Abstract
Pollen morphology is widely used in taxonomic treatments of tribe Vernonieae, and differences in exine structure and aperture form have been described for many species within the genera comprising the tribe. However, in recent years a number of new species have been described and, in the present paper, we describe in detail the pollen of 24 species of the tribe which are either endemic or uncommon to certain regions of Argentina or of Paraguay. The pollen of these species are radially symmetrical and more or less spheroidal, 3‐colporate or, in one species, 3‐porate; the exine may be tectate or semitectate and microperforate, with echinate lophae or, in the 3‐porate example, psilate lophae. Using a range of characteristics related to size, shape, wall thickness, apertures and tectum surface morphology, five of the six pollen types previously described for tribe Vernonieae, and a subtype, are recognized. Our results support the usefulness of pollen morphology in helping to determine the taxonomic position of species within tribe Vernonieae.
The Vernonieae Cass. (Asteraceae) are a pantropical tribe of 89 genera which are concentrated around two major centers of diversification: the central region of the African continent and southern Brazil. Most of the species are included in Vernonia Schreb. which is by far the largest genus of the tribe with about 1 100 species. Vernonia shows considerable variation in habit, ranging from small, scapose herbs to large trees (Bremer, Citation1994).
The most characteristic feature of the Vernonieae is found in the styles, which are slender with filiform, pilose branches and an upper style shaft bearing long sweeping hairs (Robinson, Citation1999). The pollen of the tribe also has very characteristic morphology: the tectum consists of a pattern of lophae (crests or ridges of tectum) and lacunae (cf. very wide deep lumina) or deep depressions in the tectum continuous with the lophae (Wodehouse, Citation1928; Skvarla et al., 1979). The lophae in many species of the tribe are spinose and, the spines are either disposed on the central ridge of the lophae (echinolophate) or, arranged in groups in a more or less reticulate pattern (subechinolophate). Less frequently there are species of Vernonieae where the lophate pollen lacks spines, instead, although the lophae form a reticulate pattern, they are narrow and smooth ridged (psilolophate). Between species, Vernonieae pollen is also variable in aperture form, with both tricolporate and triporate forms (Stix, Citation1960; Kingham, Citation1976; Keeley & Jones, Citation1979; Blackmore, Citation1986).
The first descriptions of Vernonieae pollen morphology were those of Steetz (Citation1864), followed by those of Fischer (Citation1890). More detailed descriptions of the main pollen forms which occur Vernonia were provided by Wodehouse (Citation1928), who described the distribution of ‘crests’ and lacunae in different pollen forms in Compositae, and also established a terminology which has become the basis for the description of the unusual and characteristic pollen morphology found in a wide number of species in the family. Kingham (Citation1976) used scanning electron microscopy to describe the surface patterns of the tropical African species of the tribe, and found a range of different pollen forms, most of which were exclusive to sections or groups. The pollen types used to describe unique combinations of pollen characteristics observed in tribe Vernonieae date initially from Keeley and Jones (Citation1979). Later Robinson (Citation1992) described several additional characteristics special to Vernonieae. So far a total of ten pollen types have been recognized for the tribe; some of these are restricted to Old World species, while others are distinctive for Central and South American taxa (Jones, Citation1979, Citation1981; Robinson, Citation1999).
Pollen morphology constitutes one of the most useful suites of characters for taxonomic studies in tribe Vernonieae, and a correlation between pollen data and the classification of the West Indian species of Vernonia has already been demonstrated by Keeley and Jones (Citation1977). Based on the variation in pollen surface morphology, Jones (Citation1979) proposed a new infrageneric classification for the American members of Vernonia. Segregation of different sections and subsections of Vernonia to new genera was realized by Robinson (Citation1988, Citation1989); in several cases the re‐circumscription was based on pollen type. The most recent classification of the New World Vernonieae, proposed by Robinson (Citation1999) is supported by a number of different features including external vegetative morphology, chromosomes, chemical composition and, importantly, pollen morphology.
Furthermore, the pollen types described for the tribe have been shown to correlate, in several cases, to other characters of taxonomic relevance, for example, chromosome number (Dematteis & Robinson, Citation1997; Dematteis & Salgado, Citation2001; Dematteis, Citation2002) or arrangement of the capitula (floral heads) (Robinson, Citation1999). In Vernonieae it has been noted (Robinson, Citation1992) that the species with tricolporate pollen generally have the base chromosome number of x = 17, while the species with triporate pollen have chromosome base numbers of x = 8, x = 9, x = 10, x = 11 or x = 13.
The surface morphology of Vernonieae pollen has been examined in a considerable number of genera and species because of its relevance to the taxonomy (Cabrera, Citation1944; Stix, Citation1960; Smith, Citation1969; Jones, Citation1970, 1979, Citation1981; Skvarla et al., Citation1977, Citation2005; Keeley & Jones, Citation1977, Citation1979; Bolick, Citation1983; Isawumi et al., Citation1996; Robinson, Citation1999; Dematteis & Salgado, Citation2001; Dematteis, Citation2003). In the last few years further new species of Vernonia have been described (Cabrera & Dematteis, Citation1999; Dematteis, Citation2000, Citation2003; Cristóbal & Dematteis, Citation2002) as these pollen has not been described in detail. There are also some species of Vernonieae which are relatively uncommon or narrowly distributed endemics, known from only one or a few populations (Jones, Citation1977; Robinson, Citation1999; Dematteis, Citation2006; Cabrera & Dematteis, Citation2008). The taxonomic position of almost all these species has yet to be established within the tribe, and their pollen morphology has not been described.
