Abstract
The family Hymenophyllaceae is represented in the study area by six species in two genera, Hymenophyllum J. E. Smith and Trichomanes L. The study was based on herbarium material and spores were studied under light microscope (LM), scanning electron microscope (SEM) and transmission electron microscope (TEM). Both genera have trilete spores, 23 to 45 μm in equatorial diameter, with an ornamentation of echinulae and cones in Hymenophyllum and of verrucae, gemmae and granules in Trichomanes. Mature spores have a sporoderm composed of a perispore, an exospore and a fibrillar endospore; the exospore is 0.5 to 2.5 μm thick, compact and with an irregular margin. In some cases radial channels and other channels associated with the middle and inner parts of the laesurae were evident. A series of cavities filled with an opaque content line the inner margin of the exospore. The perispore is 20 to 400 nm thick and unevenly differentiated along the surface of a same spore. Under TEM, two main differentially contrasted portions could be distinguished: a dark massive portion with structural components could not be distinguished, and a light portion with several plates arranged in piles. The inner surface of the perispore exhibit short scales. Globules are immersed within the perispore at some depth from the perispore surface and others connected to it by structural threads. The spore characters observed including shape, ornamentation, laesurae length and wall structure are useful in distinguishing the two genera studied, but less useful in differentiation at the species level.
The north-western part of Argentina is a large region positioned in the subtropical to tropical zone. It exhibits an altitude range from 500 to 6000 m a.s.l. and, with its diverse environments, houses the highest floristic diversity in the country. The present analysis of Hymenophyllaceae spores is part of our long-term research focus on pteridophyte spores from north-western Argentina that has so far resulted in the description of 20 of the 23 families known in the area (for a summary see Gardenal et al., Citation2007).
The Hymenophyllaceae or filmy ferns are represented in Argentina by four genera: Hymenophyllum J. E. Smith, Hymenoglossum Presl, Serpyllopsis van den Bosch and Trichomanes L., with approximately 15 species in the Andean-Patagonic forest, nine in the north-eastern area and six in north-western Argentina (Sota, Citation1972, Citation1977; Ponce, Citation1966; Ponce & Sota, Citation2008). Hymenophyllum and Trichomanes are represented by six species: H. capurroi Sota, H. tunbridgense (L.) J. Smith var. cordobense Hieronymus, T. hymenoides Hedwig, T. kraussii Hooker & Greville, T. reptans Swartz and T. tenerum Sprengel. The classification followed here (Morton, Citation1968) is different from the classification of Hymenophyllaceae suggested by Ebihara et al. (Citation2006). Morton (Citation1968) proposed six genera, two of which are the ones treated here. Ebihara et al. (Citation2006) based the classification on molecular phylogenetic analyses using chloroplast sequences and proposed nine genera of Hymenophyllaceae differentiated by characteristics of roots, veins of the lamina and characteristics and distribution of the sori.
Spores of Hymenophyllaceae are typically trilete, but abnormal forms including monolete, tetralete and intermediate spores have been observed for some specimens of Hymenophyllum (Morbelli, Citation1980; Tryon & Lugardon, Citation1991). The spores are variously ornamented and earlier studies of species in the genera Hymenophyllum, Serpyllopsis and Trichomanes from western Patagonia and north-western Argentina by Morbelli (Citation1980, Citation1983) have suggested that shape, laesurae form and ornamentation were helpful features for distinguishing taxa at genus level. Harris (Citation1955) also used spore ornamentation to distinguish groups of Hymenophyllum and Trichomanes species occurring in New Zealand. However, Large and Braggins (Citation1991) noted, based on palynological studies of species from New Zealand, that the spores of the two genera are closely similar, thus they questioned the separation of Hymenophyllum and Trichomanes based on palynological characteristics. Four wall layers were observed in the sporoderm of germinated spores: a perispore, a three-layered exospore, a pseudo-endospore, and an endospore (Lugardon, Citation1981).
We here analyse spore morphology and ultrastructure of Hymenophyllum and Trichomanes from north-western Argentina to further test the relevance of the palynological characteristics of these taxa for delimitation of taxa at the species and generic level.
