Abstract
Based on pollen analysis, 17 honey samples collected in the Caatinga area from Nova Soure city were classified by botanical origin to identify the most important floral sources. Most of the honey samples were obtained in August and September. A total of 73 pollen types were identified belonging to 30 families, 64 genera and 30 species. The families best represented by their number of pollen types were Mimosaceae (11), Caesalpiniaceae (9), Rubiaceae and Fabaceae (5 each). Predominant pollen types were: Mimosa arenosa in four samples, M. sensitiva and M. tenuiflora in one sample. Pollen from Mimosa ursina was registered for the first time in the pollen spectrum of the Caatinga vegetation. The correspondence analysis showed a similarity among the honey samples based on pollen composition. The high representation of pollen from native species such as Chamaecrista nictitans, C. ramosa, C. swainsonii and Copaifera martii (Caesalpiniaceae); Aeschynomene martii and Zornia sericea (Fabaceae); Herissantia tiubae (Malvaceae); Mimosa arenosa, M. quadrivalvis, M. sensitiva, M. tenuiflora, M. ursina, Piptadenia moniliformis and Plathymenia reticulata (Mimosaceae), and Ziziphus joazeiro (Rhamnaceae) supports the origin of these honeys from Caatinga vegetation.
The honey produced in north-eastern Brazil is rich in pollen types due to the diverse native flora used by bees for its production. There are many endemic plant species with important beekeeping potential that are good geographical markers (Santos et al., Citation2005; Borges et al., Citation2006). Although this potential is recognised throughout the north-eastern flora, particularly in the Caatinga, little is known of potential beekeeping species: moreover, according to Santos and Santos (Citation2003), the Caatinga is perhaps the only Brazilian vegetation whose flora has not had continuous and systematic pollen studies.
Honey pollen analysis allows identification of the main nectar sources used by bees for the production of honey in a region, classifying the honey botanically and geographically according to its origin (Louveaux et al., Citation1978).
According to Forcone et al. (Citation2003), analysis of the evolution of honey pollen spectrum during the honey production period is extremely useful to detect the contributions of different nectar sources over the season. This is of particular interest for hive management and allows the identification of likely periods of unifloral honey, which have high commercial value.
In Bahia, beekeeping activity has increased in recent years; however, few studies have been conducted analysing the pollen content of honey produced in the state. The pioneering works in Bahia were carried out by Barth (Citation1970b , Citation1971), and more recently by other researchers (Moreti et al., Citation2000; Sodré et al., Citation2001, 2007; Santos & Santos, Citation2003; Almeida et al., Citation2005; Novais et al., Citation2006; Lima, Citation2007).
The Caatinga represents the largest and most isolated of the South American dry forest. It covers more than 850 000 km2 in the semiarid region of north-eastern Brazil, with a high evapotranspiration potential (1500–2000 mm/year) and a low precipitation (300–1000 mm/year) concentrated in a three to five month period. These climatic conditions are the main factors that control the Caatinga vegetation. The Caatinga is characterised as low forest composed mostly of small trees and shrubs, frequently having twisted trunks and thorns, with small leaves that are deciduous in dry season. There is an ephemeral herbaceous layer present only during the short rainy season. The Caatinga extends in part through eight states of the north-eastern region and one of the south-western (state of Minas Gerais), and it is divided into eight different ecoregions depending on the vegetation type (Queiroz, Citation2006, Citation2009; Velloso et al., Citation2002).
The floral diversity of Caatinga offers the possibility of diversification in beekeeping production. In this context, pollen analysis studies are particularly relevant for indicating the nectar sources used by bees for honey production in each region and, consequently, for improving the use of bee flora in each locality. The objective of this study was to characterise pollen samples, identifying the main floral sources from honey produced in a Caatinga area located in Nova Soure, Bahia (mesoregion Nordeste Baiano, microregion Ribeira do Pombal) over a season of production.
Materials and methods
Study area
The city of Nova Soure (11° 18′ S, 38° 32′ W; altitude 136 m) is situated in the north-eastern mesoregion of Bahia, Brazil (), with an average annual temperature of 25.2°C. The Caatinga is vegetation, which predominates in the region (SEI, Citation1998). Representatives of the legume families (Caesalpiniaceae, Fabaceae and Mimosaceae) comprise 36% of species visited by Apis melliferaL. in this area. Mimosaceae is remarkable with 20% of the species. The other main species in the Caatinga vegetation in Nova Soure are Anacardium occidentale L. (introduced), Spondias venulosa Mart ex Engl. and S. tuberosa Arr. Cam. (Anacardiaceae), Syagrus coronata (Mart) Becc. (Arecaceae), Ziziphus joazeiro Mart. (Rhamnaceae), Borreria verticillata (L.) G. Mey. (Rubiaceae) (Almeida et al., Citation2006).
