Abstract
Pollen grains of 30, mainly annual, species from 134 populations of the genus Veronica (Plantaginaceae, formerly Scrophulariaceae) from the Mediterranean Region, have been studied with light and scanning electron microscopy. Three pollen types are defined based on pollen size and exine surface sculpture. In some cases within the study group pollen size can be useful in species determination. Hypothetical relationships of pollen grain size and aperture number with ploidy level, style length and corolla diameter are discussed. The ornamentation of the exine in Veronica, although generally a conservative character within the genus, gives some additional support to the most recent infrageneric classification of Veronica.
Veronica L. (Plantaginaceae sensu APG, Citation2003; formerly Scrophulariaceae) is the largest genus within tribe Veroniceae with ca. 450 species, including the Hebe complex (Albach et al., Citation2004 a, Citation2009) worldwide. The infrageneric classification of Veronica has undergone considerable changes in recent years (Albach et al., Citation2004b ; Garnock-Jones et al., Citation2007). The most recent proposal recognizes 12 subgenera (Albach et al., Citation2009), a taxonomic rank that has been rarely used for the infrageneric classification of Veronica, most of the previous classification systems have used sectional and subsectional ranks (Albach et al., Citation2004b ).
Scrophulariaceae, a family often considered to be heterogeneous and bound together by symplesiomorphies (Olmstead et al., Citation2001) has been divided extensively, following the results of DNA sequence studies. One of its segregates is included in a vastly enlarged Plantaginaceae a family which, in its new circumscription, is rather heterogeneous (there is a proposal to conserve the name Veronicaceae against Plantaginaceae: Reveal et al., Citation2008).
Previously, among the much small number of genera included in Plantaginaceae, the pollen is omniaperturate with 4–14 pores (Erdtman, Citation1952). However, the pollen of the re-circumscribed Plantaginaceae is generally isopolar, tricolpate or tricolporate with a reticulate exine a suite of characters which are also frequently encountered in other eudicotyledons. Therefore, pollen characters in this family have very limited use as taxonomic markers, because they are neither restricted to Plantaginaceae nor stable within it (Albach et al., Citation2005b ).
The first description of Veronica pollen was made by Risch (Citation1939). Subsequent studies, such as those of Cranwell (Citation1941), Varghese (Citation1968), Huang (Citation1972) and, more recently, Hong and Nilsson (Citation1983) and Hong (Citation1984), give an idea of the range of morphological variability in the pollen of Veronica and related genera.
Hong (Citation1984) described eight pollen types for 19 genera of tribe Veroniceae. His results show that the sections recognised in the traditional classification of Veronica are not characterised by a specific pollen type.
Fernández et al. (Citation1997) used light and scanning electron microscopy to study the pollen morphology of 13, mostly annual taxa, of Veronica from south-west Spain. This study shows the eurypalynous nature of the genus. Based on the surface ornamentation of the pollen grains Fernández et al. (Citation1997) recognised two morphological types: type 1. rugulate, and type 2. scabrate.
Martínez-Ortega et al. (Citation2000) studied the pollen of 30 western Mediterranean taxa belonging to two sections comprising only perennial species: Veronica sect. Veronica L. and V. sect. Veronicastrum W. D. J. Koch. They showed that pollen of each section is stenopalynous and has one or other of the two major pollen types known for the genus. The two pollen types they recognised are based on the ornamentation of the exine: type 1 (only in V. bellidioides L.): rugulate-reticulate and, type 2 (in all other studied taxa): striate reticulate-microreticulate with four subtypes. Additionally, their results showed a positive relationship between pollen size and ploidy level within every natural group of taxa within these two sections of Veronica (which mostly correspond to subsections). The results of Martínez-Ortega et al. (Citation2000) confirmed the lack of coincidence between traditional sections and specific pollen types within Veronica, previously observed by Hong (Citation1984). More recently Saeidi-Mehrvarz & Zarrei (Citation2006) examined the pollen morphology of 17 species, from five sections of Veronica from Iran, and described three different types of exine ornamentation (rugulate-perforate, microreticulate and scabrate) which, again, are not specific to section.
Further discussion on the variety of characters that have been used for taxonomic purposes in the tribe Veroniceae, as well as a comparison of Hong's (Citation1984) results with the DNA phylogeny of Veronica, and comments on evolutionary trends of pollen characters in Veronica, can be found in Albach et al. (Citation2004c ).
A careful examination of Hong (Citation1984), Fernández et al. (Citation1997), Martínez-Ortega et al. (Citation2000) and Saeidi-Mehrvarz & Zarrei (Citation2006), has highlighted the necessity to establish a coherent standard use of terminology for the description of exine sculpture in Veronica. Therefore, following Punt et al. (Citation1994, Citation2007) we have addressed this problem in the present study.
Our understanding of Veronica has changed considerably in recent years, and revisiting interpretations of pollen morphology in the genus is undoubtedly worthwhile as previously demonstrated by Albach et al. (Citation2004c ), although pollen data for Veronica at that time was still incomplete, and not the main focus of the study. We have undertaken a pollen morphological study of 30 species of Veronica using light microscopy, scanning electron and, to a lesser extent, transmission electron microscopy, in order to increase our knowledge of the pollen morphology for the genus, thereby contributing to a better understanding of the systematic value of these characters. Even now the taxon sampling for this large genus is far from complete. Nevertheless, in the present study, we have attempted to use the expanded pollen database to try and get a better idea of the degree of congruence between the classification proposed by Albach et al. (Citation2004b ), which is mainly based on phylogenetic analysis of DNA sequence data, and available pollen data. Accordingly, the analysis and discussion of our results follows this recent infrageneric classification (; Albach et al., Citation2004b , Citation2009). In discussing our results we have considered not only subgenera, but also the subsections which are mostly coincident with the “natural groups” reported by Martínez-Ortega et al. (Citation2003), and in accordance with Albach et al. (Citation2009). Therefore, the pollen data are interpreted in a context where only closely related species are compared.
Table I. Taxonomic arrangement of studied taxa of Veronica according to Albach et al. (Citation2004b , Citation2009)
Particular emphasis is given to taxonomic issues, especially regarding the more controversial cases within the study group. For example, subsections Cymbalariae and Cochlidiosperma, currently both included in subgenus Cochlidiosperma.
In this study we also consider the possibility of a relationship between pollen size and various other characteristics such as ploidy level, number of apertures, style length and corolla diameter.
Material and methods
Pollen grains from 134 populations belonging to six of the 12 subgenera in Veronica have been studied. The species studied are mostly Circum-mediterranean and mainly annuals although one perennial, Veronica filiformis (subsect. Agrestes), and some facultative perennial species belonging to Veronica subsect. Beccabunga were also studied.
All pollen material was taken from herbarium specimens. The herbarium vouchers examined are listed in ‘Specimens Investigated’ and the subgeneric and subsectional ascriptions of taxa in .
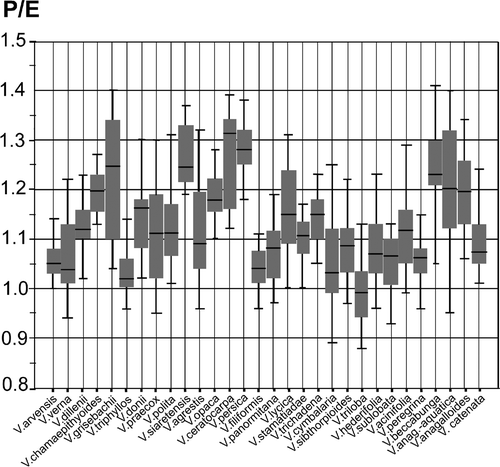
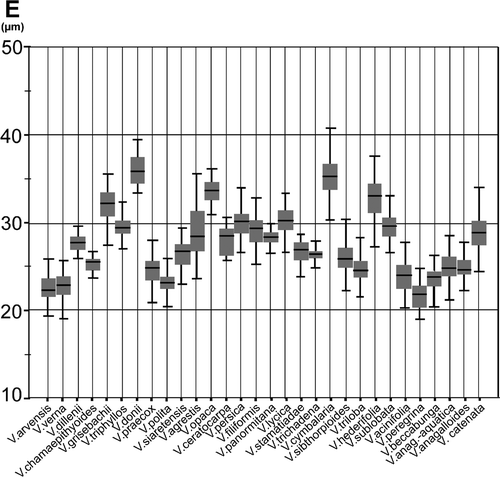
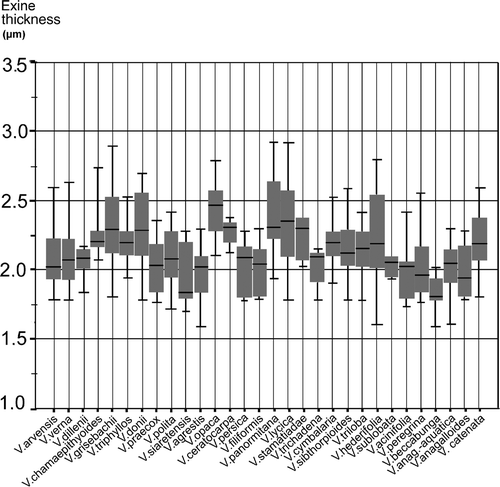
Pollen samples for light microscopy (LM) were prepared according to the acetolysis technique of Erdtman (Citation1960). Measurements were made using the digital image analysis software Image Pro-plus, V. 1.0, connected to a Nikon Optiphot 2 microscope and a Sony DCX- 930P digital video camera. Thirty measurements per population were taken for the polar axis (P) and equatorial diameter (E), from which the P/E ratio was calculated. The mesocolpial exine thickness was measured for ten pollen grains per sample (. For statistical analyses and graphic tests, the computer programs SPSS V. 1.1 and Statgraphics V. 5.90 were used.
