Abstract
Canrightiopsis with three species (C. intermedia, C. crassitesta, C. dinisii) is described from the Early Cretaceous of Portugal based on small, one-seeded berries. The fruits are derived from bisexual flowers with three stamens borne on one side of the ovary. There are no traces of a perianth. Pollen is of the Clavatipollenites-type, monocolpate, semitectate, reticulate-columellate with heterobrochate reticulum and muri with beaded supratectal ornamentation. The ovary is unilocular with a single pendant, orthotropous and bitegmic ovule. The seed is endotestal. The endotesta consists of one layer of palisade-shaped crystal cells with fibrous infillings. The fruit wall has resin bodies or cavities from presumed ethereal oil cells sometimes seen as stomata-like structures on the fruit surface. A phylogenetic analysis resolves Canrightiopsis as a close relative of extant Chloranthaceae, particularly close to extant Chloranthus and Sarcandra. All three taxa share the one-sided position of the stamens on the ovary. An evolutionary sequence from fossil Canrightia to fossil Canrightiopsis and extant Chloranthus and Sarcandra is suggested by loss of perianth, reduction in number of ovules and stamens and displacement of stamens to one side of the ovary. Canrightiopsis also shares several critical features with extant Ascarina including monoaperturate pollen and beaded supratectal ornamentation of the pollen wall.
The Chloranthaceae are a small family usually placed close to the base of the angiosperm phylogenetic tree (APG Citation2009). The family includes four extant genera, Ascarina J.R.Forst. et G.Forst., Chloranthus Sw., Hedyosmum Sw. and Sarcandra Gardner that today occur in warm temperate to subtropical and tropical regions (Endress Citation1987). The family has a long geological history with an almost worldwide distribution in the Cretaceous, and the present distribution and diversity of the family are clearly relictual. Dispersed pollen grains of Clavatipollenites Couper (compared to pollen of Ascarina; Couper Citation1960; Walker & Walker Citation1984) and Asteropollis R.W.Hedl. et G.Norris (compared to pollen of Hedyosmum; Walker & Walker Citation1984) occur very early in the history of angiosperms with records extending back to the Barremian (e.g. Couper Citation1958; Hedlund & Norris Citation1968; Walker & Walker Citation1984; Hughes Citation1994; Nichols et al. Citation2006; Friis et al. Citation2011; Martínez et al. Citation2013). In addition to numerous dispersed pollen grains, the fossil record of Chloranthaceae also includes a number of Cretaceous floral structures. From the Early Cretaceous Hedyosmum-like pistillate and staminate structures with Asteropollis-type pollen attached to the ovary or in situ in the stamens have been identified from Portugal and eastern North America (Friis et al. Citation1997, Citation2011). From the Late Cretaceous of Scania and eastern North America, inflorescences, isolated flowers, androecia and fruits closely resembling those of extant Chloranthus were assigned to three species of the extinct genus Chloranthistemon P.R.Crane, E.M.Friis et K.R.Pedersen (Crane et al. Citation1989; Herendeen et al. Citation1993; Eklund et al. Citation1997). Chloranthistemon-like floral structures are also known from the mid-Cretaceous of Germany (Friis et al. Citation2011; Hartkopf-Fröder et al. Citation2011).
Floral structures in all four extant genera of Chloranthaceae are simple (Endress Citation1987). Nevertheless, the interpretation of certain floral features and basic organisation has not been straightforward. This to some extent may reflect extensive extinction within the lineage and missing data from the geological record. Particularly the nature of the unusual tripartite androecium of Chloranthus has been much debated (Swamy Citation1953; Endress Citation1987). In Chloranthus, the androecium is typically three-lobed with a median, tetrasporangiate lobe and two lateral, bisporangiate lobes (Endress Citation1987). Endress (Citation1987) offered two hypotheses for the evolutionary origin of the androecium, both starting from a single stamen with the three lobes formed either by polymerisation, concrescence and reduction, or by lobation and fractionation of a single stamen.
New fossil fruits from the Early Cretaceous mesofossil floras of Portugal may fill in some of the missing data critical for understanding the evolutionary steps resulting in the tripartite androecium of extant Chloranthus and fossil Chloranthistemon as well as other floral features in extant Chloranthaceae. Canrightia resinifera E.M.Friis et K.R.Pedersen comprises small berries with two to five seeds. The fruits are derived from bisexual flowers with a partly epigynous, strongly reduced perianth and four or five stamens in a radial arrangement on the rim of the hypanthium. The seeds are orthotropous, bitegmic and endotestal very similar to those of Ascarina, Chloranthus and Sarcandra. There are, however, several features in Canrightia E.M.Friis et K.R.Pedersen that are not compatible with the extant genera, such as the higher number of ovules per ovary, the presence of a hypanthium and the radially arranged stamens.
Canrightiopsis is a new genus described here that shares several features with extinct Canrightia, but that also shares critical features with extant Ascarina, Chloranthus and Sarcandra not present in Canrightia. Thus, Canrightiopsis lacks a hypanthium, but has stamens (represented by scars) borne on one side of the ovary as is characteristic for extant Chloranthus and Sarcandra. Canrightiopsis also has a single ovule per ovary as in extant Chloranthaceae. Further, Canrightiopsis is similar to Chloranthus and Sarcandra in having the stamens borne in a partly epigynous position. Particularly the presence of three separate stamen scars in Canightiopsis suggests a derivation of the tripartite androecium in Chloranthus from three stamens, not from one. Monocolpate, reticulate Clavatipollenites-type pollen similar particularly to pollen of extant Ascarina was observed on the stigmatic surfaces or on the ovary wall of all three Canrightiopsis species and corroborates previous suggestions of a chloranthaceous affinity for some of the early dispersed angiosperm pollen.
Material and methods
Material
The fossil material reported here was collected from several localities (Arazede, Buarcos, Catefica, Juncal-Chicalhão, Famalicão, Vale de Água, Vila Verde) in the Lusitanian Basin, western Portugal (for information on the mesofossil floras see Friis et al. Citation2011; Mendes et al. Citation2014a).