Pollen analysis
We studied the pollen content of the 17 (No. I-XVII) honey samples produced by Apis mellifera. The honey samples were obtained from different apiaries () located in the Nova Soure region. The months June, July, November and December 2005 were not sampled because honey production did not occur in these months.
Table I. Honey samples obtained from different apiaries of Nova Soure, state of Bahia, Brazil
For pollen analysis, we followed the method described by Louveaux et al. (Citation1978). A 10 g subsample of honey was dissolved in 20 ml of distilled water and centrifuged for 10 minutes at 1048 gravity (2500 rpm), and the supernatant liquid was drawn out. The sediment was acetolysed (Erdtman, Citation1960), mounted in glycerine jelly, and sealed with paraffin. To determine frequency classes, 1200 pollen grains were counted on three slides from each sample (400 per slide). Pollen types were classified into four categories (Louveaux et al., Citation1978): predominant pollen (>45%), secondary pollen (16–45%), important minor pollen (3–15%) and minor pollen (<3%).
In addition to frequency class determination, we performed a correspondence analysis (CA, or reciprocal averaging; Hill, Citation1973), in order to verify association patterns between honey samples and pollen types, by using the PAST software – Palaeontological Statistics, Ver. 1.89 (Hammer et al., Citation2001). The main purpose of this analysis was to assess the existence of structured patterns regarding different collection times, or if samples are not structured. For this analysis, we considered only the presence/absence of pollen types in individual samples. For assessing whether the resulting patterns differ from the expected by random, we compared the eigenvalues generated by the CA with the broken-stick distribution (Jackson, Citation1993). This method provides the expected eigenvalues from a theoretical random distribution, and the comparison indicates axes with meaningful non-random patterns when their observed eigenvalues exceed the expected values.
Pollen types were identified by comparison with a reference collection from the Palynotheca of the LAMIV/UEFS (Plant Micromorphology Laboratory/Feira de Santana State University), which is a representative collection of the pollen flora of the Caatinga vegetation from north-eastern Brazil. Relevant pollen catalogues were used for auxiliary identification, published by Chávez et al. (Citation1991), Roubik and Moreno (Citation1991), Carreira and Barth (Citation2003), and Melhem et al. (Citation2003); Lima et al. (Citation2008) was used for Mimosa spp. from the Brazilian semiarid lands, and Souza (Citation2008) was used for Mitracarpus spp.
Results
A total of 73 pollen types, belonging to 30 families, 64 genera and 30 species, were identified from honey samples (; ). Pollen types that did not have their botanical affinity determined belonged to the frequency class of important minor pollen (3–15%) and minor pollen (<3%), of which many were recorded only once in the sample or had a frequency smaller than 1%. All pollen types in samples VII, IX, X and XIII had their botanical affinity determined (). Most honey samples were obtained in August and September 2005. These months are related to the rainy season (in winter) and certainly influence the growth of local vegetation and consequently the resources available to bees. Coincidentally, these were the months when the honey had predominant pollen (>45%), four in August and one in September. The other sample with predominant pollen occurred in January 2006.
Figure 2. Photomicrographs of some pollen types present in honey samples of Apis mellifera L., from Nova Soure, state of Bahia, Brazil. A. Amaranthaceae: Alternanthera type. B. Arecaceae: Syagrus coronata. C. Lamiaceae: Salvia type. D–M. Mimosaceae: D, E. Mimosa arenosa. F, G. Mimosa quadrivalvis. H. Mimosa sensitiva. I, J. Mimosa tenuiflora. K, L. Mimosa ursina (monad). M. Piptadenia moniliformis. N. Rhamnaceae: Ziziphus joazeiro. O, P. Rubiaceae: Borreria verticillata. Scale bars – 10 μm.