The purpose of our study has been to examine the pollen of 24 recently described species of Vernonia from Argentina and from Paraguay, and to consider their possible taxonomic positions within the genus based on pollen characteristics.
Material and methods
Pollen samples were obtained by removing one or two florets from herbarium specimens of each species. Voucher specimens are mainly deposited in the Herbarium of the Instituto de Botánica del Nordeste (CTES). The sources of the specimens examined are detailed in the Appendix where the taxa are grouped alphabetically by country of origin (Argentina or Paraguay). Pollen data are summarized in Table . It should be noted that because the taxonomic position within tribe Vernonieae is, for several of the taxa/collections, uncertain or in need of review, all species examined in this study are considered to belong to Vernonia sensu lato for the time being.
Table I. Vernonia pollen, summary data for species examined and pollen types distinguished (A–D; see column 13). All measures (Columns: 4, 5, 8–12) in µm; mean values in brackets. Country of origin (Column 2): A – Argentina, P – Paraguay.
The pollen was acetolysed according to Erdtman (Citation1960). For light microscopy (LM) the pollen samples were mounted in glycerol jelly on glass slides and then examined with a Zeiss Axioplan microscope. Permanent slides were deposited at the Palynological Laboratory of the Universidad Nacional del Nordeste (PAL‐CTES). Pollen measurement data were calculated from at least 30 grains per sample.
For scanning electron microscopy (SEM) acetolysed pollen grains were first washed in 96° alcohol, followed by a further wash in absolute alcohol. The samples were then air dried in Petri dishes and subsequently sputtered with gold palladium prior to observation using a JEOL 5800 LV scanning electron microscope. The terminology used for pollen grain description follows terms accepted in Punt et al. (Citation1994, Citation2006, and Citation2007). The quantitative and qualitative characters used to define the pollen types we describe are summarized in Table .
Results
General pollen morphology
Pollen grains are radially symmetric, oblate spheroidal, spheroidal or prolate spheroidal (P/E 0.93–1.07) and either, 3‐colporate or 3‐porate; equatorial outline subcircular to circular, polar outline rounded triangular, or subcircular to circular. Colpus length, the distance between the polar extremes of the abporal lacunae, ranges from 15–25 µm (long = 15–20 µm, very long = >20 µm); the abporal lacunae have interrupted interlacunar lophae at the interfaces with the poral lacuna. Pori lalongate, often slightly constricted in the central region (Figure ) or almost circular. Pollen size, polar length (P): ranges from 32.5–78.4 µm, equatorial width (E); ranges from 32.5–75.4 µm; exine thickness, excluding spines, 2.4–13.0 µm; sexine: 1.5–12.0 µm, notably wider than nexine: 0.5–1.0 µm. Tectum either continuous, comprising lophae (crests or ridges) surrounding depressions (Figure ) or discontinuous comprising lophae surrounding lacunae (Figures , ), the upper surface of the nexine is visible in the bases of the lacunae; the outline of the lacunae may be more or less regular (Figures , , , ) or notably irregular (Figures , ). The tectum surface is usually densely microperforate and spinose, spines 2–6 µm long (Figures , , ) or, less commonly, imperforate and psilate (Figures , ).
Figure 1. Pollen ofVernonia (LM). A, B. Pollen Type A – V. cupularis. Montes s. n.: (A) Slightly tilted equatorial view, showing aperture (left); (B) Polar view. C, D. Pollen Type A, subtype Aa – V. novarae. Vervoorst & Legname 4581: (C) Equatorial view; (D) Polar view, high focus. E–G. Pollen Type B – V. spicata. Schultz 7185: (E) equatorial view, mesocolpium; (F) Equatorial view showing aperture; (G) Polar view. H–J. Pollen Type D – V. amambaia. Dematteis & Schinini 867: (H) Equatorial view, aperture mid‐low focus; (I) Equatorial view, aperture high focus; (J) Polar view. K–M. Type C – V. propinqua var. canescens. Dematteis 888: (K) Equatorial view; (L) polar view, high focus; (M) Polar view, mid focus. N, O. Pollen Type E – V. brunneri. Brunner 1720: (N) Polar view; (O) Equatorial view. [All images × 1000].