Based on stratigraphic information for the Lusitanian Basin (e.g. Dinis et al. Citation2008), the plant bearing sediments at the Chicalhão site (Juncal) and the Vale de Água locality studied here belong to the basal part of the Famalicão Member of the Figueira da Foz Formation established by Dinis (Citation2001). Buarcos, Vila Verde and Arazede belong to the basal part of the Calvaria Member of the Figueira da Foz Formation established by Dinis (Citation2001). The age of the Famalicão and Calvaria members was indicated as late Aptian–early Albian by Rey et al. (Citation2006). The mesofossils from the Famalicão locality were collected in a stratigraphic position below the Calvaria Member indicating a late Aptian age (or older) for this flora. According to Jacques Rey (personal communication, June 2012), the plant bearing sediments of the Catefica locality may belong to the younger part of the Almargem Formation that is of late Aptian–early Albian age. However, the Catefica mesofossil flora shares many elements with the late Barremian–early Aptian Torres Vedras, also from the Lusitanian Basin, flora and may be slightly older than the other mesofossil floras studied here, perhaps belonging to the lower part of the Almargem Formation (late Barremian–Aptian).
Preparation and analyses of the fossil material
The fossil plant remains were extracted from the sediments by sieving in water followed by treatment in hydrofluoric and hydrochloric acid, rinsing in water and finally air dried. Measurements of fossils were made using dissecting microscope as well as from images from scanning electron microscopy (SEM) and synchrotron radiation-based X-ray tomographic microscopy (SRXTM). Pollen grains were measured using SEM images.
For SEM, fossils were mounted on aluminium stubs using nail polish, sputter coated with gold and studied using a Phillips 515 Scanning Electron Microscope at 15 kV, and a Hitachi S-4300 Field Emission Scanning Electron Microscope at 2 kV.
Internal structures were studied using SRXTM at the TOMCAT beamline of the Swiss Light Source at the Paul Scherrer Institute, Villigen, Switzerland (Stampanoni et al. Citation2006). About 30 specimens were mounted on brass stubs with a diameter of 3 mm using nail polish. All specimens were measured at 10 keV using a sCMOS detector and a 20 μm thick LAG:Ce scintillator screen. We used a 10× objective with isotropic pixel size of 0.65 μm (P0311, S174006, S174027, S174028, S174039, S174040, S174155, S174157, S174159, S174174, S174248, S174309, S174310, S174311) or 20× objective with isotropic pixel size of 0.325 μm (S174023–S174026, S174033, S174104, S174105, S174107, S174148–S174153, S174175). A more detailed outline of the technique used for coalified plant fossils is given by Friis et al. (Citation2014). Slice data derived from the scans (Hintermüller et al. Citation2010) were analysed and manipulated using AVIZO (v6.1 and v7.1) software for computed tomography.
All specimens and preparations of the new taxon from Portugal described here are housed in the palaeobotanical collections of the Swedish Museum of Natural History, Stockholm, Sweden (S) and in the palaeontological collections of the Geological Museum of Lisbon, Portugal (P).
Phylogenetic analysis
To explore the systematic affinity of the new fossils from Portugal, they were included in the morphological matrix of Doyle and Endress (Citation2010; D&E matrix in the following). We also included the closely similar fossils Canrightia resinifera and Zlatkocarpus J.Kvaček et E.M.Friis in the analysis following Doyle and Endress (Citation2014). A reduced morphological matrix including only characters that were observed for at least one of the three fossil taxa (leaving 63 out of a total of 142 characters) was analysed using PAUP* (Swofford Citation2002) to calculate pairwise mean morphological distances. SPLITSTREE 4 (Huson & Bryant Citation2006) was then used on the distance matrix to infer a neighbour-net splits graph (NNet; Bryant & Moulton Citation2002, Citation2004). The NNet’s ability to express signal incompatibility in an underlying matrix has proven to be useful in phylogenetic assessment of fossil taxa to overcome problems with homoplasy as found also in the D&E matrix (Denk & Grimm Citation2009; Friis et al. Citation2009; Mendes et al. Citation2014b).
Character plot
The evolutionary significance of character traits expressed by Canrightiopsis was evaluated by mapping 13 morphological characters on a newly generated molecular-based tree. The selected characters are traits that can be observed in the fossil material and that differ between extant members of the Chloranthaceae. All available sequence data per 24 July 2014 were harvested from gene banks using the same protocol and software environment as in Grimm and Renner (Citation2013). Information in NCBI GenBank flatfiles were read out using GBK2FAS (Göker et al. Citation2009), alignment relied on MAFFT v7 (Katoh & Standley Citation2013), strict-genus consensus sequences were generated with G2CEF (Göker & Grimm Citation2008). RAXML v7.2.8 was used to find a topology with high likelihood and establish branch support via quick, non-parametric bootstrapping using a sufficient number of bootstrap replicates (Stamatakis Citation2006; Stamatakis et al. Citation2008; Pattengale et al. Citation2009). The final matrix comprised 16 779 characters (nucleotide sites) for the five extant genera of Chloranthaceae, covering two nuclear (18S rDNA, 25S rDNA), three mitochondrial (atp1, matR, nad5) and five plastid gene regions (atpB, matK, ndhF, rbcL, rpoC2) (see also Supplemental data). MESQUITE v2.75 (Maddison & Maddison Citation2011) was then used to reconstruct possible ancestral states for the 13 selected, variable characters along the molecular based topology under maximum parsimony (MP) with the standard parsimony model where all characters are treated as unordered and under maximum likelihood (ML) using Lewis’ one-parameter Markov-model (Lewis Citation2001).
The selected morphological characters () follow Doyle and Endress (Citation2010, Citation2014) with a few exceptions. Canrightia is scored as having a partly epigynous ovary. We scored Canrightiopsis, Chloranthus and Sarcandra in a similar way based on the position of the stamens and stamen scars on the ovary, about one third to two thirds up. We also scored Chloranthus as having more than one stamen based on its full stamen and the additional lateral half stamens. We follow Doyle and Endress (Citation2014) in included the extinct Zlatkocarpus in the analysis, but changed some of the scoring for Zlatkocarpus to express the uncertainty in the interpretation of this fossil (sexuality here scored as unknown in contrast to unisexual in Doyle & Endress Citation2014; aperture membrane scored as unknown in contrast to sculptured in Doyle & Endress Citation2014).