Materials and methods
The present study was conducted on herbarium material from the Museo de Ciencias Naturales de La Plata (LP), and from the Museo Argentino de Ciencias Naturales “Bernardino Rivadavia” (BA), the Fundación Miguel Lillo (LIL), Tucumán, and the Instituto de Botánica Darwinion (SI). A new collection of additional material was also made in the study area.
The spores of Hymenophyllaceae have a thin wall that easily folds; after acetolysis only few spores kept their original shape. Taking this characteristic into account, our analysis was based on both untreated and chemically treated material. Part of the material was therefore treated with heated sodium carbonate 3% solution and then acetolysed (Erdtman, Citation1960). For light microscopy (LM) analyses, the spores were immersed in glycerine-jelly and studied with Olympus BHB and BH2 trinocular microscopes.
For scanning electron microscope (SEM) studies, both untreated and chemically-treated spores were mounted on double-sided stick tape. They were then coated with gold-palladium and studied using a JEOL JSMT-100 scanning electron microscope.
For transmission electron microscope (TEM) observations, dry material from herbarium specimens was hydrated following the technique of Rowley and Nilsson (Citation1972) using a buffer plus 1% alcian blue (AB). The material was then fixed with glutaraldehyde plus 1% alcian blue in phosphate buffer for 12 h and post-fixed with 1% osmium tetroxide in water plus 1% alcian blue. The spores were dehydrated in an acetone series and embedded in Spurr soft mixture. Sections, 3 μm thick, were stained with toloidine blue and observed under LM. Ultra-thin sections were stained with uranyl acetate (1%) for 15 minutes followed by lead citrate for 3 minutes and studied using a Zeiss T-109 transmission electron microscope.
Results
Hymenophyllum
Spores of Hymenophyllum are trilete, triangular in polar view, with proximal face conic and distal, convex in equatorial view. Equatorial diameter is 23–35 μm and the polar diameter is 16–26 μm (). Laesurae are conic in section, 20 μm long and reach the equator (). The sporoderm consist of two wall layers: an exospore that is brown-yellowish under LM, 0.3–2.5 μm thick and a thin perispore approximately 240 nm thick. Ornamentation is heteromorphic consisting mainly of heterometric echinulae and cones, with processes ranging from 830 nm to 3 μm high (, , ). Laesurae are ornamented with echinulae and cones (, , ).
Table I. Spore characteristics of Hymenophyllaceae species from north-west Argentina
Figure 1. Spores of Hymenophyllum and Trichomanes under LM. A–D. Spores of Hymenophyllum tunbridgense var. cordobense. A, B. Spores in superficial view and optical section respectively: A. The ornamentation is formed of low, scattered elements with circular outline. The laesurae have thick margins and reach the equator; B. The spore has a subtriangular outline. C, D. Spores in superficial, equatorial views and in optical section respectively; observe in (D) the proximal shape conic and the distal shape convex. E–K. Spores of Trichomanes reptans. E, F. Proximal views, in surface and optical section respectively: E. Low scattered elements with circular outline are visible. The laesurae are marked and are ¾ of the spore radius; F. The optical section allows to appreciate the circular outline. G. Distal view, with low scattered elements. H. Atypical spore with a circular laesura. I. The same spore as in (H), in optical section. J, K. Proximal, superficial and equatorial views, respectively: J. The elements of the ornamentation are scattered and occasionally fused by their bases forming short, low and narrow ridges. The laesurae are well marked; K. The spore is proximally convex and distally hemispheric. Scale bars – 10 μm.