![Figure 1. Pollen ofVernonia (LM). A, B. Pollen Type A – V. cupularis. Montes s. n.: (A) Slightly tilted equatorial view, showing aperture (left); (B) Polar view. C, D. Pollen Type A, subtype Aa – V. novarae. Vervoorst & Legname 4581: (C) Equatorial view; (D) Polar view, high focus. E–G. Pollen Type B – V. spicata. Schultz 7185: (E) equatorial view, mesocolpium; (F) Equatorial view showing aperture; (G) Polar view. H–J. Pollen Type D – V. amambaia. Dematteis & Schinini 867: (H) Equatorial view, aperture mid‐low focus; (I) Equatorial view, aperture high focus; (J) Polar view. K–M. Type C – V. propinqua var. canescens. Dematteis 888: (K) Equatorial view; (L) polar view, high focus; (M) Polar view, mid focus. N, O. Pollen Type E – V. brunneri. Brunner 1720: (N) Polar view; (O) Equatorial view. [All images × 1000].](/cms/asset/48c24d7c-4606-4e54-b064-285a555ff298/sgra_a_315330_o_f0001g.gif)
Figure 2. Pollen ofVernonia, Types A and B (SEM). A, B. Pollen Type A – V. lipeoensis. Cabrera et al. 23971: (A) Equatorial view, mesocolpium; (B) Close up of porus. C, D. Pollen Type Aa – V. novarae. Vervoorst & Legname 4581: (C) Equatorial view, mesocolpium; (D) Detail of spines. E, F. Pollen Type B – V. centauropsidea. Hilgert 2031: (E) Polar view showing the colpi (arrow) and the absence of polar lacuna; (F) Detail of lacunae and spines. Scale bars – 10 µm.
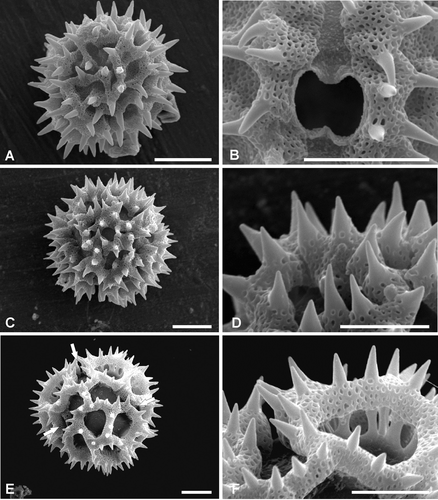
Figure 3. Pollen ofVernonia, Types C–E (SEM). A, B. Type C – V. propinqua var. canescens. Dematteis 888: (A) Polar view showing polar lacuna (arrow); (B) Detail of ‘A’ note colpate interruption to spinose lophate ridge (arrow); C, D. Pollen Type D – V. amambaia. Dematteis 867: (C) Equatorial view, mesocolpia, showing the 4 intercolpar lacunae, one interporal lacuna also visible (top centre); (D) Close up, perimeter of polar view note colpus interruption to lophate ridge (arrow). E, F. Type E – V. brunneri. Brunner 1720: (E) Mesocolpial view; (F) Close up of pore. Scale bars – 10 µm.
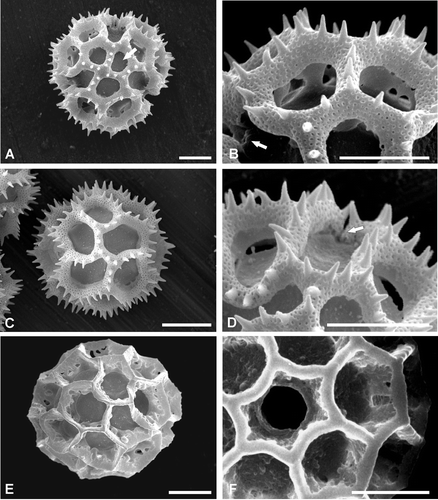
Figure 4. Diagrammatic representation of the variations to the arrangement of the lacunae in the 3 echinolophate pollen types(B–D) of Vernonia. Ai–Aiii. Pollen Type B: (Ai) Polar view, note absence of polar lacuna; (Aii) Equatorial view ‐ mesocolpium, note presence of equatorial lacuna (e); (Aiii) Equatorial view ‐ colporus, note slight lophate constriction between abporal (a) and poral (pl) lacunae. Bi–Biii. Pollen Type C: (Bi) Polar view, note presence of polar lacuna (po); (Bii) Equatorial view – mesocolpium, note absence of central mesocolpial lacuna; (Biii) Equatorial view – colporus, note lophate constrictions demarcating colpus between abporal (a) and poral (pl) lacunae. Ci–Ciii. Pollen Type D: (Ci) Polar view, note absence of polar lacuna; (Cii) Equatorial view – mesocolpium, note absence of equatorial lacuna; (Ciii) Equatorial view – colporus, note lophate constrictions demarcating colpus between abporal (a) and poral (pl) lacunae. Symbols on diagram: a – abporal lacuna; i – interpolar lacuna; e – equatorial lacuna; p – paraporal lacuna; po – polar lacuna; pl – poral lacuna.
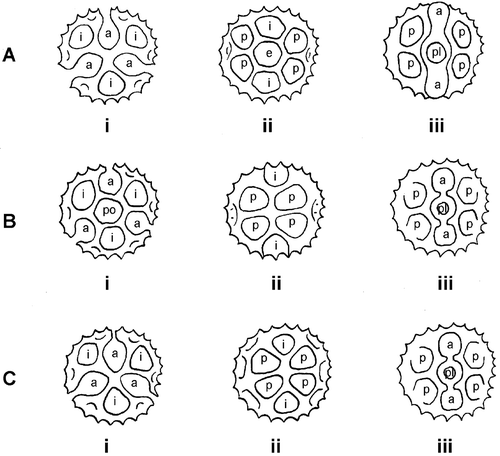
Five pollen types and one subtype are recognized following the six pollen types of Keeley and Jones (Citation1979): pollen types B, C and D are distinguished particularly by the lacunae pattern and the presence or absence of the central equatorial or central polar lacunae (see Figure ). The pollen types are described below in order of the least to the most specialized (Bolick & Keeley, Citation1994).
