapan; Thailand. Phonsena, De Wilde & Duyfjes 4002; L; *
T. postarii De Wilde & Duyfjes; Malaysia: Sabah. SAN 139470; L;
T. pubera Blume ssp. pubera; Indonesia: Java. De Wilde 21885; L; *
T. pubera Blume ssp. pubera; Indonesia: Sumatra. Korthals s.n. (L 0130557); L;
T. pubera Blume ssp. rubriflos (Cayla) Duyfjes & Pruesapan; Thailand. Phonsena, De Wilde & Duyfjes 3926; L; *
T. pubera Blume ssp. rubriflos (Cayla) Duyfjes & Pruesapan var. fissisepala Duyfjes & Pruesapan; Thailand. Maxwell 89‐553; L;
T. pubera Blume ssp. rubriflos (Cayla) Duyfjes & Pruesapan var. fissisepala Duyfjes & Pruesapan; Thailand. Kostermans 1078; L;
T. pubera Blume ssp. rubriflos (Cayla) Duyfjes & Pruesapan var. rubriflos; Thailand. Maxwell 75‐719; L;
T. pulleana Harms; Indonesia: Moluccas. Nooteboom 5777; L;
T. quinquangulata A. Gray; Malaysia: Malay Peninsula. De Wilde & Duyfjes 21967; L;
T. quinquangulata A. Gray; Indonesia: Java. De Wilde et al. 21772; L; *
T. quinquangulata A. Gray; Thailand. Maxwell 75‐693; L;
T. refracta Yueh & Huang; Brunei. BRUN 16261; L;
T. refracta Yueh & Huang; Brunei. Van Niel 4134; L;
T. rosthornii Harms; China. Farges 872; K;
T. rosthornii Harms; China. Ford 81; K;
T. rosthornii Harms; China. Teng 90473; L; *
T. schlechteri Harms; Indonesia: New Guinea. Shea 1464; L;
T. schlechteri Harms; Indonesia: New Guinea. Van Royen & Sleumer 6183; L;
T. sepilokensis Rugayah; Malaysia: Sabah. SAN 151201; L;
T. sepilokensis Rugayah; Malaysia: Sabah. SAN 143739; L;
T. sericeifolia Cheng & Yueh; China. Henry 10509; K;
T. siamensis Duyfjes & Pruesapan; Thailand. Maxwell 94‐546; L;
Trichosanthes sp. aff. laceribractea Hayata; China: Hainan; Wai‐Tak Tsang 702; K;
Trichosanthes sp. aff. laceribractea Hayata; Laos. Poilane 16778; P;
T. subrosea Cheng & Yueh; Burma. F.K.W. 7248; K;
T. tricuspidata Lour.; Indonesia: Java. Boerlage 145; L;
T. tricuspidata Lour.; Indonesia: Java. De Wilde et al. 21811; L; *
T. tricuspidata ssp. javanica; Thailand. Larsen 32315; L;
T. tricuspidata ssp. tricuspidata; Thailand. Maxwell 96‐854; L;
T. tricuspidata ssp. tricuspidata; Thailand. Palee 385; L;
T. truncata C.B. Clarke; India. King s.n. (1876); L; *
T. truncata C.B. Clarke; Thailand. Maxwell 93‐862; L;
T. truncata C.B. Clarke; Thailand. Maxwell 93‐946; L; *
T. villosa Blume; Indonesia: Java. De Wilde et al. 21883; L; *
T. wallichiana (Ser.) Wight; Nepal. Grey‐Wilson & Philips 216; K;
T. wawrae Cogn.; Indonesia: Sumatra. De Wilde & Duyfjes 20711; L;
T. wawrae Cogn.; Thailand. Maxwell 76‐381; L;
T. wawrae Cogn.; Indonesia: Java; Cogniaux 57; L;
References
REFERENCES
- Alyoshina LA 1971 Palynological data on the systematics and phylogeny of the family Cucurbitaceae Juss. – In: Pollen morphology – Cucurbitaceae, Thymelaeaceae, Cornaceae (ed. L. A. Kuprianova & M. S. Jakovlev) pp. 5–103 – Komarov Bot. Inst., USSR Acad. Sci. Leningrad
- Beevy , S and Kuriachan , P . 1996 . Pollen morphology and taxonomy of the genus Trichosanthes (Cucurbitaceae). . – J. Palynol. , 32 : 21 – 27 .
- De Wilde , WJJO and Duyfjes , BEE . 2004 . The genus Trichosanthes (Cucurbitaceae) in Sabah. . – Sandakania , 14 : 5 – 32 .