Table I. Selected morphological characters for Canrightia, Canrightiopsis, Zlatkocarpus and extant Chloranthaceae.
Results
Small elliptical to ovate berries occur abundantly in the Early Cretaceous plant bearing strata of Portugal. We here describe some of these berries characterised by the presence of stamen scars borne on one side of the fruit wall. There are no traces of a perianth or hypanthium. Each fruit has a single orthotropous and distinctly foveolate, endotestal seed. The fruits resemble in some aspects fossil berries of the extinct Canrightia also described from the Early Cretaceous of Portugal (Friis & Pedersen Citation2011), but they are distinct in a number of external and internal traits. We include these single-seeded berries in Canrightiopsis gen. nov. Currently, almost 1000 specimens of isolated fruits and isolated seeds of Canrightiopsis have been recovered from the sediments. Most are from the Famalicão locality, where about 650 specimens have been separated from the organic residue so far. Within the collection, there is variation in size of fruits, seeds and pollen as well as details of the seed wall suggesting the presence of several species. Based on our current knowledge three species, Canrightiopsis intermedia sp. nov., C. crassitesta sp. nov. and C. dinisii sp. nov., are defined. In many specimens, the fruit wall is abraded or strongly wrinkled and the scars, interpreted as stamen scars (see Description of the fossils and Discussion sections), are often difficult to detect and these specimens are unassigned at the specific level. Generic and specific diagnoses are given in the systematic section followed by a collective description of all Canrightiopsis fossils with references to species specific features.
Systematics
Angiospermae
Chloranthaceae
Canrightiopsis gen. nov.
Derivation of generic name
From opsis (Greek: appearance) to indicate close similarity in morphological appearance to fossil fruits of Canrightia.
Diagnosis
Flower bisexual. Perianth lacking. Androecium of three stamens. Stamens restricted to one side of the ovary, borne about one third to two thirds up the ovary. Stamens leaving distinct scars on the surface of the fruits. Associated pollen of Clavatipollenites-type; pollen monocolpate with long, extended aperture; pollen wall semitectate, reticulate-columellate; reticulum heterobrochate; columellae long, irregularly distributed; colpus membrane verrucate-rugulate; colpus margin indistinct. Ovary semi-inferior inferred from the position of the stamen scars; unilocular with a single pendant ovule. Stigma not or only slightly raised, indistinct or hemispherical. Ovule orthotropous, bitegmic, endotestal; endotesta thick with one layer of palisade-shaped crystal cells with fibrous infillings; single layer of larger, thin-walled cells outside endotesta; tegmen of several layers of thin-walled cells. Fruit single-seeded berry. Fruit wall with resin bodies or cavities from presumed ethereal oil cells that on the outer surface of fruit wall are seen as stomata-like structures. Embryo in mature seeds tiny.
Type species designated here
Canrightiopsis intermedia sp. nov.
Comments on the genus
The fruits and seeds of Canrightiopsis are similar to those of the extinct Canrightia in several respects. Both genera have small berries with resin bodies in the fruit wall and pendant, orthotropous ovules/seeds with a distinct endotesta composed of a single layer of palisade-shaped crystal cells with fibrous infilling. In both Canrightiopsis and Canrightia, stamen scars are placed about one to two thirds up the ovary/fruit wall. Both taxa have semitectate-reticulate and monocolpate pollen. Canrightiopsis differs, however, from Canrightia in having single-seeded berries in contrast to the two- to five-seeded berries of Canrightia. Only in one case, a two-seeded berry has been observed for a specimen that may belong to Canrightiopsis, while no one-seeded berry is known for Canrightia. Stamen scars in Canrightiopsis are not subtended by perianth parts and there is no hypanthium as there is in Canrightia. Further, in Canrightiopsis stamen scars are restricted to one side of the ovary, while stamen scars in Canrightia are radially arranged. Pollen of all Canrightiopsis species is of the Clavatipollenites-type with beaded muri, while pollen of Canrightia is of the broadly defined Retimonocolpites-type with smooth muri. Seeds of Canrightiopsis are also distinguished from those of Canrightia by their much thicker endotesta (50–120 µm in Canrightiopsis; about 20 µm in Canrightia). Crystals of the endotesta are irregularly distributed in Canrightiopsis with most crystals towards the middle and inside of the endotesta while in Canrightia, large crystals are densely packed in the outer part of the endotesta.
Canrightiopsis is distinguished from fruits, seeds and pollen of the Early and mid-Cretaceous Couperites K.R.Pedersen, P.R.Crane, Drinnan et E.M.Friis. Both taxa have one-seeded fruits, but seeds of Couperites are anatropous and exotestal and the pollen have a distinct, sharp colpus margin (see Discussion section).
The fossil fruits and seeds of Canrightiopsis also share many features with fruits and seeds of extant Ascarina, Sarcandra and Chloranthus, but are distinguished particularly based on androecial features (no stamen on the ovary/fruit wall of Ascarina; only one stamen in Sarcandra; tripartite androecium in Chloranthus).
Canrightiopsis intermedia sp. nov.
–, , ,
Figure 1. SEM images of Canrightiopsis intermedia gen. et sp. nov. Fruits, from the Early Cretaceous Famalicão locality, Portugal; holotype (S174033; sample Famalicão 25). A–F. Fruit rotated to document the position on the three stamen scars (numbered 1–3) on one side of the fruit (dorsal), stamen 2 is central, 1 and 3 in more lateral positions on the dorsal side; there are no stamen scars on the other (ventral) side; note scattered openings in the epidermis of the fruit wall representing ethereal oil cells. G–I. Magnification of the three dorsal stamen scars; numbering the same as for (A–F). Scale bars – 500 µm (A–F), 100 µm (G–I).