Type A (Figures , )
Pollen grains oblate spheroidal, spheroidal or prolate spheroidal (P/E 0.98–1.07), 3‐colporate, subechinolophate. Colpi long: between 15–20 µm, pori lalongate. Pollen size, P: 32.5 (42.5) 54.9 µm, E: 32.5 (42.1) 52.9 µm. Exine thickness, excluding spines, 2.4–6.5 µm. Tectum continuous, comprising lophae surrounding depressions (Figure ); outline of depressions irregular (Figure ). Tectum surface densely microperforate and spinose, spine length 2.4–8.0 µm, apices acute, with the exception of V. schulziana which has spines with rounded apices.
Species included: V. chaquensis, V. cichoriiflora, V. cupularis, V. lipeoensis, V. schulziana
Subtype Aa (Figures , )
Pollen grains prolate spheroidal (P/E 1.01–1.02), 3‐colporate, echinolophate. Colpi very long: 33–43 µm (notably longer than in pollen type A), with the apices visible on the polar face, pori lalongate to circular. Pollen size, P: 33.8 (38.5) 42.9 µm, E: 33.8 (37.6) 42.2 µm. Exine thickness, excluding spines, 2.6–5.2 µm. Tectum discontinuous, comprising lacunae surrounded by lophae (Figure ); outline of lacunae irregular (Figure ); number of mesocolpial lacunae variable. Tectum surface densely microperforate and spinose, spine length 2.4–4.8 µm, apices acute.
Species included: V. novarae
Type B (Figures , , )
Pollen grains oblate spheroidal, spheroidal or prolate spheroidal (P/E 0.98–1.06), 3‐colporate, echinolophate. Colpi very long: 44–77 µm, with apices visible in polar view, the colpi interrupt the lophae that separate the poral lacuna from the abporal lacunae (Figure ); pori subcircular or, sometimes, lalongate. Pollen size, P: 40.3 (57.5) 78.4 µm, E 39 (56.5) 75.4 µm. Exine thickness, excluding spines, 3.9–13.0 µm. Tectum discontinuous (Figure ), comprising lacunae surrounded by lophae (Figure ); outline of lacunae more or less regular; total number of lacunae 30 (Figure ): 3 poral, 6 abporal, 3 equatorial (central mesocolpium), 12 paraporal and 6 interpolar, polar lacunae absent. Tectum surface densely microperforate and spinose, spines with a linear distribution along the ridges of the lophae, spine length: 1.6–5.6 µm, apices acute, with the exception of V. hystricosa which has rounded apices.
Species included: V. bellula, V. centauropsidea, V. correntina, V. hassleriana, V. hystricosa, V. lanata, V. parvifolia, V. platyphylla, V. profusa, V. ramellae, V. spicata, V. teyucuarensis, V. valenzuelae
Type C (Figures , , )
Pollen grains oblate spheroidal, prolate or prolate spheroidal (P/E 0.97–1.06); 3‐colporate, echinolophate. Colpi long: 39–56.8 µm, the colpi interrupt the lophae that separate the poral lacuna from the abporal lacunae (Figure ); pori lalongate to subcircular. Pollen size, P: 40.3 (49.25) 58.5 µm, E: 37.7 (46.4) 52.9 µm. Exine thickness, excluding spines, 3.9–9.1 µm. Tectum discontinuous (Figure ), comprising lacunae surrounded by lophae (Figure ); outline of lacunae more or less regular; total number of lacunae 29 (Figure ): 3 poral, 6 abporal, 12 paraporal, 6 interporal and 2 polar or, rarely, 4 polar lacunae (two at each pole) have been recorded, equatorial lacunae absent. Tectum surface densely microperforate and spinose, spines with a linear distribution along the ridges of the lophae, spine length: 1.6–4.8 µm, apices acute.
Species included: V. hystrix, V. propinqua var. canescens, V. setososquamosa
Type D (Figures , , )
Pollen grains prolate spheroidal (P/E 1.01–1.03); 3‐colporate, echinolophate. Colpi very long: 37.0–44.5 µm long, with apices visible in polar view; the colpi interrupt the lophae that separate the poral lacunae from the abporal lacunae (Figure ); pori subcircular. Pollen size, P: 37.7 (41.0) 44.5 µm, E: 37.7 (40.0) 42.9 µm. Exine thickness, excluding spines, 2.6–6.5 µm. Tectum continuous, comprising lacunae surrounded by lophae (Figure ); outline and disposition of lacunae more or less regular (Figure ); total number of lacunae 27 (Figure ): 3 poral, 6 abporal, 12 paraporal and 6 interporal lacunae, polar lacunae absent, equatorial lacunae absent. Tectum surface densely microperforate and spinose, spines with a linear distribution along the ridges of the lophae, spine length: 2.4–4.0 µm, apices acute.