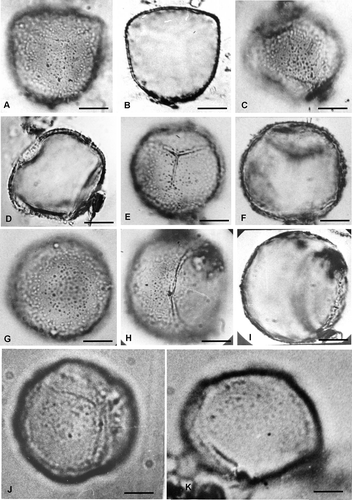
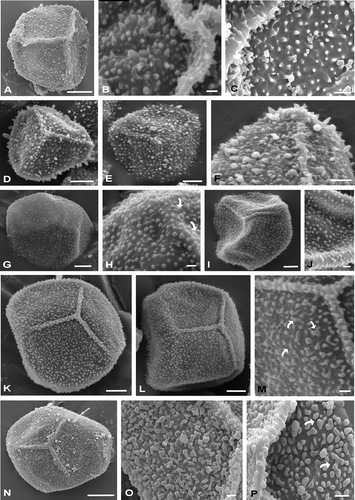
Figure 2. Spores of Hymenophyllum and Trichomanes under SEM. A–C. Spores of Hymenophyllum capurroi. A. Proximal view of a trilete spore, with triangular shape; the ornamentation is composed of echinulae, bacules and cones and the laesurae are conic in section and reach the equator. B. Detail of the laesura covered by processes of different shapes and sizes. C. Detail of the proximal face with heteromorphic processes. D–F. Spores of Hymenophyllum tunbridgense var. cordobense. D. Spore in proximal view, note the laesurae are conic in section and ornamented. E. Spore in distal view. F. Detail of the laesura. G, H. Spores of Trichomanes hymenoides. G. Distal view. H. Distal surface, some elements of the ornamentation are fused and form scattered ridges (arrows). I, J. Spores of Trichomanes kraussi. I. Proximal view. The laesurae in section are blunt; J. The distal ornamentation is composed of verrucae, gemmae and granules. K–M. Spores of Trichomanes reptans. K, L. Proximal views. The laesurae do not reach the equator. M. Proximal face in detail. Some elements of the ornamentation are fused forming scattered ridges (arrows). N–P. Spores of Trichomanes tenerum. N. Spore in proximal view; O, P. Details of proximal surfaces. Some elements are fused and form scattered ridges (arrows). Scale bars − 1 μm (O); 2 μm (B, C, H, J, M, P); 5 μm (F); 10 μm (A, D, E, G, I, K, L, N).
In TEM the exospore show less contrast than the perispore and is compact with an irregular outer surface characterised by processes of different height, shape and distribution. Within the exospore a thin, inner and denser staining zone can be distinguished ( C, , ). The exospore is also characterised by radially oriented channels (, ). The perispore is of variable thickness, 20–200 nm thick, strongly contrasted and with an irregular margin. In section, spheroids could be seen on the surface; some of them are fused to the perispore (, ). In some spores a third layer, the endospore, is clearly defined, showing a fibrilar structure ().
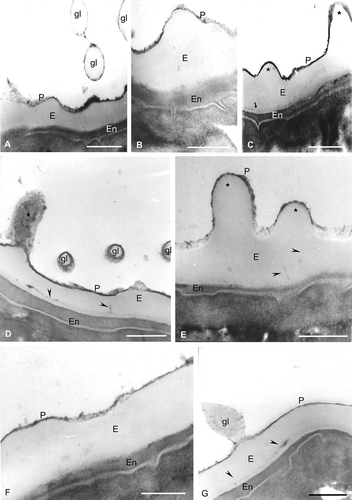
Figure 3. Spores of Hymenophyllum under TEM. Spore wall sections of Hymenophyllum tunbridgense var. cordobense from the same sporangium. The exospore (E) is compact and less contrasted than the perispore (P). In some spores, a third wall, the endospore (En), is present. A. Three globules (gl) in section, which are formed of a thin outer wall with structure and contrast similar to that of the perispore, and an inner one with contrast and structure similar to that of the exospore; the perispore (P) has regions of different contrast and thickness. B. The perispore (P) has irregular margin, different contrast and variable thickness; the endospore is present (En). C. Two processes of different height are evident (star); in the exospore (E), an inner zone with high contrast is visible (arrow); the endospore (En) is thick and highly contrasted. D. A process is seen in longitudinal section (star); three globules (gl) are visible in transversal section, which are constituted of a central body of nature and contrast similar to that of the exospore and covered by an outer layer of similar nature and contrast to that of the perispore; channels are visible in the inner region of the exospore (arrowheads); a thick endospore (En) is also visible. E. Two processes are seen also in section, with different heights (stars); in the inner zone of the exospore (E), channels are recognisable (arrowheads); the perispore (P) has an irregular margin and different contrast and thickness along its whole length; the endospore (En) margin is irregular. F. The perispore (P) has different thickness and irregular margin and the endospore (En) is highly contrasted and has a variable thickness. G. In this figure, a globule is visible (gl) that is connected to the perispore through slender threads; the globule is composed of a central body of compact nature, surrounded by a lax, less contrasted network; a highly contrasted zone is visible in the exospore (E) and in the channels (arrowheads); the endospore has irregular thickness. Scale bars – 500 nm in all figures.