Table II. Pollen data the Veronica species examined. P – polar axis (in μm); E – equatorial diameter (in μm); P/E–ratio; O-sph – oblate spheroidal; P-sph – prolate spheroidal; exine thickness – in μm. NB: For values of P, E, P/E and Exine thickness, average ± confidence limit are shown, with the range of variation in parentheses
In order to try to avoid collapse of the pollen grains through dehydration, we tested several pollen preparation techniques for scanning electron microscopy (SEM), most of them based on previous protocols, for example, Lynch & Webster (Citation1975), Sáenz de Rivas (Citation1978). Best results were obtained with the following technique: hydration of pollen in a solution 0.1% Aerosol-OT and distilled water for four days, then dehydration in an acetone series and, finally, amyl acetate. The sample was then subjected to critical point drying (CPD) in which the amyl acetate is replaced with liquid carbon dioxide. Following CPD the sample was coated with gold-palladium using a Balzers SCD 004 vacuum evaporator. For transmission electron microscopy (TEM) we used the following protocol: the anthers were first fixed in 1% glutaraldehyde buffer, followed by fixation in 0.2% osmium tetroxide, then dehydrated in an ethanol series, and then embedded in epoxy resin. Sections were cut using a Leica ‘Ultracut’ ultramicrotome, and then stained by hand with lead citrate and uranyl acetate.
To study the relationship between the size of the pollen grains, style length and ploidy level () in the species studied, detailed karyological studies were carried out on many Iberian populations (Sánchez Agudo, Citation2005). Data on style length of the studied species have been taken either from the comprehensive study by Sánchez Agudo (Citation2005) or from Martínez-Ortega et al. (Citation2009); in the case of Veronica donii from Fischer (Citation1978) and for V. siaretensis from Fischer (Citation1981).
Table III. Length of styles (μm) and levels of ploidy
Terminology
Previous publications, notably, Hong (Citation1984), Fernández et al. (Citation1997), Martínez-Ortega et al. (Citation2000) and Saeidi-Mehrvarz & Zarrei (Citation2006), have caused confusion by using conflicting terminology to describe similar exine morphology for Veronica pollen. We have attempted to redress this problem by using only terminology accepted by Punt et al. (Citation1994, Citation2007). In , alongside our recommended standard terms, we summarise the corresponding terms used by these previous authors.
Table IV. Standarised nomenclature proposed for the description of the exine sculpture in Veronica (following Punt et al., Citation2007)
Results
Pollen morphology: Symmetry, shape, size, apertures and exine
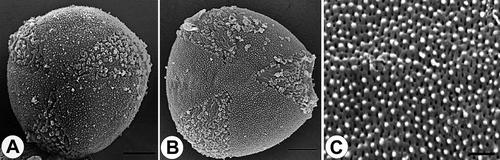
Pollen grains isopolar (), radially symmetrical, usually 3-zonocolpate, occasionally 4‐zonocolpate (Veronica filiformis, V. persica, V. hederifolia, V. cymbalaria) (), or 6-zonocolpate (Veronica persica, V. cymbalaria) (). Exceptionally, pantocolpate pollen of V. persica, with nine colpi have been observed (). Also in V. cymbalaria exceptional pantocolpate pollen grains have been observed. Colpi simple with acute apices; sometimes fused, or almost, at the apocolpium; colpi margins straight or sinuous, but often only weakly defined. Apertural membrane usually with finely perforate, granular-verrucate ectexinous elements, irregularly arranged.
Figure 10. Pollen grains of Veronica subg. Pocilla (Subsect. Agrestes). A. V. filiformis, polar view. B. V. opaca, striato-reticulate surface structure, apocolpium (SEM). C, D. V. persica: C. polar view, tricolpate grain (SEM); D. polar view, pantocolpate grain (SEM). E. V. agrestis, polar view (SEM). F. V. persica, section through pollen wall (TEM). Scale bars – 10 μm (A, C–E ); 1 μm (B, F).
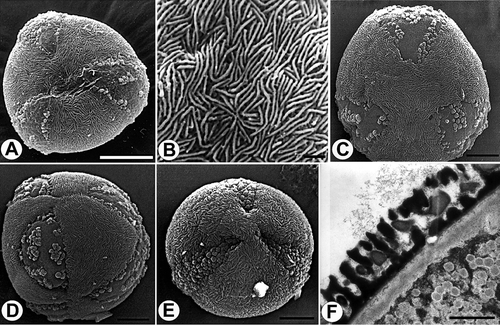
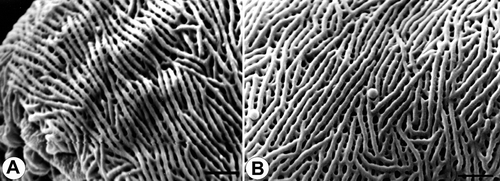
Figure 11. Pollen grains of Veronica subg. Cochlidiosperma [A–E. Subsect. Cymbalariae; I–K. Subsect. Cochlidiosperma]. A. V. cymbalaria, pantocolpate grain (SEM). B. V. trichadena, tricolpate grain, polar view (SEM). C, G and H. V. cymbalaria: C. finely scabrate-perforate surface structure, apocolpium (SEM); G. optical section, equatorial plane (LM); H. optical section, polar plane (LM). D & F. V. lycica: D. tricolpate grain, polar view (SEM); F. optical section, polar plane (LM). E. V. stamatiadae, finely scabrate-perforate surface structure, apocolpium (SEM). I. V. hederifolia, tricolpate grain, polar view (SEM). J. V. sublobata, tricolpate grain, oblique polar view (SEM). K. V. sibthorpioides, tricolpate grain, polar view (SEM). Scale bars –10 μm (F–H); 5 μm (A, B, I–K); 3 μm (D); 1 μm (C, E).
![Figure 11. Pollen grains of Veronica subg. Cochlidiosperma [A–E. Subsect. Cymbalariae; I–K. Subsect. Cochlidiosperma]. A. V. cymbalaria, pantocolpate grain (SEM). B. V. trichadena, tricolpate grain, polar view (SEM). C, G and H. V. cymbalaria: C. finely scabrate-perforate surface structure, apocolpium (SEM); G. optical section, equatorial plane (LM); H. optical section, polar plane (LM). D & F. V. lycica: D. tricolpate grain, polar view (SEM); F. optical section, polar plane (LM). E. V. stamatiadae, finely scabrate-perforate surface structure, apocolpium (SEM). I. V. hederifolia, tricolpate grain, polar view (SEM). J. V. sublobata, tricolpate grain, oblique polar view (SEM). K. V. sibthorpioides, tricolpate grain, polar view (SEM). Scale bars –10 μm (F–H); 5 μm (A, B, I–K); 3 μm (D); 1 μm (C, E).](/cms/asset/3aa38fca-f4b7-46bd-af3c-75c380b20e54/sgra_a_436650_o_f0011g.gif)
Shape ranges from oblate-spheroidal to prolate P/E 0.88 – 1.09 – 1.39, but is most frequently prolate-spheroidal (). Outline in equatorial view ranges from circular to ± ellipsoid and, in, polar view, circular, rounded triangular or tetra-lobate, somewhat dependent on the number of apertures, although the rare examples of 6-zonocolpate pollen tend to have a circular polar outline ().
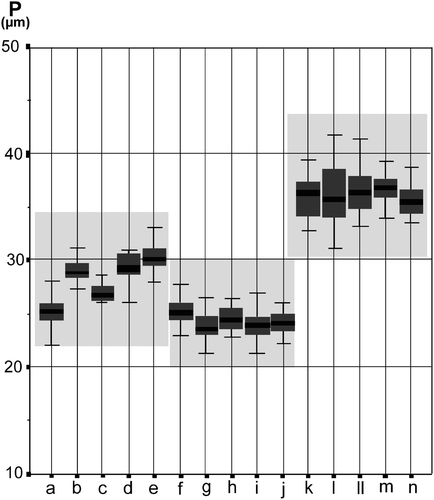
Pollen grain size () ranges in: polar axis (P) from 19.22 to 47.67 μm (mean 29.53 μm); equatorial diameter (E) from 18.79 to 46.96 μm (mean 26.46 μm). The results of the box plot graphical test for median comparisons of polar axis, equatorial width, P/E shape ratio and exine thickness are displayed respectively in . The data corresponding to the Iberian populations of subgenus Cochlidiosperma have been treated separately (, ), because they are of particular interest taxonomically.