Species included: V. amambaia
Type E (Figures , )
Pollen grains prolate spheroidal (P/E 1.02); 3‐porate, psilolophate. Pori more or less circular, 6–8.5 µm in diameter (Figure ). Pollen size, P: 49.4 (51.9) 54.6 µm, E: 45.5 (50.8) 54.6 µm. Exine thickness 6.5–9.1 µm. Tectum discontinuous, comprising narrow thin‐walled lophae surrounding lacunae (Figure ); outline and disposition of lacunae more or less regular and disposed in a geometric pattern; total number of lacunae 30–34, including 3 poral. Surface of lophae imperforate‐psilate.
Species included: V. brunneri
Key to the pollen types
-
Pollen grains psilolophate…2a
-
Pollen grains spinose & microperforate lophate…2b
-
3‐porate…Type E
-
3‐colporate…3
-
Irregular depressions or lacunae in tectum…4
-
More or less regular lacunae…5
-
Tectum continuous with depressions and lophate ridges…Type A
-
Tectum discontinuous with lacunae and lophate ridges…Type Aa
-
Pollen without equatorial lacunae…6
-
Pollen with equatorial lacunae…Type B
-
Pollen with polar lacunae…Type C
-
Pollen without polar…Type D
Discussion
The pollen types for tribe Vernonieae were established initially by Keeley and Jones (Citation1979). They described six pollen types for the genus Vernonia s. l. based mainly on variation in apertures and surface morphology. Robinson (Citation1992) later made some modifications to the original six types and, currently, ten pollen types are recognised for the tribe. In the present study five of Keeley and Jones (Citation1979) six pollen types have been found: A, B, C, D and E, as well as an additional discontinuous tectum variant of pollen Type A, which we have designated pollen Subtype Aa because we consider the exine to be similar to, but a modification of the continuous tectum of pollen Type A. Pollen Subtype Aa also has longer colpi than pollen Type A.
To date Keeley and Jones (Citation1979) six pollen types have shown differing distributions between the Old and New Worlds. Pollen Type A (subechinolophate) as well as pollen Types B (echinolophate with central mesocolpial lacunae but no polar lacunae – Figure ) and C (echinolophate with central polar lacunae but no central mesocolpial lacunae – Figure ) occur in both the Old and the New World; while pollen Type D (echinolophate without central polar or central mesocolpial lacunae – Figure ) is restricted to Brazilian taxa. The two psilophate pollen types, E and F, are almost entirely restricted to Old World species. Type F is triporate and multilacunate as in Type E, but usually the tectum of the lophae is perforate with many short spinulae along the ridges of the lophae (Robinson, Citation1992).
Most of the species in our study have not been included previously in taxonomic treatments of tribe Vernonieae and, therefore, their taxonomic position within the tribe has not been considered yet.
The morphology of the pollen described for V. amambaia differs from a previous study that recorded Type G pollen in this species (Robinson, Citation1998). Type G pollen was defined by (Robinson, Citation1998) as encompassing characteristics which distinguish V. amambaia. Like Type D pollen it is tricolporate and echinolophate, but the abporal and poral lacunae are not separated completely by lophae. However, the two specimens of V. amambaia (one a paratype) that we have examined both have Type D pollen. In the light of this conflict of opinion, pollen of additional individuals should be examined to determine, more confidently, any possible disparity in pollen morphology between the pollen grains of a range of collections of V. amambaia.
Vernonia setososquamosa has sometimes been considered as a variety of the widespread V. remotiflora L.C. Rich. (Robinson, Citation1999), but was recently restored as a species on the basis of morphological features (Cristóbal & Dematteis, Citation2003). Vernonia setososquamosa is a perennial herb, and has a shorter growth habit than that of V. remotiflora, furthermore, the tip of the phyllaries are largely aristate and these characteristics suggest that V. setososquamosa is more closely related to V. amambaia, which is also a perennial and has aristate phyllaries. The most important difference between V. setosoquamosa and V. amambaia are the corolla lobes which, in V. amambaia, have small hairs. The difference in pollen type between V. amambaia (pollen Type D) and V. setososquamosa (pollen Type C) provides an additional feature to distinguish between the two species.
The pollen type observed in V. novarae is intermediate between pollen Type A and pollen Type B, although we consider it to be a modification of Type A. This modified form is uncommon in the tribe Vernonieae and has been previously found only in the Brazilian monotypic genus Bishopalea H. Rob. (Robinson, Citation1999) to which Vernonia novarae is not related.
The species with Type B pollen have, in general, larger pollen than the remaining species of the tribe. This is coincident with cytological information which indicates that these species include the greatest proportion of polyploid entities, and the highest ploidy levels in Vernonia (Dematteis, Citation2002). The greater number of chromosomes are likely to be responsible for the larger pollen size observed in these species, this is supported by the fact that, within Type B taxa, the larger grains tend to occur in the species with higher ploidy levels, such as V. hystricosa and V. teyucuarensis, which are octoploid and decaploid respectively.