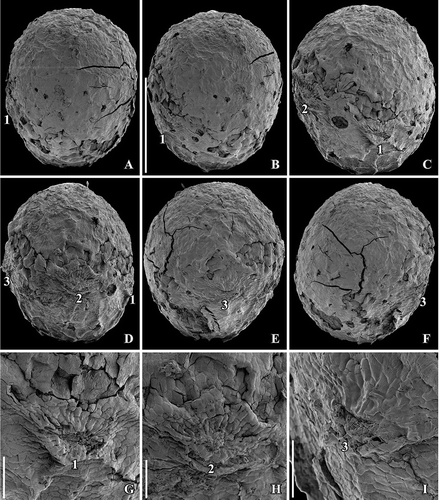
Figure 2. SEM images of Canrightiopsis intermedia gen. et sp. nov. Fruits, from the Early Cretaceous Famalicão locality, Portugal; (S174107; sample Famalicão 25). A, B. Fruit in apical (A) and dorsal (B) view showing the position on the three stamen scars (numbered 1–3), position of stigmatic area indicated by asterisk. Scale bars – 500 µm.
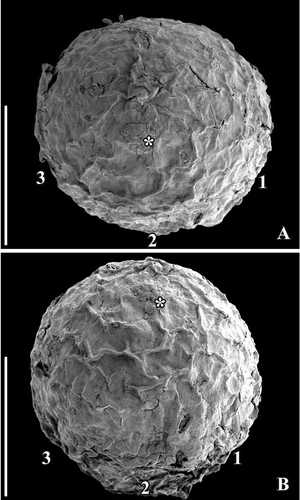
Figure 3. SEM images of Canrightiopsis intermedia gen. et sp. nov. Fruits, from the Early Cretaceous Famalicão locality, Portugal. A. Dorsal view of fruit with three stamen scars (numbered 1–3) on a slightly raised rim; position of stigma marked by asterisk (S174018; sample Famalicão 25). B. Dorsal view of fruit with three stamen scars (numbered 1–3) and a bract (b) attached to the basal part of the fruit (S174156; sample Famalicão 25). C. Fruit in partly ventral view with many openings in the epidermis of the fruit wall showing distribution of ethereal oil cells (S174004; sample Famalicão 25). D. Detail of fruit wall showing epidermal cells with wrinkled cuticle and stomata-like openings (S107700; sample Famalicão 25). E. Detail of fruit surface of holotype showing stomata-like openings (S174033; sample Famalicão 25). F, G. Details of fruit in figure (C) showing remains of resin in an abraded oil cell (F) and intact stomata-like opening over oil cell (G) (S174004; sample Famalicão 25). Scale bars – 500 µm (A–C), 100 µm (D–F), 20 µm (G).
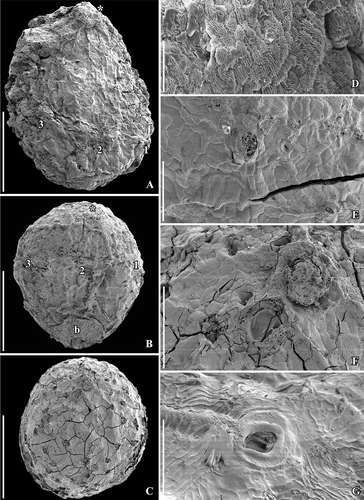
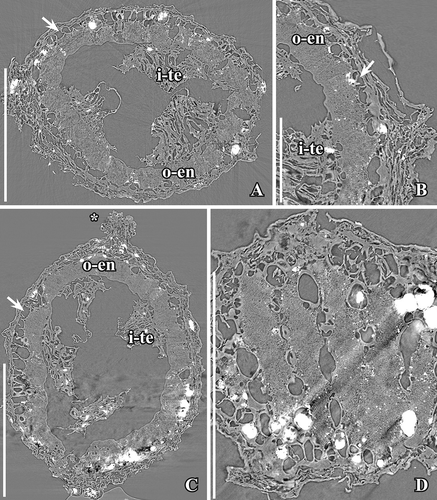
Figure 4. SEM (A–C, H) and SRXTM images (D–G, I) of Canrightiopsis intermedia gen. et sp. nov. Fruits, from the Early Cretaceous Buarcos locality, Portugal. A, B Fruit in two different views (A: dorsal to lateral view, B: dorsal view) showing three stamens (numbered 1–3) on the dorsal side of the fruit; surface of fruit with scattered holes representing the ethereal oil cells; position of stigma marked by asterisk (S174105; sample Buarcos 157). C. Detail of fruit in (A, B) showing magnification of the central stamen scar. D, E. Volume rendering (D) and orthoslice (E) of fruit in (A, B) cut longitudinally through middle of fruit exposing enclosed seed with a thick endotesta (o-en) and thin tegmen (i-te); holes are present in the outer part of endotesta; vascular supply (arrowhead) to chalaza (ch) enters through narrow opening in endotesta; micropylar area of tegmen pointed (arrow, mi); remnants of protruding stamen scar seen on dorsal side (2). F. Tangential and longitudinal orthoslice through endotesta of same specimen as shown in (A–E) showing irregular holes arranged in longitudinal grooves. G. Detail of same specimen showing the high palisade cells of endotesta (o-en) with fibrous infilling. H. Abraded fruit exposing enclosed seed with foveolate surface of the endotesta (S174104; sample Buarcos 157). I. Transverse orthoslice through specimen in (H) showing collapsed cells of fruit wall enclosing distinct endotesta with scattered holes in the outer surface and remains of endosperm in the centre. Scale bars – 500 µm (A, B, D, E, H, I), 200 µm (F, G), 50 µm (C).
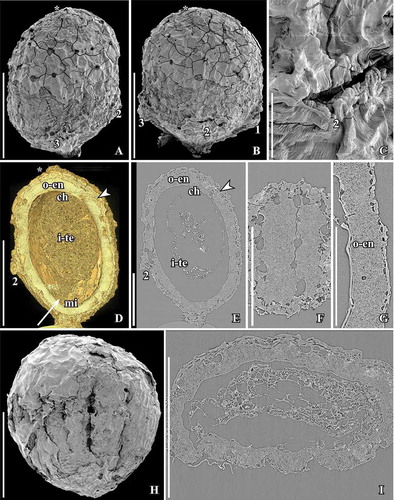
Figure 5. SEM (A–C) and SRXTM images (volume renderings D–F) of Canrightiopsis crassitesta gen. et sp. nov. Fruits, from the Early Cretaceous Catefica locality, Portugal. A, D. Holotype, view showing two of three stamen scars (numbered 2–3) borne on a slightly raised rim and wrinkled surface of fruit wall (S174311; sample Catefica 343). B. Strongly compressed and abraded fruit with enclosed foveolate seed exposed (S122089; sample Catefica 342). C. Detail of holotype showing epidermal cells with stomata-like structures representing ethereal oil cells and wrinkled cuticle. E, F. Volume renderings of holotype cut longitudinally (E: tangential, F: through middle of seed) showing foveolate surface of endotesta (E) and thick endotesta and vascular supply for chalaza (F, arrowhead). Scale bars – 500 µm (A, B, D–F), 100 µm (C).