Figure 1. Graphic test for median comparisons of polar diameter (P) for all species studied. Ascription of taxa to subgenera is indicated by rectangles. 1. V. subg. Chamaedrys. 2. V. subg. Triangulicapsula. 3. V. subg. Pellidosperma. 4. V. subg. Pocilla. 5. V. subg. Cochlidiosperma. 6. V. subg. Beccabunga.
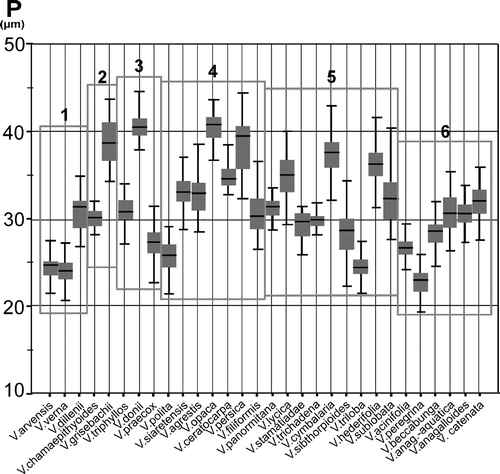
Figure 5. Graphic test for median comparisons of polar axis (P), for the Iberian populations of species in Veronica subg. Cochlidiosperma, subsect. Cymbalariae: a, b. V. panormitana; c, d. V. trichadena; e-k. V. cymbalaria.
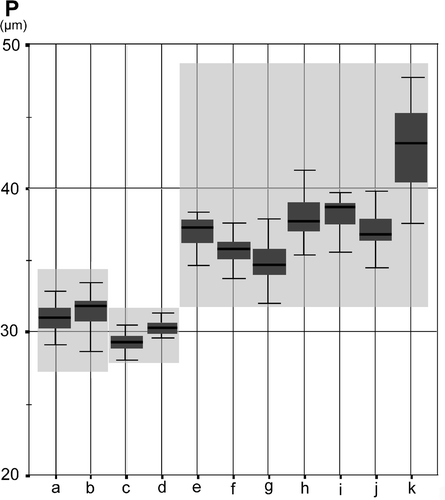
Figure 6. Graphic test for median comparisons of polar axis (P), for the Iberian populations of species in Veronica subg. Cochlidiosperma, subsect. Cochlidiosperma: a-e. V. sibthorpioides; f-j. V. triloba; k-n. V. hederifolia.
The thickness of the exine varies from 1.49 to 2.92 μm (mean value 2.06); the infratectum is columellate (, , ).
Figure 7. Pollen grains of Veronica subgenus Chamaedrys (A & E. Subsect. Microspermae; B–D. Subsect. Microspermoides). A & E. V. arvensis: A. polar view (SEM); E. section through pollen wall (TEM). B & D. V. dillenii: B. polar view (SEM); D. striato-reticulate surface structure, apocolpium (SEM). C. V. verna, polar view (SEM). Scale bars – 5 μm (A, C); 2 μm (B); 1 μm (D, E).
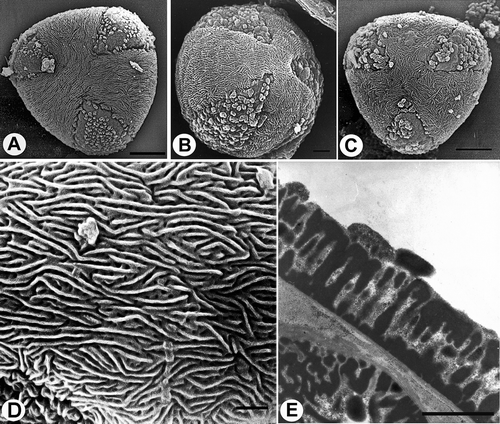
Figure 13. Pollen grains of Veronica subg. Beccabunga (Subsect. Beccabunga). A–C. V. anagalloides: A. striato-reticulate surface structure, apocolpium (SEM); B. ultrasonic fracture showing exine structure (SEM); C. ultrathin section through pollen wall showing intine thickness in apertural zone (TEM). D. V. anagallis-aquatica, ultrathin section of an anther showing two mature pollen grains, and the inner wall of the anther (TEM). Scale bars – 1 μm (A, B); 5 μm (C); 10 μm (D).
The tectum is either finely striato-reticulate (e.g. , , , ), finely scabrate-perforate (e.g. , ) or micro rugulate-perforate (, ).
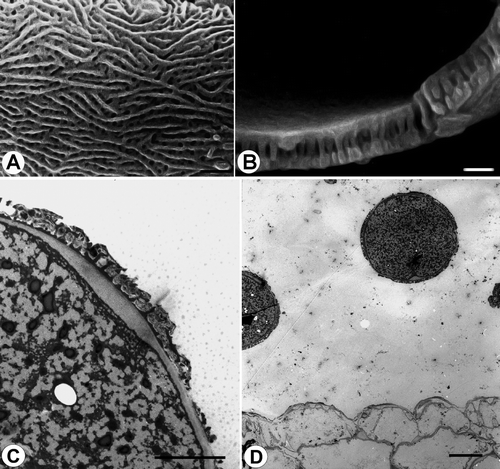
Figure 12. Pollen grains of Veronica subg. Beccabunga [A, B. Subsect. Acinifolia; C, D. V. Subsect. Peregrinae]. A, B. V. acinifolia: A. tricolpate grain, polar view; B. striato-reticulate surface structure, apocolpium (SEM). C, D. V. peregrina: C. finely rugulate surface structure, apocolpium (SEM); D. finely rugulate surface structure, apocolpium (SEM). Scale bars – 5 μm (A); 1 μm (B–D).
![Figure 12. Pollen grains of Veronica subg. Beccabunga [A, B. Subsect. Acinifolia; C, D. V. Subsect. Peregrinae]. A, B. V. acinifolia: A. tricolpate grain, polar view; B. striato-reticulate surface structure, apocolpium (SEM). C, D. V. peregrina: C. finely rugulate surface structure, apocolpium (SEM); D. finely rugulate surface structure, apocolpium (SEM). Scale bars – 5 μm (A); 1 μm (B–D).](/cms/asset/1ed1f60d-c0f8-408c-90e3-08813d290e57/sgra_a_436650_o_f0012g.gif)
Pollen types
Three pollen types are recognised, based on exine sculpture:
Type I
Finely striato-reticulate (see for comparisons with previous non-standard terminologies (i.e. Punt et al., Citation1994, Citation2007)
Basically this type of exine sculpture has a pattern in which parallel or sub-parallel smooth muri are cross-linked to form a reticulum in the grooves between the striae; the connecting muri of the fine reticulum between the striae may be on a single plane or slightly different planes (Punt et al., Citation1994, Citation2007). Although there is some variation in the degree of intercrossing of the muri between the striae in the pollen exine of the species we studied, is not sufficient enough to allow any definition of subtypes, as has been done previously for pollen of other species of Veronica (Martínez-Ortega et al., Citation2000).
Species included: Veronica arvensis (, E), V. verna (), V. dillenii (, D), V. chamaepithyoides (), V. grisebachii (), V. polita, V. siaretensis, V. agrestis (), V. opaca (), V. ceratocarpa, V. persica (, D), V. filiformis (), V. beccabunga, V. anagallis-aquatica, V. anagalloides, V. catenata, V. acinifolia (, ).
Type II
Scabrate-perforate.
The finely scabrate-perforate tectum is covered by very small, more or less regularly distributed, supratectal elements, less than 1 μm in height.
Species included: Veronica triphyllos (), V. donii (), V. praecox (), V. panormitana, V. lycica (, ), V. stamatiadae (), V. trichadena (), V. cymbalaria (, , , ), V. sibthorpioides (), V. triloba, V. hederifolia (), V. sublobata ().
Type III
Micro rugulate-perforate.
The tectum comprises small closely packed, elongate elements ≤ 1 μm in diameter. The elements are interspersed by irregularly distributed perforations linked by microchannels.
Species included: V. peregrina (, D)
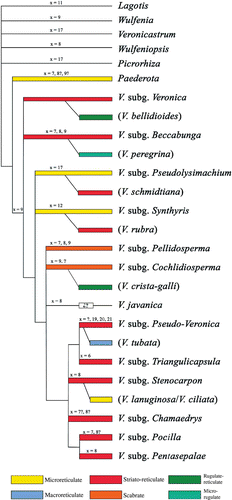
In we summarise the six main exine ornamentation types represented in Veronica using our own data plus data from Hong (Citation1984); Fernández et al. (Citation1997); Martínez-Ortega et al. (Citation2000); Saeidi-Mehrvarz & Zarrei (Citation2006). We have mapped the ornamentation types onto a cladogram which shows the relationships of Veroniceae based on analyses of DNA sequences, from both nuclear and plastid regions, according to data by Albach et al. (Citation2001, Citation2004a , Citation2004c , Citation2005b , Citation2005c , Citation2009).