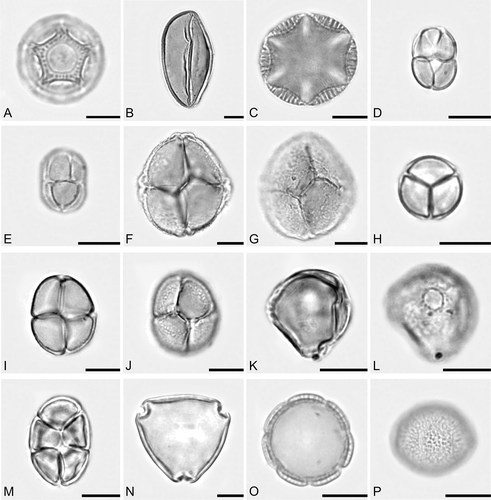
Table II. Pollen types and their frequency-class in the honey samples (I–XVII) from Bahia, Brazil. D – predominant pollen (>45%), S – secondary pollen (16–45%), I – important minor pollen (3–16%), m – minor pollen (<3%)
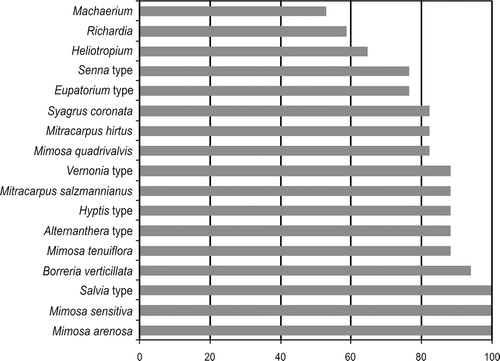
Of the 30 families represented in the pollen spectrum of honey from Nova Soure, those that occurred in more than 50% of samples include Lamiaceae and Mimosaceae found in all samples, Asteraceae, Rubiaceae and Caesalpiniaceae in 94% of samples; Amaranthaceae in 88.2%; Arecaceae in 82.4%, Fabaceae in 76.5%, Boraginaceae in 64.7%, and Anacardiaceae, Myrtaceae, Sapindaceae and Sterculiaceae in 58.8% of samples. However, the contribution of the pollen types from each family was variable among the samples, and many of these families were represented in the pollen spectrum only by important minor pollen or minor pollen (). The families most representative in the number of pollen types in the honey analysed were Mimosaceae with 11 types, Caesalpiniaceae with nine, and Fabaceae and Rubiaceae with five.
Of the 17 honey samples analysed, six showed predominant pollen types. In the other samples, the pollen spectrum was characterised by the presence of secondary pollen (16–45%), important minor pollen (3–15%), and minor pollen (<3%) (). Predominant pollen types belong to the family Mimosaceae and include Mimosa arenosa (, E), which prevailed in four samples (ranging from 48.4 to 73.8%), and M. sensitiva (80.2%) () and M. tenuiflora (83.6%) (, J), which each predominated in one sample.
There was an average of 24 pollen types per honey sample, with sample II (May) being one of poorest at 15 pollen types; however, the pollen spectrum in this sample was characterised by the presence of three secondary pollen types: Alternanthera type (28.8%) (), Borreria verticillata (27.7%) (, P) and Mimosa arenosa (29.6%). Moreover, samples with high diversity pollen were III, V and XIV, all with 30 pollen types and produced in May, August and September 2005, respectively.
The pollen types Mimosa arenosa, M. sensitiva and Salvia type (), present in all honey samples, have characterised honey from the study area. Borreria verticillata was not only observed in sample I (March 2005). Seventeen pollen types were observed in more than half of the honey samples ().
Secondary pollen types were represented by Alternanthera type (Amaranthaceae), Borreria verticillata (Rubiaceae), Eupatorium type (Asteraceae), Hyptis and Salvia types (Lamiaceae), Myrcia type (Myrtaceae), Mimosa arenosa, M. sensitiva and Piptadenia moniliformis (Mimosaceae) (), and Ziziphus joazeiro (Rhamnaceae) (). In addition to the pollen types cited, pollen from representative species of other families from Caatinga flora were also observed, such as Schinus sp. (Anacardiaceae), Syagrus coronata (Arecaceae) (), Croton sp. (Euphorbiaceae), Herissantia sp. (Malvaceae), and Serjania sp. (Sapindaceae).