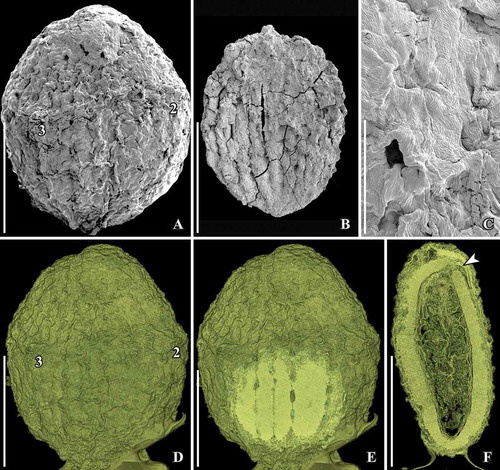
Figure 6. SEM images of Canrightiopsis dinisii gen. et sp. nov. Fruits, from the Early Cretaceous Chicalhão site, Portugal (sample Chicalhão 125). A. Fruit with two of three stamen scars exposed (numbered 1–2) and slightly raised, rounded stigmatic area (asterisk) (holotype, P0311). B. Detail of holotype showing stamen scar (1). C. Slightly abraded fruit with two of three stamen scars (numbered 1 and 3) exposed; a further, larger scar-like structure (arrowhead) is different from the stamen scars in other specimens; stigmatic area (asterisk) indistinct (P0312). D. Detail of larger scar of fruit shown in (C) probably representing oil cell opening or damage to the fruit wall. E. Details of fruit shown in (C) showing stigmatic area (asterisk) and epidermal cells of fruit wall. F. Detail of holotype showing epidermal cells of fruit wall with stomata-like openings. Scale bars – 500 µm (A, C), 100 µm (B, D, E), 50 µm (F).
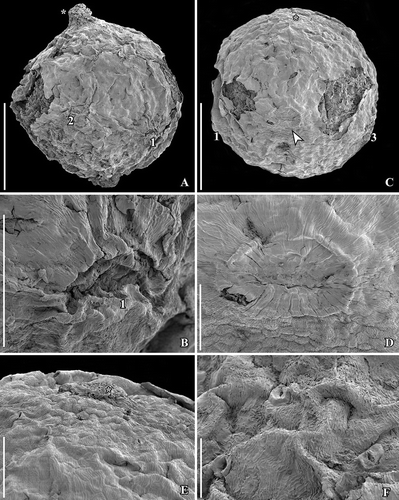
Figure 7. SEM images of Canrightiopsis intermedia gen. et sp. nov. Stigmatic area and pollen, from the Early Cretaceous Famalicão locality, Portugal (sample Famalicão 25). A. Stigmatic area covered by amorphous substance with pollen grains embedded (S174018). B. Group of monocolpate pollen clustered on fruit surface showing apertures (S174005). C. Pollen grains close to stigmatic area of fruits in (A). D. Pollen grains on surface of fruit in (S174004). E. Pollen grains on surface of fruit showing reticulum, columellae and beaded muri (S107702). F. Pollen grains on surface of fruit with pollen wall folded over aperture; reticulum well exposed showing scattered columellae (S107703). Scale bars – 100 µm (A), 50 µm (B), 10 µm (C–F).
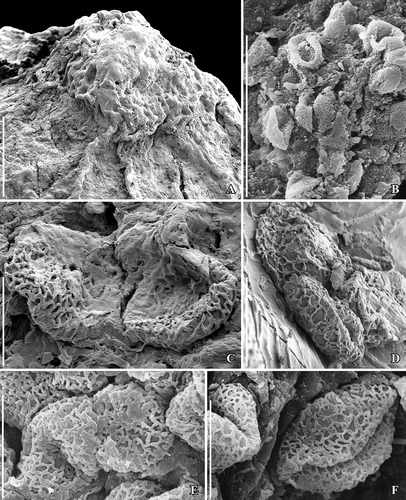
Figure 8. SEM images of Canrightiopsis crassitesta gen. et sp. nov. Pollen, from the Early Cretaceous Catefica locality, Portugal. A–D. Pollen grains from surface of fruit shown in showing heterobranchiate reticulum and beaded supratectal ornamentation; pollen with wall folded over aperture (A, B); pollen with exposed aperture (C) showing verrucate aperture membrane and indistinct margins; detail of pollen wall showing supratectal ornamentation (D) (S122089; sample Catefica 342). E–G. Pollen grains from surface of holotype (S174311; sample Catefica 343). Scale bars – 10 µm (A), 5 µm (B, C, E–G), 2.5 µm (D).
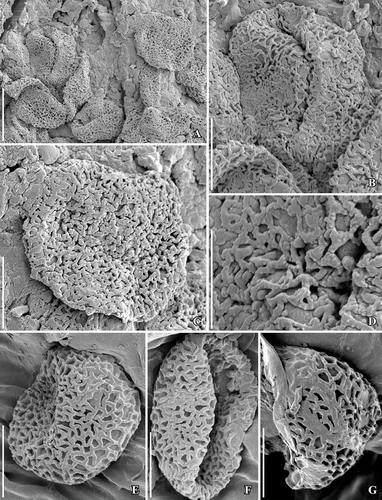
Figure 9. SEM images of Canrightiopsis dinisii gen. et sp. nov. Stigmatic area and pollen, from the Early Cretaceous Chicalhão site, Portugal (holotype P0311; sample Chicalhão 125). A. Raised stigmatic area with numerous pollen grains embedded in amorphous substance. B–C. Details of pollen from stigmatic area showing monocolpate aperture and reticulate tectum (B) as well as supratectal ornamentation. Scale bars 100 µm (A), 20 µm (B), 10 µm (C).