Figure 14. Principal exine ornamentation patterns represented in Veronica mapped onto a cladogram of relationships within tribe Veroniceae based on Albach et al. (Citation2001, Citation2004a , Citation2004c , Citation2005b , Citation2005c , Citation2009). Principal chromosome base numbers represented in each subgenus are indicated above branches. (Pollen data summarised from Hong, Citation1984, Fernández et al., Citation1997; Martínez-Ortega et al. Citation2000; Saeidi-Mehrvarz & Zarrei, Citation2006 and this paper.)
Discussion
Over a number of years various characters have been considered as useful for the infrageneric classification of Veronica, for example, life cycle and inflorescence position, but subsequently have been shown to be homoplasious and associated with the parallel evolution of specific life strategies (Albach et al., Citation2004a ; Citation2004c ). Currently base chromosome number (Albach et al., Citation2009), ultrastructure of the seed testa (Martínez-Ortega & Rico, Citation2001; Muñoz-Centeno et al., Citation2006) and selected phytochemical data (Albach et al., Citation2005a , Citation2005b ; Jensen et al., Citation2005; Taskova et al., Citation2004) are being used to assess relationships among taxa within the genus, and to provide additional support for molecular trees.
Pollen morphological characters, probably due to their frequently conservative nature, especially characters such as symmetry, shape, size and number of apertures, do not seem to provide much additional support to infrageneric studies in Veronica. Nevertheless, in this study we have re-considered their potential value in the classification of Veronica, in particular, exine sculpture and pollen size. As we will see, in a few cases, the exine ornamentation provides at least some additional support to the most recent phylogenetic hypothesis and infrageneric classification (Albach, Citation2004b ).
Systematic and phylogenetic usefulness of exine sculpture
The study of Albach et al. (Citation2004c ) inferred an ancestral micro-reticulate tectum in tribe Veroniceae. Within Veronica this character state is typically representative of subgenus Pseudolysimachium (Opiz) Buchenau, with only one exception, V. schmidtiana Regel, which has pollen with a striato-reticulate pattern (Hong, Citation1984). A micro-reticulate tectum is also typical of the pollen in subgenus Synthyris (Benth.) M. M. Mart. Ort., Albach, M. A. Fisch., with the exception of V. rubra (Douglas) M. M. Mart. Ort. & Albach, which again has pollen with a striato-reticulate exine (Hong, Citation1984). The micro-reticulate tectum is also known for the pollen of two species in subgenus Stenocarpon (Boriss.) M. M. Mart. Ort., Albach, M. A. Fisch.: V. lanuginosa Benth. and V. ciliata Fisch. (Hong, Citation1984).
The striato-reticulate pattern is, in fact, the most widely represented exine ornamentation type in Veronica. It is present in most subsections of subgenus Veronica with the exception of V. bellidioides L. which shows a, possibly derived, rugulate-reticulate pattern (Martínez-Ortega et al., Citation2000). It is also typical of the pollen in subgenus Beccabunga although Saeidi-Mehrvarz & Zarrei (Citation2006) described the exine ornamentation of V. beccabunga as micro-reticulate (in our opinion this may have been a mistake; see Hong, 1984: ). Within subgenus Beccabunga the pollen of V. peregrina, according to our data, with a micro-rugulate exine is exceptional. Other subgenera which typically have striato-reticulate pollen include subgenus Pseudoveronica J. B. Armstr. [with the exception of V. tubata (Diels) Albach, where the pollen has a macro-reticulate exine (Hong, Citation1984), probably a derived characteristic and, as far as we know, the only example within Veronica], and finally all species of subgenera Triangulicapsula, Pocilla and Pentasepalae (Benth.) M. M. Mart. Ort., Albach, M. A. Fisch. (Martínez-Ortega et al., Citation2000).
From the above summary it is clear that, in Veronica, the ornamentation of the pollen ectexine is usually a conservative character (), and there are many examples: in Veronica subgenus Triangulicapsula, a group with several characters which are outstandingly divergent from the remaining subgenera in the genus, notably the base chromosome number and the form of the capsule, both of which are unique within Veronica but, nevertheless, the subgenus has a generically widespread exine type (Type I). In subgenus Beccabunga, a group which includes morphologically highly variable annual and perennial species, including three types of seed coat ultrastructure patterns (Muñoz-Centeno et al., Citation2006), varying composition of iridoid compounds between species (Jensen et al., Citation2005), and a notable range of ecological preferences. Furthermore, three differing base chromosome numbers are represented within the subgenus (Sánchez Agudo, Citation2005; Albach et al., Citation2009) (). This high level of heterogeneity is not reflected in the pollen morphology of subgenus Beccabunga where, although two pollen types (Types I and III) have been recognised, Type III is restricted to a single species, V. peregrina, which is notably deviant in other characters. With the exception of pollen, these data suggest a high degree of evolutionary plasticity for many of the characters found in the representatives of subgenus Beccabunga; this might correspond with a rapid diversification in speciation over a wide range of habitats. Yet another example of this is found in subgenus Chamaedrys, a group which includes both annual and perennial species. Traditionally, these were thought to be phylogenetically unrelated and, as a result, placed in very different sections of the genus, which have two types of seed coat ornamentation (Muñoz-Centeno et al., Citation2006). However, they have a uniform pattern of pollen exine ornamentation. Thus the subgenera Beccabunga and Chamaedrys could also be considered as further examples of the conservative nature of exine sculpture within the Veronica.
The scabrate-perforate (Type II) exine ornamentation pattern in Veronica is restricted to the subgenera Cochlidiosperma and Pellidosperma. Interestingly, the margin of the seeds in these subgenera (but also in subgenus Pocilla) turn inwards to form cyathiform (subg. Cochlidiosperma) or cymbiform (subg. Pellidosperma) seeds (Muñoz-Centeno et al. 2006) which would also seem to support the possibility of a close relationship.
Subgenus Beccabunga, is the unique among the subgenera studied in that it includes two pollen types: I and III, although the latter is restricted to V. peregrina. Fernández et al. (Citation1997) described the exine ornamentation of V. peregrina as scabrate. Further collections of V. peregrina should be examined to ascertain the stability of the micro rugulate-perforate type in the pollen of this species. Furthermore, because of its other morphological singularities, notably an apparently derived chromosome number (Albach et al., Citation2009), and a seed coat sculpture which also seems to be derived (Muñoz-Centeno et al., Citation2006), more studies are needed to elucidate the phylogenetic and taxonomic position of this species.
Systematic and phylogenetic usefulness of pollen size
In a small number of cases pollen size has been used to help distinguish between species of perennial Veronica, for example, between V. allionii Vill. and V. officinalis L., and between V. chamaedrys L. ssp. chamaedrys and V. micrantha Hoffmanns. & Link (Martínez-Ortega et al., Citation2000). However, in general size variability is minor and, consequently, the parameters for polar axes and equatorial diameters have a low discriminant value within the genus. Nevertheless, the present study demonstrates that pollen size characters can be useful taxonomic tools in helping to differentiate controversial taxa. Our own observations suggest that polar axis is less altered by acetolysis, or air drying, than the equatorial diameter which, due to the orientation and re-action of the colpi, is more directly affected by dehydration changes to volume. Therefore, we consider polar length to be a more reliable parameter.
Among the annual Veronica species we studied there are several cases in which the size of the pollen can be used to identify species within taxonomically complex groups; for example, within subgenus Chamaedrys, subsection Microspermoides, the morphologically similar species V. verna and V. dillenii are clearly distinguished by pollen size (, ). Although, in this example, the difference in pollen size is not related to ploidy level (both have the same chromosome number, 2n = 2x = 16) there are other cases where size difference can be related to a difference in ploidy level. For example, Veronica opaca and V. polita are morphologically closely similar and, consequently, often misidentified. However, V. opaca has a chromosome number of 2n = 4x = 28, while in V. polita the chromosome number is 2n = 2x = 14. The diploid V. polita has much smaller pollen grains (av. polar axis 25.48 μm) than the tetraploid V. opaca (av. polar axis 40.25 μm). Within subsection Cymbalariae differences in pollen size allow the distinction of V. stamatiadae from V. lycica, although both species have the same ploidy level, and, according to DNA analyses, are closely related (Albach, Citation2007). Within the same subgenus, in subsection Cochlidiosperma, almost every one of the species studied can be distinguished by notable pollen size differences () even though they are closely related (Albach, Citation2007) and some species seem very similar in their morphology.
Our results for pollen size agree with the view of Martínez-Ortega et al. (Citation2000) that size is a pollen character of limited phylogenetic use in Veronica, although this conflicts with the opinion of Hong (Citation1984). The limited usefulness of pollen size as a character in a phylogenetic context may well be because at best it simply reflects its relationship with other characters, such as ploidy level or, possibly, style length and selfing rates, which are discussed in the next two sections.