Pollen Type C occurs mostly in the New World taxa of Vernonia Subsect. Flexuosae ( = Chrysolaena H.Rob.) and Vernonia Sect. Stenocephalum ( = Stenocephalum Sch. Bip.) two groups distributed in southern South America. However, they are not closely related and differ considerably in many morphological features, such as the inflorescence pattern, shape of the capitula, and chromosome number. Section Stenocephalum species have spicate inflorescences, cylindrical heads and a base chromosome number of x = 17, while Chrysolaena has cymose inflorescences, campanulate heads and a base chromosome number of x = 10 (Dematteis, Citation1997).
Pollen Type E occurs frequently in Old World species of Vernonieae, but in the New World, has been found only in four taxa from Paraguay: Pacourina edulis Aubl. (Robinson, Citation1999), Vernonia echitifolia Mart. ex DC. (Dematteis & Robinson, Citation1997), V. rojasii Cabrera (Dematteis & Salgado, Citation2001) and V. brunneri (Robinson, Citation1999); because of this it has been previously suggested (Robinson, Citation1999; Dematteis & Salgado, Citation2001), that the presence of Type E pollen in these New World species indicates that they are probably more closely related to Old World members of Vernonieae than to other New World taxa.
Conclusions
In our study we have found five of the six pollen types recognized for Vernonia s.l., in South America, including pollen Type E which is only rarely recorded in New World Vernonieae. The pollen types recognised for Vernonieae tend to occur in different groups of the tribe and, consequently, often indicate the possible taxonomic position of a species. Multidisciplinary analyses of the species examined are still incomplete. Nevertheless, our pollen studies of recently described Argentinean and Paraguayan species of Vernonia again indicate how potentially useful pollen characters are in delimiting natural groups within the Vernonieae.
Specimens investigated
Vernonia centauropsidea Hieron.
Argentina, Salta: Dept. Santa Victoria, National Park Baritú. Hilgert 2031 (MCNS).
V. chaquensis Cabrera
Argentina, Chaco: Estancia La Lonja. Schulz 13696 (CTES).
Paraguay, Alto Paraná: 12 km NE of Hernadarias. Schinini et al. 28183 (CTES).
V. correntina Cabrera & Cristóbal
Argentina, Corrientes: 8 km N of Curuzú Cuatía. Schinini & Ahumada 13895 (CTES).
V. cupularis Chodat
Argentina, Misiones: Dept. Santa Ana, Loreto. Montes s.n. (CTES).
Misiones: Dept. San Pedro, Montecarlo. Schwindt 1071 (CTES).
V. lipeoensis Cabrera
Argentina, Jujuy: Dept. Ledesma, on the road to Valle Grande, Abra de Cañas. Cabrera et al. 23971 (LIL isotype).
V. novarae Cabrera
Argentina, Salta: Dept. Santa Victoria, on road from Los Toldos to Lipeo, 13 km from Los Toldos. Vervoorst & Legname 4581(CTES).
Salta: Dept. Santa Victoria. On the road to San José river. O. Ahumada & J. Aguero 8177 (CTES).
V. schulziana Cabrera
Argentina, Chaco: Dept. 1° de Mayo, Colonia Benítez. Schulz 3757 (CTES isotype).
Chaco: Dept. 1° de Mayo, Colonia Benítez. Schulz 921 (CTES paratype).
V. setososquamosa Hieron.
Argentina, Salta: Dept. Rosario de Lerma, Dique Las Lomitas. Novara 10877 (CTES).
Salta: Dept. Orán, between Orán and Saucelito. Spegazzini & Girola s. n. (CTES).
V. spicata Cabrera
Argentina, Misiones: Dept. San Ignacio, Santo Pipó. Schulz 7185 (CTES).
V. teyucuarensis Cabrera
Argentina, Misiones: Dept. San Ignacio, Teyú Cuaré. Dematteis 476 (CTES).
Misiones: Dept. San Ignacio, Horacio Quiroga's house. Dematteis 515 (CTES).
V. amambaia (H.Rob.) Dematteis
Paraguay, Amambay: 25 km N of Pedro Juan Caballero, on the road to Colonia Estrella. Dematteis & Schinini 867 (CTES).
V. brunneri (H.Rob.) Cabrera
Paraguay, Concepción: Tagatiya‐mi river. Brunner 1720 (G isotype).
V. bellula Dematteis
Paraguay, Canindeyú: between Ypé‐Hú and Capitán Bado, 10 km from Itanará. Fernández Casas & Molero 5989 (CTES isotype).
V. cichoriiflora Chodat
Paraguay, San Pedro: 35 km N of San Estanislao. A. Krapovickas & C. L. Cristóbal 34263 (CTES).
V. hassleriana Chodat
Paraguay, Itapúa: Capitán Meza. Montes 7161 (CTES).
V. hystricosa Cabrera & Dematteis
Paraguay, Amambay: 30 km S of Bella Vista, on the road to the Aquidabán river. Dematteis et al. 905 (CTES).
Amambay: on the road to Colonia Estrella, 25 km N of Pedro Juan Caballero. Dematteis et al. 911 (CTES).