In the correspondence analysis, the variance accumulation patterns obtained by comparing the eigenvalues to randomly expected values showed a pattern similar to a random distribution, demonstrating the similarity of the samples, i.e., following a highly stochastic pattern (). Only in the first axis (21.1% explained variance) did sample I separate, and to a lesser degree sample VIII. In axis 2 (14.9%), sample VIII was separated again, and samples II and XV were separated to a lesser degree. These four samples (I, II, VIII and XV) are differentiated from other types by each having unique pollen types. Sample I is distinguished from the others by five exclusive pollen types (Cordia, Tamarindus indica, Alchornea, Trichilia hirta and Leucaena leucocephala). Sample VII differs by Jacquemontia and Helicteres velutina; sample II by Eucalyptus and VI by Ceiba erianthos, Camptosema, Erytrina and Prosopis juliflora, while samples II and VI share Chamaecrista swainsonii ().
Figure 4. Observed and expected eigenvalues in a correspondence analysis (CA) of 17 honey samples (I–XVII) in relation to presence/absence of pollen types. Expected eigenvalues estimated using the broken-stick distribution.
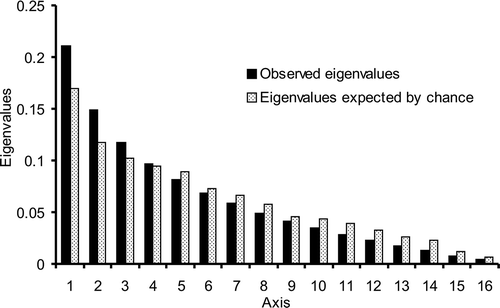
Figure 5. Biplot from correspondence analysis (CA) of honey samples from Nova Soure, Bahia, Brazil. Legend: ▪ honey samples.
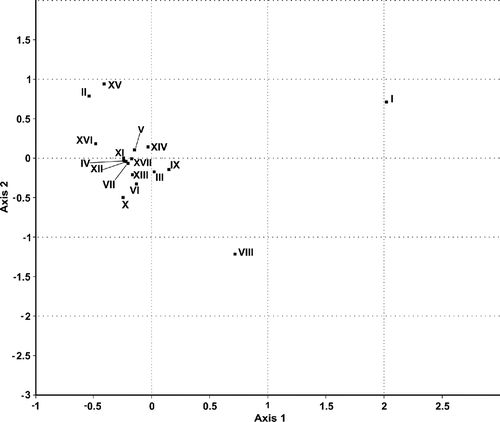
Pollen types Ricinus communis, Plathymenia reticulata, Psidium type and Microtea that appeared in focus on axis 1 were shared between samples I or VIII with other samples. Sample I shares three pollen types with other samples: Ricinus communis (I and VIII), Plathymenia reticulata (I and XIV) and Psidium type (I, V and VII). Sample VIII shares the pollen type Microtea with sample IV ().
Discussion
The relationship between climatic factors, availability of bee species and honey has been observed in other regions with similar environmental characteristics to Nova Soure (Carvalho & Marchini, Citation1999; Andrada & Tellería, Citation2002; Basílio & Romero, Citation2002). Moreover, Battesti and Goeury (Citation1992) had verified that climatic conditions influence the melliferous and polleniferous potential of the local flora.
The pollen spectrum of the honeys from Nova Soure presented pollen types of several typical species of Caatinga's flora, some endemic, as was the case of Caesalpiniaceae, Chamaecrista nictitans (L.) Moench, C. ramosa (Vogel) H.S.Irwin & Barneby, C. swainsonii (Benth.) H.S.Irwin & Barneby (endemic), and Copaifera martii Hayne; Fabaceae, Aeschynomene martii Benth. and Zornia sericea Moric.; Malvaceae, Herissantia tiubae (K. Schum.) Brizicky (endemic); Mimosaceae, Mimosa arenosa (Willd.) Poir, M. quadrivalvis L., M. sensitiva L., M. tenuiflora (Willd.) Poir, M. ursina Mart., Pityrocarpa moniliformis (Benth.) Luckow & Jobson (=Piptadenia moniliformis Benth.), and Plathymenia reticulata Benth.; and Rhamnaceae, Ziziphus joazeiro Mart. (endemic) (Giulietti et al., Citation2002; Sampaio, Citation2002). The presence of these pollen types related to those species points botanical origin of the honey to Caatinga's flora.