Comments
The specimens of Hymenophyllum capurroi, Capurro 105 (LP), and H. tunbridgense var. cordobense, Castillón s/n (LP), produced atypical spores. In H. capurroi, the spores are tetralete while there are monolete and trilete hyaline forms with smooth surface and thin walls in H. tunbridgense var. cordobense.
Trichomanes
Spores of Trichomanes are trilete, globose in polar view and with both proximal and distal faces that are convex in equatorial view. Equatorial diameter is 25–45 μm; the polar diameter is 25–32 μm (). Laesurae are 24 μm long, representing ¾ of the equatorial radius. In LM, the sporoderm is divided into a light yellow exospore, 0.9–3.7μm thick, and a perispore, 30–400 nm thick. The ornamentation consists of verrucae, gemae and granules, 788 nm to 1.6 μm high. Some processes are fused at their bases, creating a pattern of scattered, thin, low ridges (, ).
SEM studies reveal that the ornamentation elements include verrucae, gemmae and granules (, , ). The same sculptural elements are also present on the laesurae (, M). Some of the elements are fused, forming scattered ridges (, , ).
Laesurae are blunt in TEM-section (). The exospore is compact, less contrasted than the perispore, 900 nm to 3.7 μm thick, with radially oriented, simple or ramified channels. The perispore is strongly contrasted, 30–400 nm thick, continuous and with an irregular margin (, , ). Fragments of membranes were evident at the inner surface of the perispore (). Spheroids, free or laterally fused to each other and to the perispore, are visible in ultrathin sections (, C, D). Some spores exhibit a thick, two-layered endospore with a fibrilar structure (, G). shows a spore with a circular laesura.
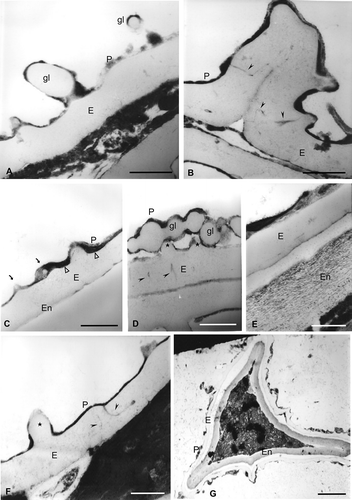
Figure 4. Spores of Trichomanes under TEM. Wall sections of spores of Trichomanes hymenoides with different degree of maturation from a sporangium. The exospore is compact with irregular margin less contrasted than the perispore. In some spores a third wall is evident: the endospore (En). A. Two globules (gl) in section are visible, whereas a big one is fused to the exospore (E). They consist of: a thin outer wall of similar structure and contrast to that of the perispore (P) and an inner wall with the same contrast as that of the exospore; the perispore has areas with different contrast and is continuous in the whole surface. B. Section through a laesura. Channels are present at the edges of the commissure; in non-apertural areas some channels, both single or branched (arrowheads), are seen in connection with the perispore; processes of ornamentation are visible on the laesura surface. C. Globules are immersed in the perispore (P, arrows); membrane fragments (arrowheads) are in the base of the perispore. D. In this section, channels with a radial orientation within the exospore (arrowheads) are visible; two low processes (stars) are also visible; the perispore (P) in some areas has different contrast and thickness; numerous globules (gl) are free or fused to each other or to the perispore. E. A thick endospore (En) with a fibrilar structure is evident; the exospore (E) is compact and less contrasted than the perispore, while the perispore has different thickness, contrast and structure along the surface; the lighter areas have a structure formed of fibres while the dark areas have a compact structure. F. Within the exospore, the sculpture process appears (star). Large channels (arrowheads) with a dark content appear within the exospore and contact the perispore at several places. The perispore (P) is uniformly developed. G. In this spore the perispore (P) is thin, and irregularly developed along the surface; the exospore (E) has irregular margin. A thick, fibrilar endospore (En) is evident and the laesura is located in the lower part of the figure. Scale bars − 500 nm in all figures.