V. hystrix Chodat
Paraguay, Amambay: 25 km N of Igatimí. Dematteis & Schinini 852 (CTES).
Amambay: Colonia Marizcal López, near Capitán Bado. Schinini et al. 35505 (CTES).
V. lanata (Chodat) Cabrera
Paraguay, Cordillera: Eusebio Ayala. Schinini 2211 (CTES).
Cordillera: Itacurubí. Schinini 2633 (CTES).
V. parvifolia (Chodat) Cabrera
Paraguay, Cordillera: Emboscada. Bordas 1257 (CTES).
Cordillera: Tobatí, Huguaty Rozado ‐ Itá Espejo. Bordas 4397 (CTES).
Paraguarí: Tuyá‐Quindy Church. Schinini 2783 (CTES).
V. platyphylla Chodat
Paraguay, Amambay: 40 km N of Pedro Juan Caballero, on the road to Arroyo Estrella. Dematteis & Schinini 865 (CTES)
V. profusa Dematteis & Cabrera
Paraguay, Amambay: near Pedro Juan Caballero. Schinini et al. 30440 (CTES holotype).
V. propinqua var. canescens (Chodat) Dematteis
Paraguay, Amambay: Chiriguelo. Dematteis et al. 888 (CTES)
Amambay: Colonia Estrella. Schinini & Dematteis 33530 (CTES).
V. ramellae Cabrera
Paraguay, Paraguarí: in valle fluminis Y‐acá. Hassler 6674 (G holotype).
V. valenzuelae Chodat
Paraguay, Cordillera: Piribebuy and Tobatí, Serranía Y‐aguaí‐guazú. Fiebrig 859 (G)
Amambay: Chiriguelo. Schinini & Dematteis 33467 (CTES).
Acknowledgements
We would like to express our appreciation to the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and the Secretaría General de Ciencia y Técnica of the Universidad Nacional del Nordeste, for the grants which have supported this research. We are indebted to our two reviewers for their many helpful comments and suggestions. We would also like thank Cristina Salgado and Carolina Peichoto of the SEM service, Universidad Nacional del Nordeste, for taking the SEM micrographs of the pollen for us.
References
- Blackmore , S. 1986 . The identification and taxonomic significance of lophate pollen in the Compositae. . Can. J. Bot. , 64 : 3101 – 3112 .
- Bolick , M. R. 1983 . Exine structure of Trichospira verticillata (L.) Blake (Compositae) and its implications for the tribal position of the genus. . Am. J. Bot. , 70 : 463 – 465 .
- Bolick , M. R. and Keeley , S. C. 1994 . Pollen morphology and classification of the Vernonieae (Compositae). . Acta Bot. Gall. , 141 : 279 – 284 .
- Bremer , K. 1994 . Asteraceae. Cladistics and classification , Portland, OR : Timber Press .
- Cabrera , A. L. 1944 . Vernonieas Argentinas (Compositae). . Darwiniana , 6 : 265 – 379 .
- Cabrera , A. L. and Dematteis , M. 1999 . Novedades en el género Vernonia Schreb. (Compositae‐Vernonieae) para la Flora del Paraguay. . Candollea , 54 : 103 – 110 .
- Cabrera , A. L. and Dematteis , M. 2008 . “ Compositae. VI. Asteroideae, Vernonieae. ” . In Flora del Paraguay , Edited by: Stauffer , F and Ramella , L . Genève : Conserv. & Jard. Bot./St. Louis: Mo. Bot. Gard .
- Cristóbal , C. L. and Dematteis , M. 2002 . Una nueva especie de Vernonia (Asteraceae) del nordeste de Argentina y sur de Brasil. . Darwiniana , 40 : 51 – 55 .
- Cristóbal , C. L. and Dematteis , M. 2003 . “ Asteraceae, Tribu I. Vernonieae. ” . In Flora Fanerogámica Argentina , Edited by: Hunziker , A. T . Vol. 83 , 3 – 53 . Córdoba : Mus. Bot. .
- Dematteis , M. 1997 . Cromosomas en Vernonia platensis y especies afines (Asteraceae). . Bonplandia , 9 : 259 – 264 .
- Dematteis , M. 2000 . Vernonia bellula (Compositae‐Vernonieae) una nueva especie para la flora del Paraguay. . Candollea , 55 : 307 – 310 .
- Dematteis , M. 2002 . Cytotaxonomic analysis of South American species of Vernonia (Vernonieae: Asteraceae). . Bot. J. Linn. Soc. , 139 : 401 – 408 .
- Dematteis , M. 2003 . New species and new combinations in Brazilian Vernonieae (Asteraceae). . Taxon , 52 : 281 – 286 .
- Dematteis , M. 2006 . Vernonanthura warmingiana (Asteraceae: Vernonieae), a new species from Brazil. . Brittonia , 58 : 182 – 188 .
- Dematteis , M. and Robinson , H. 1997 . Chromosome studies and taxonomic considerations in Acilepidopsis (Vernonieae, Asteraceae). . Phytologia , 83 : 366 – 370 .
- Dematteis , M. and Salgado , C. R. 2001 . Pollen morphology and chromosome number of Vernonia rojasii (Vernonieae, Asteraceae). . Compositae Newsl. , 36 : 69 – 76 .