Possible relationships between aperture number and/or pollen size with ploidy level
Fernández et al. (Citation1997) suggested that the predominance of four colpi instead of three within some populations of V. persica Poir. (2n = 4x = 28) and V. hederifolia (2n = 6x = 54), could be related to the fact that they are polyploids. However, no connection between chromosome number and aperture number was observed in any of the perennial species of Veronica studied by Martínez-Ortega et al. (Citation2000). Furthermore, our results so far have not provided any evidence to support a relationship between aperture number and ploidy level. We have observed the presence, in some taxa, of pollen with four or six colpi but this is not necessarily associated with polyploid species; for example, the pollen of Veronica filiformis often has four colpi but the chromosome number for the species is 2n = 2x = 14.
There does, however, appear to be a much stronger relationship between pollen size and ploidy level. Martínez-Ortega et al. (Citation2000) suggested that, within every natural group, higher ploidy level would be reflected in larger sized pollen grains. For example, in V. chamaedrys from V. subsect. Multiflorae, the average polar axis is 34.3 μm and the chromosome number is 2n = 4x = 32; however, within V. chamaedrys subsp. chamaedrys some diploid taxa are insufficiently known and cannot be clearly assigned to this taxon (Albach et al., Citation2009), but the samples studied by Martínez-Ortega et al., Citation2000 were tetraploids. In previous reports (Martínez-Ortega et al., Citation2000; Albach et al., Citation2009) pollen size and chromosome number have been given for a number of Veronica species including, in subsection Multiflorae Benth., V. micrantha where the pollen has an average polar axis of 30.7 μm, and a chromosome number of 2n = 2x = 16. In subsection Veronica, V. officinalis has an average polar length of 30.4 μm and a chromosome number of 2n = 4x = 36; only three reports of exceptional diploid plants are known (Albach et al., Citation2009). Also from subsection Veronica, the species V. allionii has an average polar axis of 22.8 μm and a chromosome number of 2n = 2x = 18. From V. subsect. Pentasepalae Benth. V. orsiniana Ten. (av. polar axis 37.6 μm) has a chromosome number of 2n = 2x = 16, and in V. sennenii (Pau) M. M. Mart. Ort. & E. Rico the average polar axis is 39.3 μm and the chromosome number 2n = 8x = 64.
In the annual Veronica species which we have studied a relationship between pollen size and ploidy level within subsections has been confirmed for some species but not for others.
Detailed pollen morphological and karyological studies (Sánchez Agudo, Citation2005) were conducted on some Iberian populations of subgenus Cochlidiosperma. In subsection Cymbalariae () the pollen sizes for V. trichadena and V. panormitana were similar, and both species had the same chromosome number (2n = 2x = 18). It should be noted, however, that a tetraploid population of V. trichadena was reported by Albach et al. (Citation2009). By comparison, in the same subsection, V. cymbalaria, which is either tetraploid (2n = 4x = 36) or hexaploid (2n = 6x = 54), had consistently bigger pollen grains. Nevertheless, in another population of V. cymbalaria, from Menorca, the situation is reversed; pollen of the only tetraploid population studied (K), is significantly larger than that of the hexaploid populations, which were studied. Interestingly recent DNA analysis (Albach, Citation2007) suggests different phylogenetic origins for the tetraploid and hexaploid lineages of V. cymbalaria. shows the median comparisons of polar axis for the Iberian populations of the species belonging to Veronica subsect. Cochlidiosperma. Here the diploid V. triloba (2n = 2x = 18) has smaller pollen (av. 27.9 μm) than the hexaploid V. hederifolia (2n = 6x = 54), with an average pollen size of 32.2 μm. In these two widely distributed species, the correlation between pollen size and chromosome number remains quite homogeneous among all studied populations. In contrast, the narrow endemic V. sibthorpiodes (subsect. Cochlidiosperma), shows relatively high inter-populational variation with regard to pollen size (A–E).
In Veronica subg. Pocilla pollen size of some of the species from subsection Agrestes also shows notable correlation with ploidy level, for example, V. polita (2n = 2x = 14) has significantly smaller pollen grains (av. 25.4 μm) than V. opaca (2n = 4x = 28) where the average pollen size is 40.2 μm. However, the pollen in some other species, for example, V. siaretensis (2n = 2x = 14), are similar in size (32.8 μm) to those of V. agrestis (2n = 4x = 28), but significantly larger than those of another diploid V. polita (av. pollen size 25.4 μm). Moreover, within the same subsection the pollen of the diploid V. ceratocarpa (2n = 2x = 14) is comparatively large (av. 35 μm).
In spite of the frequently positive relationship between pollen size and ploidy level observed in Veronica (i.e., higher ploidy level = larger pollen size), there are notable exceptions. In Veronica subgenus Triangulicapsula, subsection Digitate (), the opposite occurs: the pollen of the tetraploid V. chamaepithyoides (2n = 4x = 24) is smaller (29.9 μm) than the pollen (av. size 38.5 μm) of the diploid V. grisebachii (2n = 4x = 12). This, and other examples we have given, suggest that there may be other factors affecting pollen size, and these are discussed in the next section.
In subgenus Pellidosperma, subsect. Pellidosperma we have chromosome and pollen size data for the diploid species V. triphyllos (av. pollen size 30.7 μm) and V. praecox (av. pollen size 27.0 μm) but, although the pollen is much larger (av. 40.4 μm), the chromosome number for V. donii is not yet known and so we are unable to form any conclusions regarding ploidy level and pollen grain size, for this group of species.
Our results are also inconclusive for subgenus Beccabunga, as we do not know the ploidy levels of the specimens used for the pollen studies. In subsection Beccabunga two ploidy levels are described in the literature for V. beccabunga and V. anagalloides with a chromosome base number of x = 9. However, the tetraploid level is rare in V. beccabunga and, we suggest that V. anagalloides is most probably diploid in the Iberian Peninsula, so our samples are, in both cases, likely to be diploids. V. anagallis-aquatica and V. catenata are tetraploids with 2n = 4x = 36. In subsection Acinifolia, Veronica acinifolia is a diploid (2n = 2x = 14) and, in subsection Peregrinae, the chromosome number in V. peregrina is 2n = 52 which, according to Hofelich (Citation1935), probably implies a hexaploid level (6x; x = 9) with a subsequent loss of two chromosomes.
Pollen grain size in relation to style length and corolla diameter
Our survey of style length and corolla diameter, relative to pollen size, has produced some interesting data. As noted above morphometric analysis distinguishes the pollen of V. dillenii (V. subsect. Microspermoides) from that of V. verna () because it is, on average, larger. In this example, the difference cannot be explained by ploidy levels because both species have the same chromosome number, 2n = 16. However, it is possible that it might be a result of the difference in style length between the two species: the styles of V. dillenii (0.9–1.5 mm) are much longer than those of V. verna (0.2–0.6 mm). In subgenus Triangulicapsula: subsect. Digitatae the pollen of the tetraploid V. chamaepithyoides is, on average, smaller than the pollen of the diploid V. grisebachii. Therefore, it is interesting to note that in V. grisebachii the style length varies between 2–4 mm and the corolla diameter is between 7–13 mm, while in V. chamaepithyoides average style length is 0.4–0.6 mm and average corolla diameter is 2–5 mm. This shows a priori a positive relationship between pollen grain size, corolla diameter and style length within Veronica subsect. Digitatae. A similar result is found in V. subsect. Pellidosperma where there are significant differences in the size of the pollen grains of the three species studied (). Veronica donii has the largest pollen grains while those of V. triphyllos and V. praecox are similar in size but much smaller than those of V. donii. Three different base numbers (x = 7, 8, 9) have been reported for subgenus Pellidosperma. The number of chromosomes of the Turkish endemic V. donii remains unknown, while V. triphyllos (2n = 2x = 14) and V. praecox (2n = 2x = 18) are diploids with different base numbers. Therefore, any connection between ploidy level and pollen size cannot be inferred at present. Nevertheless, it is interesting to note, in relation to pollen size, that the style in V. donii is much longer (6–8 mm) than in either V. praecox (0.8–2 mm), or V. triphyllos (0.5–2 mm). With regard to corolla diameters, in V. donii these vary between 20–24 mm, while in V. praecox they are between 4–7 mm and in V. triphyllos between 5–10 mm. These data suggest a strong connection between style length, corolla diameter and pollen size. This is especially interesting in the case of the phylogenetically closely related V. donii and V. triphyllos with very different pollen sizes, but with an apparently positive relationship between pollen grain size, corolla diameter and style length. Finally, V. polita and V. siaretensis, in Veronica subsect. Agrestes, also show a positive relationship between pollen grain size, corolla diameter and style length.
It is interesting to note, however, that where positive correlations have been observed between pollen size, style length and stigma depth in closely related species of Polygonum, it has been suggested that it might reflect a phyletic, rather than a functional, relationship (Cruden & Lyon, Citation1985) and this could also be the case in Veronica.