Comments
Within this genus, atypical spores are found in a Trichomanes hymenoides specimen, Capurro 145, a T. kraussii specimen, Capurro 250, a T. reptans specimen, Castillon s/n, and in a T. tenerum specimen, Castellanos 14. These atypical spores are monolete, tetralete and trilete, thin-walled, smooth and hyaline.
Discussion and conclusions
Hymenophyllum and Trichomanes may be distinguished by certain characteristics of the sporophyte including indusial morphology. Our studies show that spore morphology may also be useful in taxonomic separation of the genera.
Spores of the Hymenophyllaceae have chlorophyll and germinate quickly, sometimes within the sporangia before the wall splits (Stokey, Citation1940) and are thus usually multicellular. In our study we also found multicellular spores that had germinated within the sporangium. These all have a thick endospore, which might correspond to the type of sporoderm present in primitive spores of Ophioglossaceae (Lugardon, Citation1981).
Spores produced by Trichomanes species from north-western Argentina range in size from 25–45 μm in diameter, whereas spores of Hymenophyllum from the same area range in size from 23–35 μm in diameter. The spores of Hymenophyllum and Trichomanes are generally trilete, although some specimens produced atypical monolete, tetralete spores or trilete spores with hyaline walls. Such abnormal and intermediate forms between monolete and trilete were also mentioned by Morbelli (Citation1980) for specimens from the Andean region of Argentine Patagonia, as well as in specimens of Hymenophyllum from North America, Jamaica and South Africa (Tryon & Lugardon, Citation1991). The spherical bodies, as seen here and associated with the perispore in both genera, are similar to the globules described by Lugardon (Citation1981), which, according to Tryon and Lugardon (Citation1991), are common on the surface of many pteridophyte spores. Ultrastructural features show some variation, but our study revealed that the degree of contrast and thickness in the perispore varies in the same spore, which may suggest that some variations correspond to different stages of wall differentiation. We have not observed any pseudo-endospore as described by Lugardon (Citation1981) and Tryon and Lugardon (Citation1991) for Hymenophyllaceae.
Subtle characteristics, such as shape, ornamentation and laesurae, distinguish the spores of the two genera. Spores of Hymenophyllum are characterised by their triangular shape and conical proximal faces, high sculptural processes with sharp tips, and laesurae that are conical in section and reach the equator, while spores of Trichomanes have a spheroidal shape and convex proximal faces, low processes with rounded tips, and short laesurae that are blunt when seen in section. Therefore, both palynological and morphological characteristics of the sporophyte are useful characters that should be included in systematic analyses of the group. This is in contrast to the conclusions by Large and Braggins (Citation1991), who noted that identification to the genus level based on spore morphology was questionable due to the homogeneity of the palynological characters.
Harris (Citation1955) grouped the spores of Hymenophyllum and Trichomanes species from New Zealand into three categories, based on their ornamentation seen in LM: smooth to fine granulate-papillate, fine to moderate granulate-papillate, and granulate to papillate. Our studies indicate that there is an inverse correlation between spore size and height of the ornamentation processes in specimens from the study area. This is in agreement with observations reported by Harris (Citation1955) for the species from New Zealand.