- Erdtman G 1952 Pollen morphology and plant taxonomy. Angiosperms. – Almquist & Wiksell Stockholm
- Huang , L‐Q , Yang , B and Yue , C‐X . 1997 . Pollen morphology of Trichosanthes and its taxonomic significance. . – Acta Phytotax. Sin. , 35 : 125 – 135 .
- Huang L‐G Yue C‐X Yang B Cheng C‐Y Li N‐H Ruan C Qu L‐J Gu H‐Y 1998 Systematic studies on Trichosanthes L. (Cucurbitaceae). – In: Proceed. Cross‐Strait Symp. Flor. Div. Conserv. Taipei 1996 (ed. S.‐T. Chiu & C.‐I. Peng), pp. 75–83 – Natl. Mus. Nat. Sci. Taichung Taiwan
- Huang T‐C 1972 Pollen flora of Taiwan. – Bot. Dept. Press, Natl Taiwan Univ. Taipei
- Ikuse M 1956 Pollen grains of Japan. – Hirokawa Publ. Co. Tokyo
- Jeffrey , C . 1964 . A note on pollen morphology in Cucurbitaceae. . – Kew Bull. , 17 : 473 – 477 .
- Jeffrey C 1980 The Cucurbitaceae of eastern Asia. – R. Bot. Gards Kew
- Jeffrey C 1990 An outline classification of the Cucurbitaceae. – In: Biology and utilization of the Cucurbitaceae (ed. D. M. Bates, R. W. Robinson & C. Jeffrey) pp. 449–463, Appendix Cornell Univ. Press Ithaca N.Y
- Keraudren , M . 1968 . Recherches sur les Cucurbitacées de Madagascar. . – Mém. Mus. Natl Hist. Nat. NS, Sér. B, Bot. , 16 : 126 – 330 .
- Keraudren‐Aymonin M Straka H Friedrich B 1984 Fam. 184–188. Microscopie électronique á balayage et addenda. – In: Palynologia Madagassica et Mascarenica (ed. M.‐T. Cerceau‐Larrival, M. Keraudren‐Aymonin, D. Lobreau‐Callen, H. Straka & B. Friedrich) pp. 117–136 – Trop. Subtrop. Pflanzenwelt 51. Akad. Wissensch. Lit., Mainz / F. Steiner, Wiesbaden/Stuttgart
- Keraudren‐Aymonin , M , Straka , H and Simon , A . 1969 . Palynologica Madagassica et Macarenica: Fam. 184–188. . – Pollen Spores , 11 : 299 – 332 .
- Kocyan A Zhang L‐B Renner S 2004 Towards a densely sampled phylogeny of Cucurbitaceae wordwide. – In: Botany 2004. Int. Meet. BSA, AB‐LS, ASPT & AFS Ann. Meet., Salt Lake City, 2004 (ed. Org. Comm.). Syst. Sect. Abstr. 326 & Poster., – www.botanyconference.org
- Khunwasi C 1998 Palynology of the Cucurbitaceae. – Diss. Naturwissensch. Fak. L‐F‐Univ., Innsbruck
- Mandal , S and Chanda , S . 1981 . Pollen atlas of Kalyani, West Bengal, with reference to aeropalynology. . – Trans. Bose Res. Inst. , 44(3) : 35 – 62 .
- Marticorena , C . 1963 . Material para una monografía de la morfología del polen de Cucurbitaceae. . – Grana Palynol. , 4 : 78 – 91 .
- Punt W Blackmore S Nilsson S Le Thomas A 1994 Glossary of pollen and spore terminology. – LPP Contrib. Ser. 1. LPP Found., Utrecht
- Rugayah and De Wilde , WJJO . 1999 . Conspectus of Trichosanthes (Cucurbitaceae) in Malesia. . – Reinwardtia , 11 : 227 – 280 .
- Van der Ham RWJM 1990 Nephelieae pollen (Sapindaceae): form, function, and evolution. – Leiden Bot. Ser. 13., Natl. Herb. Neerl. Leiden
- Van der Ham , RWJM . 1999 . Pollen morphology of Bayabusua (Cucurbitaceae) and its allies. . – Sandakania , 13 : 17 – 22 .
- Yue , C‐X and Zhang , Y‐L . 1986 . Studies on the pollen morphology of Chinese Trichosanthes. . – Bull. Bot. Res. (China) , 6(2) : 21 – 35 .
- Yueh , C‐H and Cheng , C‐Y . 1974 . A preliminary study on the Chinese medicinal species of genus Trichosanthes L. . – Acta Phytotax. Sin. , 12 : 418 – 447 .
- Yueh , C‐H and Cheng , C‐Y . 1980 . The Chinese medicinal species of the genus Trichosanthes L. . – Acta Phytotax. Sin. , 18 : 333 – 352 .