It was observed that the taxa of the Mimosaceae are important for beekeeping in Nova Soure, not only in the number of pollen types but also as the single family with predominant pollen. The same importance of this family was observed in the pollen spectrum of honey from other regions of Bahia (Barth, Citation1970a , Citation1971; Moreti et al., Citation2000; Santos & Santos, Citation2003; Almeida et al., Citation2005; Sodré et al., Citation2007). The genus Mimosa is particularly important in the pollen spectrum, and its pollen types were the only predominant pollen, appearing as secondary pollen, important minor pollen and minor pollen. In the six honey samples with predominant pollen, M. arenosa occurred in four (48.4% to 73.8%); the others were M. sensitiva (80.2%) and M. tenuiflora (83.6%). The species related to these types, according to Queiroz (Citation2009), are common in degraded environments.
Although these samples with predominant pollen suggest a unifloral origin (Louveaux et al., Citation1978), it is important to emphasise that little is known about the floral biology of the Mimosa species represented in the pollen spectrum, their real nectariferous contribution to honey production or the relationship between pollen and nectar in the pollen spectrum. Moreover, Mimosa pollen is highly over-represented in honeys (Barth, Citation1989). For these reasons, those honey samples were not considered as unifloral honey.
As secondary pollen, Mimosa arenosa was in four samples and M. sensitiva present in five samples as secondary pollen. The other secondary pollen, which belongs to the Mimosaceae, was Piptadenia moniliformis in honey sample III. According to Freitas and Silva (Citation2006), Pityrocarpa moniliformis (=Piptadenia moniliformis) offers pollen and nectar as floral resources.
The pollen of Mimosa ursina was registered for the first time in the pollen spectrum of honey from the Caatinga. According to Lima et al. (Citation2008), M. ursina has pollen grains in dyads and monads, an unusual feature for the Mimosa genus, which may have prevented its identification in previous works. Another pollen, which has also not been recorded in other pollen spectrum of honey from the Caatinga, is M. quadrivalvis despite being mentioned as a species of beekeeping interest in the semiarid climate (Santos et al., Citation2006), having contributed in the honey of Nova Soure as important minor pollen and minor pollen ().
The fact is that the Mimosaceae family stands out from honey produced in other regions of Bahia. Barth (Citation1970a ) found pollen type Mimosa scabrella predominant in two honey samples from Bahia. Moreti et al. (Citation2000) highlighted M. verrucosa and M. scabrella as predominant types in honey samples from some municipalities of Recôncavo Baiano. In honey samples from the northern coast of Bahia, Sodré et al. (Citation2003) showed that M. scabrella and M. verrucosa were the most frequent pollen types in the samples analysed.
The pollen type Mimosa arenosa, quite representative in this work, can refer to pollen types of M. arenosa (Willd.) Poir. alternative M. caesalpiniifolia Benth. that are indistinguishable by their pollen grains (Lima et al., Citation2008). Pollen types belonging to this group were found by Barth (Citation1970a ), who reported the occurrence of M. caesalpiniifolia as the predominant pollen in a honey sample in the municipality of Castro Alves (Bahia), and by Santos and Santos (Citation2003), who found M. arenosa as the predominant and secondary pollen for honey from the microregion of Paraguassu (Bahia).
The pollen types belonging to the family Asteraceae had low representation in the honey pollen spectrum from Nova Soure, both in the number of pollen types and number of pollen grains per sample, appearing in the pollen spectrum as important minor pollen and minor pollen. Only in honey sample III did Vernonia type participate as secondary pollen, the most frequent among the honey samples. Almeida et al. (Citation2005) also documented the presence of Asteraceae pollen types in honeys from same research area. Although the Asteraceae family is one of the richest in number of species and the most visited by social bees in different regions (Andrada & Tellería, Citation2002; Bhusari et al., Citation2005; Fagúndez & Caccavari, Citation2006), it did not have a significant contribution to honey production in Nova Soure.
The similarity between the samples studied can be observed through the correspondence analysis that did not detect a clear pattern of differentiation among samples (), suggesting that they are practically homogeneous, and the few differences observed are explained mainly by random sampling error (). The only samples that show some differentiation (I, II, VIII and XV) were based on pollen types represented only as minor pollen, some of which were recorded only once in the pollen spectrum (). Minor and trace pollen, due to its extremely low numbers, should be considered carefully before any assessment is made of their presence in honey sample (Lieux, Citation1978). A possible explanation for the random variation of samples in the study is the number of uncontrolled factors which can influence the presence of pollen types in honey samples, such as availability at the time, unbalance in the abundance of flowers among plants species, bee collection preferences, and others. In fact, there is a lack of studies addressing more specifically these questions with controlled experiments, which are needed to understand the system for using community ecology methods.