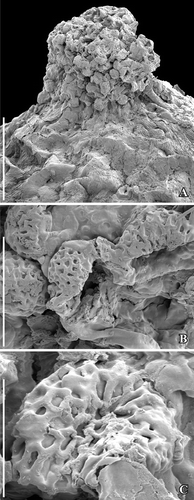
Figure 10. SEM (A–E) and SRXTM images (F) of Canrightiopsis intermedia gen. et sp. nov. Fruits and seeds, from the Early Cretaceous Famalicão locality, Portugal (sample Famalicão 25). A, B. Fruits with fruit wall collapsed over the foveolate surface of the endotesta (A: S107707; B: S174026). C–E. Foveolate seeds with holes in endotesta in longitudinal grooves (C: S174024; D: S174025; E: S174023). F. Longitudinal and tangential section of endotesta (221 consecutive orthoslices) showing holes in endotesta and polygonal and isodiametric facets of palisade cells. Scale bars – 500 µm (A–E), 100 µm (F).
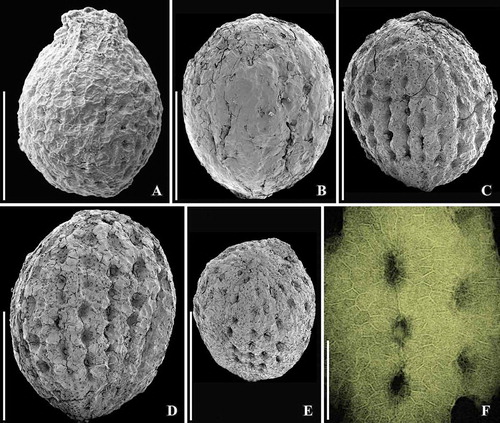
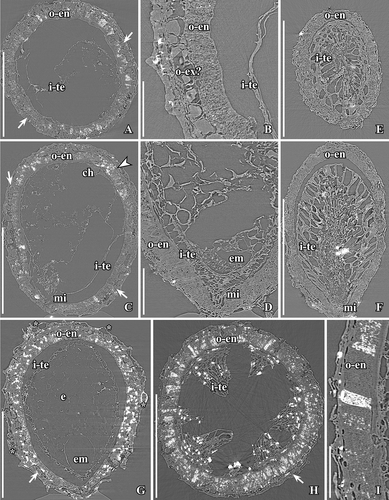
Figure 11. SRXTM images of Canrightiopsis intermedia gen. et sp. nov. (A–D, G–I) and Canrightiopsis sp. (E, F). Internal structures, from the Early Cretaceous Famalicão locality, Portugal (sample Famalicão 25). A–C. Transverse (A, B) and longitudinal (C) orthoslices through holotype showing fruit and enclosed seed; outer cells of fruit wall collapsed; endotesta (o-en) consists of a single layer of crystal cells with fibrous infilling; larger cells to the outside of the endotesta appears infilled with amorphous substance and may represent exotesta (o-ex? and arrows); internally, there are remnants of tegmen (i-te); subapical position of chalaza (ch) and micropyle (mi) towards the base seen in longitudinal orthoslice; remnants of embryo is preserved in the micropylar part of the seed; secondary crystal infilling (probably pyrite), particularly in endotesta is seen as white flecks (S174033). D. Longitudinal orthoslice through fruit with seed in micropylar area, showing palisade-shaped cells of endotesta (o-en), pointed micropyle (mi) formed from tegmen (i-te) extending through micropylar opening in endotesta and remnants of embryo is preserved in the micropylar part of the seed (em) (S174148). E, F. Transverse (E) and longitudinal (F) orthoslices through fruit with enclosed seed showing cells of endotesta (o-en) and radially elongated cells of tegmen (i-te), perhaps representing an endothelium (S174149). G. Longitudinal orthoslice through fruit and seed showing position of oil cells (asterisk) in the fruit wall; seed wall composed of endotesta (o-en) and tegmen (i-te) and larger cells (arrow) of possible exotesta, hollow space indicating position of embryo (em) and remains of endosperm (e) (S174006). H. Transverse orthoslice through fruit and seed showing endotesta (o-en) filled secondarily with crystals and fragmented tegmen (i-te) (S174004). I. Longitudinal orthoslice showing detail of seed wall with palisade-shaped cells of endotesta (o-en); one of the cells almost completely filled by secondary crystals seen as white flecks (S174005). Scale bars – 500 µm (A, C, E–H), 100 µm (B, D, I).
Derivation of specific epithet
From inter and medium (Latin: between and middle) to indicate an intermediate form between fossil Canrightia and extant Chloranthus.
Specific diagnosis
As for the genus with the following addition: Fruit elliptical to ovate. Endotesta moderately thick. Stigmatic area only slightly raised.
Dimensions
Fruits 1.1–1.3 mm long and 0.9–1.15 mm broad. Pollen 13–14.5 µm in equatorial diameter. Endotestal cells about 50–90 µm high.
Holotype designated here
S174033 (sample Famalicão 25); illustrated here in –I, A–C.
Paratypes designated here
S107700–S107708, S174004–S174006, S174016–S174028; S174107, S174108, S174148, S174150–S174158, S174364–S174368 (sample Famalicão 25). Totally, about 50 specimens, several specimens stored in slide boxes under the same number.
Other specimens
S 174104, S174105 (sample Buarcos 157); S174174 (sample Vale de Água 331).
Type locality
Famalicão, Portugal (39° 42ʹ 16ʺ N; 08° 46’ 12ʺ W).
Type horizon and age
Early Cretaceous (Aptian; below the Calvaria Member of the Figueira da Foz Formation).
Distinguishing features for Canrightiopsis intermedia. — Canrightiopsis intermedia is distinguished from C. crassitesta and C. dinisii mainly by its thinner endotesta.
Canrightiopsis crassitesta sp. nov.
, , .