The lack of a correlation between pollen volume and style length in comparisons of both related and unrelated plants has been shown for several plant species (Cruden & Lyon, Citation1985), however, it would seem to be inconsistent with the hypothesis that pollen grains must contain sufficient resources to sustain the growth of a pollen tube to an ovule (Cruden & Lyon, Citation1985). In contrast, a positive relationship between pollen grain size and stigma depth (distance from the external surface of the stigma to the transmission tissue in the style, which reflects the distance that a pollen tube has to grow to reach the resources in the style) has been reported (Cruden & Lyon, Citation1985; Cruden, Citation2000). There is also considerable evidence supporting the fact that there are types of resources (i.e., starch) in the style and transmission tissue, which do not occur in the stigma and that are used by growing pollen tubes (Cruden, Citation2000, and examples cited therein). The underlying assumption here is that larger pollen grains contain more nutrients than smaller ones and this allows their pollen tubes to grow through deep stigmas that cannot be traversed by pollen tubes from small pollen grains (Cruden, Citation2000). Measurements of stigma depth are unfortunately not available for any species Veronica, although it seems that there are very slight differences in stigma depth among the species (pers. obs.). Nutrient uptake of pollen tubes in Veronica has not been studied yet, so no conclusions related to any of these parameters can be drawn.
Corolla diameter and style length, at least, could be expected to be inversely related to selfing rates. It is well-known that there is strong correlation between pollen/ovule ratios (P/O) and breeding systems; thus P/O ratio decreases from xenogamous to facultative xenogamous to autogamous species (Cruden, Citation2000). Additionally, as pollen size increases, pollen quantity decreases and selfing rates increase (Cruden, Citation2000). In Veronica no data are available for pollen ovule ratios and neither are the breeding systems well understood; further in depth studies exploring all these parameters, as well as their relationships with pollen size, are needed.
Conclusions
Our results for pollen size support a previous opinion that it is character of limited phylogenetic use in Veronica. The limited usefulness of pollen size as a character in a phylogenetic context may be because at best it simply reflects a close relationship with other characters, such as ploidy level and, possibly, style length and corolla diameter.
Other important areas for investigation that have been highlighted in our study include further studies of Veronica peregrina to elucidate its phylogenetic and taxonomic position. It is a species that shows many morphological singularities including a pollen exine type not observed in any of the other species which we examined, a probably derived chromosome number, and a pattern of seed coat sculpture that is also probably derived. In subgenus Pellidosperma, subsect. Pellidosperma the chromosome number for Veronica donii needs to be established before any conclusions regarding ploidy level and pollen grain size, for this group of species, can be reached. Furthermore, our results are also inconclusive for subgenus Beccabunga, as we do not know the ploidy levels of all the specimens used in our pollen studies. Finally, there are, as yet, no data available for pollen ovule ratios in Veronica, and the breeding systems are not well understood; further in depth studies exploring all these parameters, as well as their relationships with pollen size, are needed.
Acknowledgements
The authors would like to express their gratitude to the curators of the following herbaria: G/ HGI/ JACA/ MA/ MGC/ MPU/ SALA/ W/ WU. Thanks are also due to Miguel Jerez former technician of the Scanning Electron Microscopy Service of the Real Jardín Botánico de Madrid (Spain). We specially thank Dr. D. Albach and Dr. M. M. Harley for their constant and generous collaborations and their helpful comments to improve the manuscript. This work was funded by the projects Flora iberica VI (REN2002-04634-C05-02) and Flora iberica VII (CGL2005-05471-C04-03/BOS). We are also very grateful for comments made by the referees.
Specimens investigated
[For herbarium acronyms, see Holmgren & Holmgren (Citation1998). Key: [S] material used for scanning electron microscopy, [T] material used for transmission electron microscopy, [C] material used for chromosome count.]
V. acinifolia L. Spain: Badajoz: La Codosera, 28 March 1999, L. Delgado et al., SALA (108481) [C]; Girona: Caldes de Malavella, 11 May 1999, L. Delgado et al., SALA (109220) [C] [S]; León: Albiles, 18 May 1972, J. Izco, MA (332987); Salamanca: Aldea del Obispo, 17 April 1998, J. A. Sánchez Agudo, SALA (109241) [C]; Ciudad Rodrigo, 2 May 1998, J. A. Sánchez Agudo, SALA (109222); Gallegos de Argañán, 21 April 1978, E. Rico, SALA (14483).
V. agrestis L. Spain: Jaén: Mancha Real, 10 May 1998, E. Rico et al., SALA (109217) [C]; Logroño: Valgañón, 1 May 1999, G. García Baquero, SALA (109356) [C]; Lugo: Monforte, 14 April 1991, J. Amigo & M. I. Romero, MA (503412); Salamanca: Pelabravo, 29 April 1998, L. Delgado & J. A. Sánchez Agudo, SALA (109253) [S] [C].
V. anagallis-aquatica L. Spain: Ávila: Pinar de Hoyocasero, 16 June 1998, J. A. Sánchez Agudo, SALA (109326); Badajoz: Cheles, 12 April 1980, B. Casaseca et al., SALA (50804); Huesca: Belsué, 11 June 1970, P. Montserrat & L. Villar, JACA (219870); Navarra: Plana de Sasi, 1 July 1975, L. Villar, JACA (10065475); Logroño: Agoncillo, Río Leza, 20 May 1985, D. Belmonte, SALA (42699); Salamanca: Montemayor del Río, 26 June 1985, E. Rico & A. Guillén, SALA (36270); Salamanca: Pelabravo, 9 September 1989, E. Rico et al., SALA (59082) [S] [T]; Zamora: Guarrate, 31 May 1998, J. A. Sánchez Agudo, SALA (109344).
V. anagalloides Guss. Spain: Cáceres: Matalobos, 13 June 1982, A. Valdés, SALA (78599); Huelva: Almonte, 19 June 1978, S. Castroviejo & E. Valdés-Bermejo, SALA (44209); León: Bercianos del Real Camino, 2 June 1983, T. E. Díaz & A. Penas, SALA (83661); Segovia: Cantalejo, 3 July 1983, T. Romero, SALA (38899); Teruel: Lavajo de Hornos, 6 July 1958, P. Montserrat, JACA (66858); Zamora: Cuelgamures, 17 June 1981, X. Giráldez, SALA (30450) [S].
V. arvensis L. Spain: Ávila: San Martín del Pimpollar, 16 June 1998, J. A. Sánchez Agudo, SALA (109251); Huesca: Valle de Benasque, La Renclusa, 13 May 1986, X. Giráldez, SALA (43971). Salamanca: Ciudad Rodrigo, 2 May 1998, J. A. Sánchez Agudo, SALA (109409); Salamanca: Ituero de Azaba, 17 April 1998, J. A. Sánchez Agudo, SALA (109242) [S] [T]; Toledo: Los Yébenes, Sierra del Rebollarejo, 9 May 1998, E. Rico et al., SALA (109254); Valladolid: Olmedo, pinar de Ordoño, 7 April 1998, L. Delgado et al., SALA (109257) [S].
V. beccabunga L. Spain: Burgos: La Puebla de Arganzón, 27 September 1984, P. Montserrat, JACA (234784); León: Peñalba de Santiago, 14 June 1998, J. A. Sánchez Agudo, SALA (108480) [S]; Huesca: San Juan de Plan, 24 May 1981, G. Montserrat, JACA (48981); Navarra: Belagua, 25 July 1977, F. Amich et al., SALA (13366); Segovia: Cedillo de la Torre, 5 June 1985, A. R. Burgaz & A. Izuzquiza, SALA (42724); Zamora: Venialbo, 17 May 1981, X. Giráldez, SALA (30452).
V. catenata Pennell Spain: Girona: Avinyonet de Puigventós, 29 April 1995, J. Font, HGI (8567); Mallorca: Capdepera, 6 April 1977, J. Duvigneaud, MA (303839) [S]. France: Dep. Savoie, Boveyron, 22 May 1972, E. Berger, SALA (88494).
V. ceratocarpa C. A. Mey. Armenia: Meghri, reg. Zangezur mountain ridge near Bugariaz, 9 June 1978, E. Gabrieliam, WU. Turkey: Erzurum (A9), Senkaya, 10 July 1976, A. Öztürk, WU; Strassenböschung, Bachufer, 4 August 1982, Sorger & Buchner, W [S].
V. cymbalaria Bodard Spain: Cáceres: () Casar de Cáceres hacia Monroy, L. Delgado et al., SALA (109378); Huesca: () Agüero, 25 March 1982, P. Montserrat & R. Fanlo, SALA (43463); Málaga: () Cartajima, 14 May 2000, L. Delgado et al. SALA (109202); Málaga: Cartajima 14 May 2000, L. Delgado et al. SALA (109203) [C]; Málaga: () Montejaque, 14 May 2000, L. Delgado et al. SALA (109204) [C]; Menorca: () Ferreríes, 20 April 2001, L. Delgado et al., SALA (109383) [C]; (); Menorca: Entre Ses Voltes y Algendar, 17 April 1957, P. Montserrat, SALA (49607) [C]; Sevilla: () Morón de la Frontera, 25 March 1982, M. Ladero et al., SALA (26842).