These pollen classes cannot be considered a good character to support the separation of the samples because their occurrence in the pollen spectrum is normally considered accidental and some species associated with these pollen types are useful only to identify the honey's geographical origin. The pollen composition of the majority of the remaining samples is quite homogeneous and corresponds to a set with many pollen types in common. The small differences within these groups of samples can be completely explained by the random variation of sampling nature.
Conclusions
Apis mellifera utilises a diversity of resources available in the Caatinga vegetation from Nova Soure, with 73 pollen types belonging to 30 families, 64 genera and 30 species identified in the pollen spectrum. Pollen types Lamiaceae (Salvia type) and Mimosaceae (Mimosa arenosa and M. sensitiva) were present in all honey samples. Mimosaceae was the most important family to beekeeping activity in the study area, as in richness pollen types, as the only to have predominant pollen.
In Nova Soure, plant resources to production of honey by Apis mellifera are represented by great contribution of endemic flora, mainly species of the Mimosa genus, whose pollen types are predominant in some samples.
The correspondence analysis showed a similarity among the honey samples based on pollen composition, with the few differences observed explained mainly by random sampling error.
Acknowledgements
The authors thank Eng. Alberto Magno M. de Almeida (EBDA, Nova Soure) for valuable cooperation in acquiring honey samples and rainfall data; Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB) for grant to PP Oliveira (BOL0015/2005); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for grant to FAR Santos (Proc. 304545/2007-4).
References
- Almeida , A. M. M. , Carvalho , C. A. L. , Abreu , R. D. , Santos , F. A. R. , Araújo , R. C. M. S. and Oliveira , P. P. 2005 . Espectro polínico de amostras de mel de Apis mellifera L. provenientes de Nova Soure, Bahia . Rev. Agric. , 80 : 131 – 147 .
- Almeida , A. M. M. , Carvalho , C. A. L. , Abreu , R. D. , Santos , F. A. R. , Silva , A. M. , Oliveira , P. P. and Araújo , R. C. M. S. 2006 . Plantas visitadas por Apis mellifera L. em Nova Soure, Bahia . Magistra , 18 : 152 – 161 .
- Andrada , A. C. and Tellería , M. C. 2002 . Botanical origin from south of Cálden district (Argentina) . Grana , 41 : 58 – 62 .
- Barth , O. M. 1970a . Análise microscópica de algumas amostras de mel. 1 – Pólen dominante . An. Acad. Bras. Ciênc. , 42 : 351 – 366 .
- Barth , O. M. 1970b . Análise microscópica de algumas amostras de mel. 3 – Pólen isolado . An. Acad. Bras. Ciênc. , 42 : 747 – 772 .
- Barth , O. M. 1971 . Análise de algumas amostras de mel. 6: Espectro polínico de algumas amostras de mel dos estados da Bahia e do Ceará . Rev. Bras. Biol. , 31 : 431 – 434 .
- Barth , O. M. 1989 . O pólem no mel brasileiro , Rio de Janeiro : Gráfica Luxor .
- Basílio , A. M. and Romero , E. J. 2002 . Variaciones anuales y estacionales em el contenido polínico de la miel de um colmenar . RIA , 31 : 41 – 58 .
- Battesti , M.-J. and Goeury , C. 1992 . Efficacité de l'analyse mélitopalynologique quantitative pour la certification des origines géographique et botanique des miels: Le modèle des miels corses . Rev. Palaeobot. Palynol. , 75 : 77 – 102 .
- Borges , R. L. B. , Lima , L. C. L. , Oliveira , P. P. , Silva , F. H. M. , Novais , J. S. , Dórea , M. C. and Santos , F. A. R. 2006 . “ O pólen no mel do semi-árido brasileiro ” . In Apium plantae , Edited by: Santos , F. A. R. 103 – 118 . Recife, PE : IMSEAR .
- Bhusari , N. V. , Mate , D. M. and Makade , K. H. 2005 . Pollen Apis honey from Maharashtra . Grana , 44 : 216 – 224 .
- Carreira , L. M. M. and Barth , O. M. 2003 . Atlas de pólen da vegetação de Canga da Serra de Carajás (Pará, Brasil) , Belém, PA : Mus. Para. Emílio Goeldi .