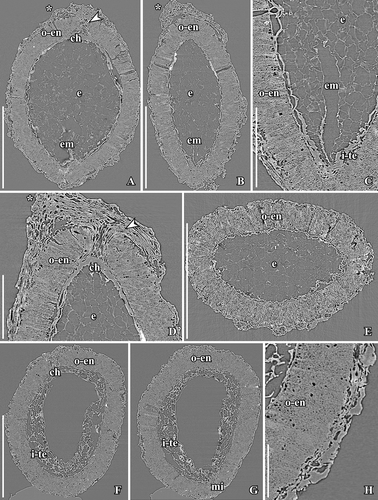
Figure 12. SRXTM images of Canrightiopsis crassitesta gen. et sp. nov. Internal structures, from the Early Cretaceous Catefica locality, Portugal (sample Catefica 49). A–E. Longitudinal (A–D) and transverse (E) orthoslices of fruit in different directions showing thin-walled cells of collapsed fruit wall and thick endotesta (o-en); palisade cells of endosperm with fibrous infilling and scattered crystals (best seen in C); endosperm (e) partly preserved inside the seed and a narrow hollow in the endosperm marks the position of the embryo (em); stigmatic area marked by asterisk and vascular bundle to chalaza (ch) by arrowhead (S174159). F–G. Longitudinal orthoslices of fruit in two directions showing thin-walled cells of collapsed fruit wall, thick endotesta (o-en), and thin-walled cells of tegmen (i-te); apical chalaza (ch) seen in (F) and basal micropyle (mi) seen in (G) (S174039). H. Detail of fruit and seed wall in same specimen as in (F, G), showing the collapsed cells of the fruit and the high endotestal cells (o-en). Scale bars – 500 µm (A, B, E–G), 200 µm (C, D), 100 µm (H).
Derivation of specific epithet
From crassus (Latin: thick, solid) for the thick endotesta.
Specific diagnosis
As for the genus with the following addition: Fruit elliptical to ovate. Endotesta thick. Stigmatic area only slightly raised.
Dimensions
Fruits 0.85–1.05 mm long and 0.6–0.8 mm broad. Pollen 10.3–12.4 µm in equatorial diameter. Endotestal cells about 80–120 µm high.
Holotype designated here
S174311 (sample Catefica 343); illustrated here in , D–F, A–C.
Paratypes designated here
S174039, S174159 (sample Catefica 49), S174310 (sample Catefica 154); S122089 (sample Catefica 342).
Type locality
Catefica, Portugal (39° 03ʹ 30ʺ N; 09°14ʹ 30ʺ W).
Type horizon and age
Early Cretaceous (late Barremian–early Albian; Almargem Formation).
Distinguishing features for Canrightiopsis crassitesta. — Canrightiopsis crassitesta is distinguished from C. intermedia by its thicker endotesta and from C. dinisii by its more elliptical to ovate fruits and generally thicker endotesta.
Canrightiopsis dinisii sp. nov.
, ,
Figure 13. SRXTM images of Canrightiopsis dinisii gen. et sp. nov. Internal structures, from the Early Cretaceous Chicalhão site, Portugal (holotype, P0311, sample Chicalhão 96). A, B. Transverse orthoslices through fruit with enclosed seed in overview (A) and detail (B); fruit wall of partly collapsed, thin-walled cells; note layer of larger thin-walled cells (arrow) between outer fruit wall and endotesta perhaps representing outer cells of testa; fragmented tegmen (i-te) preserved inside the endotesta. C. Longitudinal orthoslice of fruit showing partly preserved cells of fruit wall toward the apex and section through the stigma (asterisk); seed wall consists of endotesta (o-en) and fragmented tegmen (i-te) and perhaps outer testa (arrow). D. Tangential and longitudinal orthoslice through seed showing the foveolate-grooved surface of endotesta. Scale bars – 500 µm (A, C), 250 µm (D), 100 µm (B).
Derivation of specific epithet
In honour of Jorge Manuel Leitão Dinis for his contribution to the Cretaceous geology of Portugal.
Specific diagnosis
As for the genus with the following addition: Fruit almost spherical. Endotesta thick, stigmatic area distinct.
Dimensions
Fruits 1.1 mm long and 0.9 mm broad. Pollen 14 µm in equatorial diameter. Endotestal cells about 100 µm high.
Holotype designated here
P0311 (sample Chicalhão 125); illustrated here in , A–C, A–D.
Paratypes designated here
P0312 (sample Chicalhão 125).
Other specimens
P0095, P0096 (sample Chicalhão 96), P0313, P0314 (sample Chicalhão 127); P0315, P0316, P0317, P00318, P0319, P0320, P0321, P0322, P0323, P0324, P0325, P0326 (sample Chicalhão128).
Type locality
Chicalhão opencast clay pit complex near Juncal, Portugal (39° 35ʹ 34.8ʺ N 08° 54ʹ 19.2ʺ W).
Type horizon and age
Early Cretaceous (late Aptian–early Albian, Famalicão Member of the Figueira da Foz Formation).
Distinguishing features for Canrightiopsis dinisii. — Canrightiopsis dinisii is distinguished from C. intermedia and C. crassitesta in its more spherical shape and distinct stigmatic area. It is further distinguished from C. intermedia by its much thicker endotesta.
Canrightiopsis sp.
A number of specimens cannot be included with certainty in any of the three species established here for Canrightiopsis. Most of them are abraded fruits or isolated seeds where information on stamen scars, pollen or seed wall is missing. We refer to these specimens only as Canrightiopsis sp. Canrightiopsis is most abundant in the Famalicão flora with about 600 specimens assigned to Canrightiopsis sp.: S107695–S107699, S107709, S107710, S174149, S174369–S174378 (sample Famalicão 25). Many of them probably belong to C. intermedia. The genus is also common in the Catefica mesofossil flora with about 150 specimens assigned to Canrightiopsis sp.: S174379, S17434380, S174396 (sample Catefica 49); S174381 (sample Catefica 50); S174382 (sample Catefica 51); S174383 (sample Catefica 150); S174384 (sample Catefica 152); S174385 (sample Catefica 153); S174309, S174386 (sample Catefica 154); S174387 (sample Catefica 242); S174388 (sample Catefica 342); S174389 (sample Catefica 343); S174390 (sample Catefica 359); S174391 (sample Catefica 360); S174392 (sample Catefica 361); S174393 (sample Catefica 362); S174394 (sample Catefica 381); S174395 (sample Catefica 382). The genus is less common in the other mesofossil floras. Twenty-two specimens are recorded from the Arazede locality: S174457 (sample Arazede 372), S174458 (sample Arazede 374); seven specimens from the Buarcos locality: S174459, S174460 (sample Buarcos 157), S174461 (sample Buarcos 209); and four specimens from the Vila Verde 2 locality: S174463 (sample Vila Verde 2 439, S174464 (sample Vila Verde 2 441), S174465 (sample Vila Verde 2 442). Several specimens are stored in slide boxes under same number.