The characteristics of the spores and their variations are useful when determining the hybrid nature of the species; therefore, further experiments on spore cultures may be useful for testing the apogamic condition in Hymenophyllum capurroi, H. tunbridgense var. cordobense, Trichomanes hymenoides, T. kraussii, and T. tenerum. This agrees well with Morzenti (Citation1966), who stressed the importance of studying these spore types in order to detect the hybrid and/or apogamic nature of ferns in the Asplenium heterochroum-resiliens complex.
Specimens investigated
Hymenophyllum capurroi. Argentina. Prov. Tucumán: Dpto. Chicligasta, Capurro 105, 8-VI-1945 (LP, Holotypus), MP 1127.
Hymenophyllum tunbridgense var. cordobense. Argentina. Prov. Tucumán: Dpto. La Casita, Castillón s/n, 11-VII-1911, (LIL 40854), MP 1027; Idem, Castillón 2253, 2-XII-1920, alt. 1838 m, (LIL), MP. 1058; Alpachiri, Estancia Los Pinos, Capurro 87, 5-VI-1945 (LIL), MP 1055; Castillón 11658, 11-VII-1911 (LIL), MP 1056.
Trichomanes hymenoides. Argentina. Prov. Tucumán: Cerro Aconquija, Capurro 145, 14-VI-1945 (LIL), MP 1059; Dpto. Monteros, Sota 4077, I-1965 (LP), MP 1113a; Arroyo Celeste, Fabris 6606, X-1966 (LP), MP 1112a.
Trichomanes krausii. Argentina. Prov. Salta: Urundel, Capurro 278, 4-VII-1945 (LP, BA), MP 1111a; Idem, Capurro 250, 4-VII-1945 (LP, BA), MP 1109a; Ibidem, Capurro 256, 4-VII-1945 (LP, BA), MP 1110 a; Dpto. Orán, Río Santa María, Las Juntas, Castellanos 2, 28-VII-1944 (LIL), MP 1060.
Trichomanes reptans. Argentina. Prov. Tucumán: La Quebrada, Castillón s/n, 11-VII-1911 (LIL 40818), MP 1061; Aconquija, Camino a Tafí del Valle, Capurro s/n, 18-X-1948 (BA 28048, LP), MP 1107a; La Quebrada de Los Sosas, Capurro s/n, X-1962 (BA 58069, LP), MP 1108a.
Trichomanes tenerum. Argentina. Prov. Jujuy: Dpto. Ledesma, Camino a Valle Grande, Abra de Cañas, 1700 m, Cabrera et al. 25674, 31-X-1974 (LP), MP 1102a; Idem Cabrera et al. 25674, 31-X-1974 (LP), MP 1102b. Prov. Salta: Dpto.Orán, Quebrada del Acheral, Castellanos 14, 29-VII-1944 (LIL), MP 1062. Prov. Misiones: Dpto. Gral. Belgrano, Ruta 14, e/ Yrigoyen y 2 Hermanas, cabecera Arroyo Campiña de América, Sota et al. 6148 (LP), 15-XII-1970, MP 1103a; San Pedro, Las Ratas, Capurro s/n, s/fecha, (BA 57282, LP), MP 1106; Dpto. Iguazú, Ruta 101, Parque Nacional Iguazú y San Antonio, Sota et al. 6126, 14-XII-1970 (LP), MP 1106a; Bernardo de Irigoyen, Torres 124, 7-XII-1958 (LP), MP 1105a, Tobuna El Piñalito, Capurro 953, 2-II-1952 (LP), MP 1104a.
Acknowledgements
The authors wish to thank Lic. Rafael Urrejola of the SEM Unit at the Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, and Ing. Luis Zimmerman of the TEM Unit of the Instituto de Biología Celular, Facultad de Medicina, Universidad de Buenos Aires. This contribution was funded by the National Council of Scientific and Technological Research, CONICET (PIP #5044), the National Agency of Science and Technology Promotion, ANPCyT (PICT #12758) and Universidad Nacional de La Plata (project # 451).