V. chamaepithyoides Lam. Spain: Madrid: Madrid, April, L. Neyra, MA (344600); Madrid, Altos de Amaniel, 2 April 1920, C. Vicioso, MA (112037) [S].
V. dillenii Crantz. France: Dép. Allier, près de Peyrolles, 19 May 1991, R. Deschâtres, MA (563733) [S]; Spain: Girona: Viladrau, 10-05-1999, L. Delgado et al., SALA (110142) [C]. Switzerland: Alpes, Grisons, Engiadina Bassa, 17 July 1990, E. Rico et al., SALA (109233). Turkey: Bursa (A2), Bursa, Ulu Dag, 22 June 1964, A. Huber-Morath, G.
V. donii Römpp Turkey: Muğla, zwischen dem “Gökbel-Pass” der Landkarten und Yatağan, 28 March 1978, G. & M. A. Fischer, W [S].
V. filiformis Sm. Austria: Oberösterreich, Pyphrnpaβ; 13 May 1994, E. Vitek, SALA (109410); Great Britain: Scotland, Inverness, Loch Ness, 29 April 2000, X. Giráldez, SALA (123737) [S].
V. grisebachii Walters Turkey: Phrygie, Ouchak, 26 May 1857, Balansa MPU [S]; Bulgaria: Distr. Kardzali, M. Rhodopae Orientalis: in arenosis loco dicto ZeleznaVrata, 24 April 53, N. Stojanov & B. Kitanov, MA (182293).
V. hederifolia L. Spain: () Jaén: Torres, 8 April 1999, L. Delgado et al., SALA (109255) [C]. Segovia: () San Miguel Bernuy, 9 March 2000, L. Delgado & J. A. Sánchez Agudo, SALA (109411) [S]; Salamanca: () Salamanca, 3 May 1999, L. Delgado, SALA (109309); Toledo: (l) Ontígola, 11 April 1999, L. Delgado et al., SALA (109276) [C] [S]; Valladolid: () Olmedo, 7 April 98, E. Rico et al., SALA (109308).
V. lycica E. B. J. Lehm. Turkey: Antalya (C2), Susuzdag, 21 June 1965, M. A. Fischer, W [S]; Antalya (C2), Akdäg bei Elmali, 24 June 1967, F. Sorger, W; Antalya (C2), S. Sinekcibeli Passhöhe, 23 April 1984, M. A. Fischer, W.
V. opaca Fries Finland: Alaudis, Frújtrau, 1880, J. Arrhenius, MA (112290). Rumania: Kojojna, 1 July 2000, X. Girádez et al., SALA (109360) [S].
V. panormitana Tineo Spain: Menorca: () Ferreríes, 20-04-01, L. Delgado et al., SALA (105882) [C] [S]; () Ferreríes, 19 February 2000, P. Fraga, Hº P. Fraga.
V. peregrina L. Spain: Cáceres: Valdecañas de Tajo, 23 April 2000, E. Rico, SALA (109320) [C] [S]; Girona: Girona, 11 May 1999, L. Delgado et al., SALA (109318). Zamora: Carbajales de Esla, 22 April 2002, P. Bariego et al., SALA (109392) [S].
V. persica Poir. Spain: Alicante: Denia, 15 April 1992, A. Barber, SALA (90657); Cáceres: Villanueva de la Vera, 29 March 1989, A. Amor, SALA (80188); Logroño: Leza del río Leza, 12 January 1979, F. Amich, SALA (20490); Salamanca: Ituero de Azaba, 17 April 1998, J. A. Sánchez Agudo SALA (100143) [S].
V. polita Fries Spain: Cádiz: Benaocaz, Arroyo Seco, 29 March 1984, A. Aparicio & S. Silvestre, SALA (48018); Lérida: L'Urgell, Boldú, 26 March 1986, J. Boixadera et al., SALA (43059); Salamanca: Ciudad Rodrigo, 2 May 1998, J. A. Sánchez Agudo, SALA (100145) [C]; Segovia: San Miguel de Bernuy, 7 April 1998, E. Rico et al., SALA (109206) [C] [S]; Zamora: Dehesa El Cubeto, 17 February 1983, X. Giráldez, SALA (30527).
V. praecox All. Spain: Córdoba: Lucena, 15 March 1987 [collector not indicated], MGC (22040); Jaén: Mancha Real, 10 May 1998, L. Delgado et al., SALA (109287); Segovia: San Miguel de Bernuy, 7 April 1998, E. Rico et al. SALA (109289) [S]; Valladolid: Villardefrades, 21 May 1980, J. Fernández Diez SALA (21626).
V. siaretensis E. B. J. Lehm. Iran: Tangerah, Golestan National Park, 21 April 1995, H. Akhani, W.
V. sibthorpioides Debeaux, Degen. & Hervier Spain: Granada: () Sª de Baza, 9 April 1999, L. Delgado et al., SALA (109304) [C]; Jaén: () Pico Almadén, 8 April 1999, L. Delgado et al., SALA (109361) [C]; () Pico Almadén, 8 April 99, L. Delgado et al., SALA (109274); () Sª Cazorla, Sierra del Pozo, Pico Cabañas, 10 April 1999, L. Delgado et al., SALA (109273) [C]; () Sierra Mágina, 8 April 1999, L. Delgado et al., SALA (109281).
V. stamatiadae M.A. Fisch. Greece: Dodecanese Islands, Ro Island off Kasterllorizo, 12 April 1974, W. Greuter W.
V. sublobata M. A. Fisch. Austria: Niederösterreich, Wechselgebiet, 4 April 1994, E. Vitek, W. Bulgaria: Regio Vitosae, Lozenska planina, 26 May 1973, D. Peev, MA (333219) [S].
V. trichadena Jord. & Fourr. Spain: Mallorca: () Establiments, 6 March 1947, P. Ferrer, MA (112238) [S]; () Pont d'Inca, 14 March 1919, F. Bianor, MA (112236).
V. triloba Opiz. Spain: Ciudad Real: () Venta de Cárdenas, 11 April 1999, L. Delgado et al. SALA (109275) [C]; Jaén: () Término municipal de Cambil-Arbuniel, 13 May 2000, L. Delgado et al., SALA (109265); Segovia: () Fuente el Olmo de Fuentidueña, 2 April 2003, J. A. Sánchez Agudo, SALA; () San Miguel de Bernuy, 2 April 2003, L. Delgado et al., SALA (109351); Toledo: () Ontígola, L. Delgado et al. SALA (109270) [C] [S].
V. triphyllos L. Spain: Ávila: Crespos, 6 April 1984, I. Barrera et al. SALA (42725); Salamanca: El Maíllo, 4 April 1976, E. Rico, SALA (10810); Valladolid: Olmedo, pinar de Ordoño, 7 April 1998, E. Rico et al., SALA (109237) [S]; Alava: Corres, 5 May 1985, I. Martínez de Icaya & G. Morante, SALA (44019).
V. verna L. Spain: Ávila: La Herguijuela, 16 June 1999, E. Rico, SALA (109229) [S]; Salamanca: Pelabravo, Las Morelas, 29 April 1998, L. Delgado & J. A. Sánchez Agudo SALA (109225); Valladolid: Olmedo, pinar de Ordoño, 7 April 1998, E. Rico et al. SALA (109223) [C]; Alava: Corres, Larintxo, 5 May 1985, I. Martínez de Icaya & G. Morante SALA (44018).
References
- Albach , D. C. 2007 . Amplified fragment length polymorphisms and sequence data in the phylogenetic analysis of polyploids: Multiple origins of Veronica cymbalaria (Plantaginaceae) . New Phytol. , 176 : 481 – 498 .
- Albach , D. C. and Chase , M. W. 2001 . Paraphyly of Veronica based on sequences from the internal transcribed spacers (ITS) of nuclear ribosomal DNA. J. Plant Res . 114 : 9 – 18 .
- Albach , D. C. and Chase , M. W. 2004 . Incongruence in Veroniceae (Plantaginaceae): Evidence from two plastid and a nuclear ribosomal DNA region . Molec. Phyl. Evol. , 32 : 183 – 197 .
- Albach , D. C , Grayer , R. J. , Kite , G. C. and Jensen , S. R. 2005a . Veronica: Acylated flavone glycosides as chemosystematic markers . Biochem. Syst. Ecol. , 33 : 1167 – 1177 .
- Albach , D. C. , Jensen , S. R. and Özgökce , F. 2005b . Veronica: Chemical characters for the support of phylogenetic relationships based on nuclear ribosomas and plastid DNA sequences data . Biochem. Syst. Ecol. , 33 : 1087 – 1106 .
- Albach , D. C. , Martínez-Ortega , M. M. and Chase , M. W. 2004a . Veronica: Parallel mophological evolution and phylogeography in the Mediterranean . Plant Syst. Evol. , 246 : 177 – 194 .