- Erdtman , G. 1960 . The acetolysis method. A revised description. . Sv. Bot. Tidskr. , 54 : 561 – 564 .
- Fischer , H. 1890 . Beiträge zur vergleichenden Morphologie der Pollenkörner , Berlin : J. U. Kern .
- Isawumi , M. A. , El‐Ghazaly , G. and Nordenstam , B. 1996 . Pollen morphology, floral microcharacters and taxonomy of the genus Baccharoides Moench (Vernonieae, Asteraceae). . Grana , 35 : 205 – 230 .
- Jones , S. B. 1970 . Scanning electron microscopy of pollen as an aid to the systematics of Vernonia (Compositae). . Bull. Torrey Bot. Club , 97 : 325 – 335 .
- Jones , S. B. 1977 . “ Vernonieae – systematic review. ” . In The biology and chemistry of the Compositae. I , Edited by: Heywood , V. H , Harborne , J. B and Turner , B. L . 503 – 521 . London/New York : Acad. Press .
- Jones , S. B. 1979 . Synopsis and pollen morphology of Vernonia (Compositae: Vernonieae) in the New World. . Rhodora , 81 : 425 – 447 .
- Jones , S. B. 1981 . Synoptic classification and pollen morphology of Vernonia (Compositae: Vernonieae) in the Old World. . Rhodora , 83 : 59 – 75 .
- Keeley , S. C. and Jones , S. B. 1977 . Taxonomic implications from of external pollen morphology to Vernonia (Compositae) in the West Indies. . Am. J. Bot. , 64 : 576 – 584 .
- Keeley , S. C. and Jones , S. B. 1979 . Distribution of the pollen types in Vernonia (Vernonieae: Asteraceae). . Syst. Bot. , 4 : 195 – 202 .
- Kingham , D. L. 1976 . A study of the pollen morphology of tropical African and certain other Vernonieae (Compositae). . Kew Bull. , 31 : 9 – 26 .
- Kremp , G. O. W. 1965 . Morphologic encyclopedia of palynology , Tucson, AZ : Univ. Ariz. Press .
- Punt , W. , Blackmore , S. , Nilsson , S. and Le Thomas , A. 1994 . Glossary of pollen and spore terminology , Utrecht : LPP Found. Univ. Utrecht . LPP Contrib. Ser. 1
- Punt , W. , Hoen , P. P. , Blackmore , S. , Nilsson , S. and Le Thomas , A. 2006 . Glossary of pollen and spore terminology. . Rev. Palaeobot. Palynol. , 143 : 1 – 181 .
- Punt, W., Hoen, P. P., Blackmore, S., Nilsson, S. & Le Thomas, A. (2007). Glossary of pollen and spore terminology. www.bio.uu.nl/∼palaeo/glossary/glos‐int.htm (www.bio.uu.nl/~palaeo/glossary/glos-int.htm) .
- Robinson , H. 1988 . Studies in the Lepidaploa complex (Vernonieae: Asteraceae). IV. The new genus Lessingianthus. . Proc. Biol. Soc. (Washington DC) , 100 : 929 – 951 .
- Robinson , H. 1989 . Acilepidopsis, a new genus of Vernonieae from South America (Asteraceae). . Phytologia , 67 : 289 – 292 .
- Robinson , H. 1992 . The Asteraceae of the Guianas, III: Vernonieae and restoration of the genus Xiphochaeta. . Rhodora , 94 : 348 – 361 .
- Robinson , H. 1998 . Two new species of Lepidaploa (Vernonieae, Asteraceae). . Phytologia , 84 : 40 – 42 .
- Robinson , H. 1999 . Generic and subtribal classification of American Vernonieae , Washington DC : Smith. Inst. Press . Smith. Contrib. Bot., 89
- Skvarla , J. J. , DeVoreb , M. L. and Chissoe , W. F. 2005 . Lophate sculpturing of Vernonieae (Compositae) pollen. . Rev. Palaeobot. Palynol. , 133 : 51 – 68 .
- Skvarla , J. J. , Turner , B. L. , Patel , V. C. and Tomb , A. S. 1977 . “ Pollen morphology in the Compositae and in morphologically related families. ” . In The biology and chemistry of the Compositae. Vol. I , Edited by: Heywood , V. H , Harborne , J. B and Turner , B. L . 141 – 448 . London/New York : Acad. Press .
- Smith , E. C. 1969 . Pollen characteristics of African species of Vernonia. . J. Arnold Arbor. , 50 : 469 – 473 .
- Steetz , J. 1864 . “ Crystalopollen und Ambassa. ” . In Naturwissenschaftliche Reise nach Mossambique auf Befehl seiner Majestäts des Königs Friedrich Wilhelm. IV. Vol. 6, Botanik, P. 2 , Edited by: Peters , W. C. H . 363 – 364 . Berlin : G. Reimer .
- Stix , E. 1960 . Pollenmorphologische Untersuchungen an Compositen. . Grana Palynol. , 2 : 41 – 104 .
- Wodehouse , R. P. 1928 . Phylogenetic value of pollen grain characters. . Ann. Bot. , 168 : 891 – 934 .