References
- Ebihara , A. , Dubuisson , J. , Iwatsuki , K. , Hennequin , S. and Ito , M. 2006 . A taxonomic revision of Hymenophyllaceae . Blumea , 5 : 221 – 280 .
- Erdtman , G. 1960 . The acetolysis method. A revised description . Sv. Bot. Tidskr. , 54 : 561 – 564 .
- Gardenal , P. , Morbelli , M. A. and Giudice , G. E. 2007 . Morphology and ultrastructure of heterosporous Filicophyta spores from north-west Argentina . Grana , 46 : 65 – 77 .
- Harris , W. F. 1955 . A manual of the spores of New Zealand Pteridophyta , Wellington : Dept. Sci. & Industr. Res. Bull. 116 .
- Large , M. F. and Braggins , J. E. 1991 . Spore atlas of New Zealand ferns & fern allies , Wellington : SIR Publ. N. Zeal. J. Bot. Suppl .
- Lugardon , B. 1981 . “ TEM observations on the sporoderm of Hymenophyllaceae and Hymenophyllopsidaceae ” . In 5th Int. Palynol. Congr., Cambridge 1980. Abstracts , Edited by: Chaloner , W. G. and Sheerin , A. 236 Cambridge & St. Ives : Call Print. Group .
- Morbelli , M. A. 1980 . Morfología de las esporas de Pteridophyta presentes en la región Fuego-patagónica, República Argentina , Tucumán : Fac. Ci. Nat. & Inst. M. Lillo UNT. Opera Lilloana 28 .
- Morbelli , M. A. 1983 . “ Estudio de esporas de Pteridofitas del Noroeste de Argentina. Hymenophyllaceae ” . In V Simp. Argentino Paleobot. & Palinol., Buenos Aires 1983. Libro Resúm. , Edited by: Org. Comm . 36 La Plata : Mus. Cie. Nat .
- Morton , C. V. 1968 . The genera, subgenera and sections of the Hymenophyllaceae . Contr. U.S. Natl. Herb. , 38 : 153 – 214 .
- Morzenti , V. M. 1966 . Morphological and cytological data on southeastern United States species of the Asplenium heterochroum-resiliens complex . Am. Fern J. , 56 : 167 – 177 .
- Ponce , M. M. 1996 . “ Pteridophyta ” . In Catálogo de las plantas vasculares de la República Argentina. I , Edited by: Zuloaga , F. O. and Morrone , O. 1 – 79 . St. Louis, MO : Mo. Bot. Gard. Press. Monogr. Syst. Bot. 60 .
- Ponce , M. and Sota , E. R. de la. 2008 . “ Hymenophyllaceae ” . In Catálogo de las plantas vasculares del Cono Sur. Vol. 1. Pteridophyta, Gymnospermae y Monocotyledonae , Edited by: Zuloaga , F. , Morrone , O. and Belgrano , M. 62 – 75 . St. Louis, MO : Mo. Bot. Gard. Press. Monogr. Syst. Bot. 107 .
- Rowley , J. R. and Nilsson , S. 1972 . Structural stabilization for electron microscopy of pollen from herbarium specimens . Grana , 12 : 23 – 30 .
- Sota , E. R. de la . 1972 . Sinopsis de las Pteridofitas del Noroeste de Argentina I . Darwiniana , 17 : 11 – 103 .
- Sota , E. R. de la. 1977 . “ Pteridophyta ” . In Flora de la Provincia de Jujuy , Edited by: Cabrera , A. L. 14 – 275 . Buenos Aires : INTA. Colecc. Cie. INTA 13(2) .
- Stokey , A. G. 1940 . Spore germination and vegetative stage of the gametophytes of Hymenophyllum and Trichomanes . Bot. Gaz. (Chicago) , 101 : 759 – 790 .
- Tryon , A. F. and Lugardon , B. 1991 . Spores of the Pteridophyta: Surface, wall structure and diversity based on electron microscope studies , New York : Springer .