- Carvalho , C. A. L. and Marchini , L. C. 1999 . Plantas visitadas por Apis mellifera L. no vale do rio Paraguaçu, município de Castro Alves, Bahia . Rev. Brás. Bot. , 22 : 333 – 338 .
- Chávez , R. P. , Wiechers , B. L. and Villanueva , G. R. 1991 . Flora palinologica de la reserva de la biosfera de Sian Ka'An Quintana Rôo, México , Chetumal, Q. Roo : CIQRO .
- Erdtman , G. 1960 . The acetolysis method. A revised description . Sv. Bot. Tidskr. , 54 : 561 – 564 .
- Fagúndez , G. A. and Caccavari , M. A. 2006 . Pollen analysis of honeys from the central zone of the Argentine province of Entre Ríos . Grana , 45 : 305 – 320 .
- Forcone , A. , Bravo , O. and Ayestarán , M. G. 2003 . Intraannual variations in the pollinic spectrum of honey from the lower valley of the River Chubut (Patagônia, Argentina) . SJAR , 1 : 29 – 36 .
- Freitas , B. M. and Silva , E. M. S. 2006 . “ Potencial apícola da vegetação do semi-árido brasileiro ” . In Apium plantae , Edited by: Santos , F.A.R. 19 – 32 . Recife, PE : IMSEAR .
- Giulietti , A. M. , Harley , R. M. , Queiroz , L. P. , Barbosa , M. R. V. , Neta , A. L. B. and Figueiredo , M. A. 2002 . “ Espécies endêmicas da Caatinga ” . In Vegetação e flora da Caatinga , Edited by: Sampaio , E. V. S. B. , Giulietti , A. M. , Virgínio , J. and Gamarra-Rojas , C. F. L. 103 – 118 . Recife, PE : APNE .
- Hammer , Ø. , Harper , D. A. T. and Ryan , P. D. 2001 . PAST: Paleontological statistics software package for education and data analysis . Palaeontol. Electron , 4 : 1 – 9 .
- Hill , O. M. 1973 . Reciprocal averaging: An eigenvector method of ordination . J. Ecol. , 61 : 237 – 249 .
- Jackson , D. A. 1993 . Stopping rules in Principal Components Analysis: A comparison of heuristical and statistical approaches . Ecology , 74 : 2204 – 2214 .
- Lieux , M. H. 1978 . Minor honeybee plants of Louisiana indicated by pollen analysis . Econ. Bot. , 32 : 418 – 432 .
- Lima , L. C. L. 2007 . Espécies de Mimosa L. (Leguminosae) do semi-árido nordestino: palinologia, fenologia, biologia floral e potencial apícola , Feira de Santana, BA : UEFS Grad. Progr. Bot., PhD. Diss .
- Lima , L. C. L. , Silva , F. H. M. and Santos , F. A. R. 2008 . Palinologia de espécies de Mimosa L. (Leguminosae - Mimosoideae) do semi-árido brasileiro . Acta Bot. Bras. , 22 : 794 – 805 .
- Louveaux , J. , Maurizio , A. and Vorwohl , G. 1978 . Methods of melissopalynology . Bee World , 59 : 139 – 157 .
- Melhem , T. S. , Cruz-Barros , M. A. V. , Corrêa , A. M. S. , Makino-Watanabe , H. , Silvestre-Capelato , M. S. F. and Gonçalves-Esteves , V. L. 2003 . Variabilidade polínica em plantas de Campos do Jordão (São Paulo, Brasil) . Bol. Inst Bot. , 16 : 1 – 104 .
- Moreti , A. C. C. C. , Carvalho , C. A. L. , Marchini , L. C. and Oliveira , P. C. F. 2000 . Espectro polínico de amostras de mel de Apis mellifera L. coletadas na Bahia . Bragantia , 59 : 1 – 6 .
- Novais , J. S. , Lima , L. C. L. and Santos , F. A. R. 2006 . Espectro polínico de méis de Tetragonisca angustula Latreille, 1811 coletados na Caatinga de Canudos, Bahia, Brasil . Magistra , 18 : 257 – 264 .
- Queiroz , L. P. 2006 . “ The Brazilian Caatinga: Phytogeografical patterns inferred from distribution data of the Leguminosae ” . In Neotropical savanas and seasonally dry forests: Plant diversity, biogeography, and conservation , Edited by: Pennington , R. T. , Lewis , G. P. and Ratter , J. A. Vol. 69 , 121 – 157 . Boca Raton, FL : CRC. Syst. Assoc. Sp .