- Albach , D. C. , Martínez-Ortega , M. M. , Fischer , M. A. and Chase , M. W. 2004b . A new classification of the Veroniceae -- problems and a possible solution . Taxon , 53 : 429 – 452 .
- Albach , D. C. , Martínez-Ortega , M. M. , Fischer , M. A. and Chase , M. W. 2004c . Evolution of the Veroniceae: A phylogenetic perspective . Ann. Mo. Bot. Gard. , 91 : 275 – 302 .
- Albach , D. C. , Martínez-Ortega , M. M. , Delgado , L. , Weiss-Schneeweiss , H. , Özgökce , F. and Fischer , M. A. 2009 . Chromosome number in Veroniceae: Review and several new counts . Ann. Mo. Bot. Gard. , 95 : 543 – 566 .
- Albach , D. C. , Meudt , H. M. and Oxelman , B. 2005b . Piecing together the “new” Plantaginaceae . Am. J. Bot. , 92 : 297 – 315 .
- Albach , D. C. , Utteridge , T. and Wagstaff , S. J. 2005c . Origin of Veroniceae (Plantaginaceae, formerly Scrophulariaceae) on New Guinea . Syst. Bot. , 30 : 412 – 423 .
- APG (Angiosperm Phylogeny Group) . 1998 . An ordinal classification for the families of flowering plants . Ann. Mo. Bot. Gard. , 85 : 531 – 553 .
- APG (Angiosperm Phylogeny Group) . 2003 . An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II . Bot. J. Linn. Soc. , 141 : 399 – 436 .
- Cranwell , L. M. 1941 . New Zealand pollen studies. 1. Key to the pollen grains of families and genera in the native flora . Rec. Auckland Inst. Mus. , 2 : 280 – 308 .
- Cruden , R. W. 2000 . Pollen grains: Why so many? . Plant Syst. Evol. , 222 : 143 – 165 .
- Cruden , R. W. and Lyon , D. L. 1985 . Correlations among stigma depth, style length, and pollen grain size: Do they reflect function or phylogeny? . Bot. Gaz. (Chicago) , 146 : 143 – 149 .
- Erdtman , G. 1952 . Pollen morphology and plant taxonomy: Angiosperms , Stockholm : Almqvist & Wiksell .
- Erdtman , G. 1960 . The acetolysis method. A revised description . Sv. Bot. Tidskr. , 54 : 561 – 564 .
- Fernández , I. , Juan , R. and Pastor , J. 1997 . Morfología polínica de Veronica L. (Scrophulariaceae) en el suroeste de España . Acta Bot. Malacit. , 22 : 65 – 72 .
- Fischer , M. A. 1978 . “ Veronica ” . In Flora of Turkey 6 , Edited by: Davis , P. H. 689 – 753 . Edinburgh : Univ. Press .
- Fischer , M. A. 1981 . “ Veronica ” . In Flora Iranica 147 , Edited by: Rechinger , K. H. 25 – 165 . Graz : Akad. Wiss .
- Garnock-Jones , P. , Albach , D. C. and Briggs , B. 2007 . Botanical names in Southern Hemisphere Veronica (Plantaginaceae): Sect. Derwentia, sect. Detzneria, and sect. . Hebe. Taxon , 56 : 571 – 582 .
- Hideux , M. J. and Ferguson , I. K. 1976 . “ The stereostructure of the exine and its evolutionary significance in Saxifragaceae s.l ” . In The evolutionary significance of the exine , Edited by: Ferguson , I. K. and Muller , J. 327 – 377 . London : Acad. Press. Linn. Soc. Symp. Ser .
- Hofelich , A. 1935 . Die Sektion Alsinebe Grisebach der Gattung Veronica in ihren chromosomalen Grundlagen . Jahrb. Wiss. Bot. , 81 : 541 – 572 .
- Holmgren, P. K. & Holmgren, N. H. (1998) [continuously updated]. Index Herbariorum: A global directory of public herbaria and associated staff. Bronx, NY: New York Botanical Gard. Virtual Herbarium http://sweetgum.nybg.org/ih/ (Accessed: 2 June 2008 ).
- Hong , D.-Y. 1984 . Taxonomy and evolution of the Veroniceae (Scrophulariaceae) with special reference to palynology. Copenhagen: Counc. Nord. Publ. Bot . Opera Bot. , : 75
- Hong , D.-Y. and Nilsson , S. 1983 . On the validity of the genus Cochlidiosperma Reichenb. (Scrophulariaceae), as supported by additional palynological evidence . Acta Phytotax. Sin. , 21 : 146 – 150 .
- Huang , T.-C. 1972 . Pollen flora of Taiwan , Taipei : Bot. Dept. Natl. Taiwan Univ. Press .
- Jensen , S. R. , Albach , D. C. , Ohno , T. and Grayer , R. J. 2005 . Veronica: Iridoids and cornoside as chemotaxonomic markers . Biochem. Syst. Ecol. , 33 : 1031 – 1047 .
- Lynch , S. P. and Webster , G. L. 1975 . A new technique of preparing pollen for scanning electron microscopy . Grana , 15 : 127 – 136 .
- Martínez-Ortega , M. M. and Rico , E. 2001 . Seed morphology and its taxonomic significance in some Veronica species (Scrophulariaceae) from the Western Mediterranean . Plant Syst. Evol. , 228 : 15 – 32 .
- Martínez-Ortega , M. M. , Sánchez Agudo , J. A. and Rico , E. 2009 . “ Veronica ” . In Flora iberica. 13 , Edited by: Benedí , C. , Rico , E. , Güemes , J. and Herrero , A. 360 – 434 . Madrid : R. Jard. Bot. Madrid/CSIC .
- Martínez-Ortega , M. M. , Sánchez Sánchez , J. and Rico , E. 2000 . Palynological study of Veronica Sect. Veronica and Sect. Veronicastrum (Scrophulariaceae) and its taxonomic significance . Grana , 39 : 21 – 31 .
- Martínez-Ortega , M. M. , Sánchez Agudo , J. A. and Rico , E. 2003 . Sobre el tratamiento taxonómico de Veronica L. (Scrophulariaceae) para flora iberica . An. Jard. Bot. Madrid , 60 : 236 – 241 .
- Muñoz-Centeno , L. M. , Albach , D. C. , Sánchez Agudo , J. A. and Martínez-Ortega , M. M. 2006 . Systematic significance of seed morphology in Veronica (Plantaginaceae): Phylogenetic perspective . Ann. Bot. , 98 : 335 – 350 .
- Olmstead , R. G. , de Pamphilis , C. W. , Wolfe , A. D. , Young , N. D. , Elisons , W. J. and Reeves , P. A. 2001 . Disintegration of the Scrophulariaceae . Am. J. Bot. , 88 : 348 – 361 .
- Praglowski , J. and Punt , W. 1973 . An elucidation of the micro-reticulate structure of the exine . Grana , 13 : 45 – 50 .
- Punt , W. , Blackmore , S. , Nilsson , S. and Le Thomas , A. 1994 . Glossary of pollen and spore terminology. Utrecht: Lab. Palaebot. Palynol., Utrecht Univ . LPP Found. Contrib. Ser. , : 1
- Punt, W., Blackmore S., Nilsson, S. & Le Thomas, A. (2007). Glossary of pollen and spore terminology. 3 ed. Utrecht: Lab. Palaebot. Palynol., Utrecht Univ. LPP Found., Contrib. Ser. 1. http://www.bio.uu.nl/∼palaeo/glossary/ (www.bio.uu.nl/~palaeo/glossary/) (Accessed: 17 June 2009 ).
- Reveal , J. L. , Olmstead , R. and Judd , W. S. 2008 . (1812–1813) Proposal to conserve the name Veronicaceae (Magnoliophyta), and to conserve it against Plantaginaceae, a “superconservation” proposal . Taxon , 57 : 643 – 644 .
- Risch , C. 1939 . Die Pollenkörner der in Deutschland wild wachsenden Scrophulariaceen . Ver. Dtsch. Bot. Ges. , 57 : 108 – 121 .
- Sáenz de Rivas , C. 1978 . Polen y esporas (Introducción a la palinología y vocabulario palinológico) , Madrid : H. Blume .
- Sánchez Agudo , J. A. 2005 . “ Estudio biosistemático de Veronica sect. Pocilla Dumort. y V. sect. Beccabunga (Hill) Dumort. ” . In (Scrophulariaceae) en la Península Ibérica , Salamanca : Bot. Dept., Salamanca Univ. PhD Diss .
- Saeidi-Mehrvarz , S. and Zarrei , H. 2006 . Pollen morphology of some species of the genus Veronica (Scrophulariaceae) in Iran . Wulfenia , 13 : 1 – 10 .
- Taskova , R. M. , Albach , D. C. and Grayer , R. J. 2004 . Phylogeny of Veronica -- a combination of molecular and chemical evidence . Plant Biol. , 6 : 673 – 682 .
- Varghese , T. M. 1968 . Studies in the family Scrophulariaceae. II. Pollen morphology . J. Palynol. , 4 : 91 – 97 .