- Queiroz , L. P. 2009 . Leguminosas da Caatinga , Feira de Santana, BA : UEFS/Kew: R. Bot. Gard .
- Queiroz , L. P. , Conceição , A. A. and Giulietti , A. M. 2006 . “ Nordeste semi-árido: Caracterização geral e lista das fanerógamas ” . In Diversidade e caracterização das fanerógamas do semi-árido brasileiro , Edited by: Queiroz , L. P. , Conceição , A. A. and Giulietti , A. M. 15 – 360 . Recife, PE : IMSEAR .
- Roubik , D. W. and Moreno , J. E. 1991 . Pollen and spores of Barro Colorado Island , St. Louis, MO : Mo. Bot. Gard. Monogr. Syst. Bot. 36 .
- Sampaio , E. V. S. B. 2002 . “ Uso das plantas da Caatinga ” . In Vegetação e flora da Caatinga , Edited by: Sampaio , E. V. S. B. , Giulietti , A. M. , Virgínio , J. and Gamarra-Rojas , C. F. L. 49 – 90 . Recife, PE : APNE .
- Santos , F. A. R. , Oliveira , A. V. , Lima , L. C. L. , Barros , R. F. M. , Schlindwein , C. P. , Martins , C. F. , Camargo , R. C. R. , Freitas , B. M. and Kiill , L. H. P. 2005 . “ Apícolas ” . In Espécies da flora nordestina com importância econômica potencial , Edited by: Sampaio , E. V. S. B. , Pareyn , F. G. C. , Figueroa , J. M. and Santos , A. G. Jr. 15 – 26 . Recife, PE : APNE .
- Santos , F. A. R. , Oliveira , J. M. , Oliveira , P. P. , Leite , K. R. B. and Carneiro , C. E. 2006 . “ Plantas do semi-árido importantes para as abelhas ” . In Apium Plantae , Edited by: Santos , F. A. R. 61 – 86 . Recife, PE : IMSEAR .
- Santos , M. C. Jr and Santos , F. A. R. 2003 . Espectro polínico de amostras de méis coletadas na microrregião do Paraguassu, Bahia . Magistra , 15 : 79 – 85 .
- SEI, Superintendência de Estudos Econômicos e Sociais d Bahia . 1998 . Atributos climáticos do estado da Bahia , Salvador, BA : Secr. Plan. Ciênc. Tecnol. Est. Bahia. Sér. Estud. Pesq. 38 .
- Sodré , G. S. , Marchini , L. C. , Moreti , A. C. C. C. and Carvalho , C. A. L. 2001 . Análises polínicas de méis de Apis mellifera L. 1758 (Hymenoptera: Apidae) do litoral norte do estado da Bahia . Rev. Agric. , 76 : 215 – 225 .
- Sodré , G. S. , Marchini , L. C. , Moreti , A. C. C. C. and Carvalho , C. A. L. 2003 . Análises multivariadas com base nas características físico-químicas de amostras de méis de Apis mellifera L. (Hymenoptera: Apidae) da região litoral norte no estado da Bahia . Arch. Latinoam. Prod. Anim. , 11 : 129 – 137 .
- Sodré , G. S. , Marchini , L. C. , Carvalho , C. A. L. and Moreti , A. C. C. C. 2007 . Pollen analysis in honey samples from the two main producing regions in the Brazilian northeast . An. Acad. Bras. Ciênc. , 79 : 381 – 388 .
- Souza , E. B. 2008 . Estudos sistemáticos em Mitracarpus (Rubiaceae – Spermacoceae) com ênfase em espécies brasileiras , Feira de Santana, BA : UEFS Grad. Progr. Bot. PhD. Diss .
- Velloso , A. L. , Sampaio , E. V. S. B. , Giulietti , A. M. , Barbosa , M. R. V. , Castro , A. A. J. F. , Queiroz , L. P. , Fernandez , A. , Oren , D. C. , Cestaro , L. A. , Carvalho , A. J. E. , Pareyn , F. G. C. , Silva , F. B. R. , Miranda , E. E. , Keel , S. and Gondim , R. S. 2002 . Ecorregi[otilde]es: Propostas para o bioma Caatinga , Recife : APNE. The Nature Conservancy - Brasil .