Description of the fossils
No flowers that can be linked to the fossil fruits have been discovered in any of the Early Cretaceous mesofossil floras, but distinct scars from stamens (see later) have been observed in all three species (, , , , , ) and show that the fruits are derived from bisexual flowers. A single fruit of Canrightiopsis intermedia from Famalicão has one small, fan-shaped subtending bract preserved adhering to the base of the fruit () showing that the fruit is developed from an almost sessile flower. This is also supported by the morphology of the isolated fruits that lack stalks or have remnants of a very short stalk (). There is no other information on how the fruits were borne on the plant. The specimen with subtending bract preserved is important in allowing observations on the orientation and position of the ovule and androecium, although the orientation cannot be established with certainty without developmental information.
The fruits of Canrightiopsis intermedia and C. crassitesta are typically ovate to elliptical, sometimes flattened on one side (, , , , ) while fruits of C. dinisii are almost spherical (). The surface is typically strongly wrinkled and it may be difficult to observe details on the fruit wall. Internal details were studied using SRXTM for 19 specimens from Famalicão (S174006, S174023–S174028, S174033, S174107, S174148–S174153, S174155, S174157, S174159, S174175), six specimens from Catefica (S172309, S174039, S174040, S174248, S174310, S174311), two specimens from Buarcos (S174104, S174105), one specimen from Vale de Água (S174174) and one specimen from Chicalhão (P0311). Based on these studies, it was possible to reconstruct morphology of seeds enclosed in the fruits and thus to link the fruits to isolated seeds co-occurring associated with the fruits. There are also a number of specimens where seeds have part of the fruit wall preserved.
No stamens are found attached, but in better preserved specimens three distinct scars are present about one third to two thirds up on the fruit. The scars are restricted to one side of the fruit and typically protrude from the fruit surface (, , , , , ). The size and general appearance of the scars indicate that they are from stamens. One specimen of Canrightiopsis intermedia (S174156) with a fan-shaped subtending bract still attached to the fruit has the three stamen scars on the same side as the subtending bract () demonstrating that the stamens are probably on the dorsal side of the carpel, although this cannot be established with certainty as developmental sequences are lacking. Because some of the fruits are strongly wrinkled or partly abraded, the scars are not always clear. In some specimens, there is a distinct rim on the androecium side of the ovary supporting the scars (), but this is not always the case and, except for the occasional rim, there are no features that could indicate traces of a hypanthium. The scars are all of the same dimension indicating that they are scars from equally developed stamens. They are placed with one scar in a central position (no. 2 in –) and one scar on both sides (no. 1 and no. 3 in –) of the central scar with a distance between the scars of about 300 µm (, ). Sometimes, there are also other scar-like structures that appear mostly to be from burst oil cells, but could also be from various damages to the fruit wall. In one of the two fruits of C. dinisii from the Chicalhão mesofossil flora, an additional, prominent scar is present (). It is distinguished from the other scars in being bigger and not protruding. The nature of this scar is uncertain, but the possibility that it represents a scar from an aborted stamen cannot be ruled out. Another possibility is that it is a burst oil cell or damage to the epidermis. Vascular bundles supplying the stamens are rarely preserved, but can be seen in a specimen from Vale de Água.
Pollen is observed on the surface of several fruits or on the stigmatic surface (–). Pollen grains are semitectate-reticulate and monocolpate, almost circular in equatorial outline and 10–15 µm in diameter (Canrightiopsis intermedia: 13–14.5 µm; C. crassitesta: 10.3–12.4 µm; C. dinisii: 14–14.9 µm). The reticulum is heterobrochate. Details of the pollen sculpture are well-exposed in some specimens of C. intermedia () and specimens of C. crassitesta (), while grains on the stigma surface of C. dinisii () are embedded in a secretion that impedes detailed observations. It is, however, clear that the pollen of all three species of Canrightiopsis is of the Clavatipollenites-type with beaded muri. Muri are narrow with rounded profile and an ornamentation of low supratectal elements. Columellae are long and irregularly distributed. The colpus membrane is covered by irregular verrucate-rugulate elements that also have a beaded ornamentation. The colpus margin is indistinct and there is a gradual transition from the verrucate-rugulate elements of the colpus membrane to the open reticulum of the intercolpi areas ().
The gynoecium is unilocular, apparently monocarpellate with a single subapical and rounded stigma that is usually only slightly raised and about 100 µm in diameter. In Canrightiopsis dinisii, it is distinct and hemispherical (, ). It is uncertain whether a similar raised stigma was also present in the other species, but has been abraded during fossilisation. Considering the numerous specimens present in the Famalicão and Catefica mesofossil floras, it is, however, most likely that the stigma is indistinct in the other species of Canrightiopsis. Pollen is sometimes attached to the stigma and often embedded in an amorphous substance (, ) suggesting that the stigma was wet.
The fruits are one-seeded berries, 0.85–1.3 mm long and 0.6–1.15 mm broad, with a thin fruit wall of small parenchyma cells (, , –). The soft tissue of the fruit is usually strongly compressed or not preserved and often, the outer cuticle of the fruit wall is collapsed over the hard endotesta of the seed (). Scattered on the outer surface of the fruits, are numerous stomata-like structures. In well preserved specimens, they are intact with smooth cells in contrast to the other epidermal cells of the fruit wall that usually show distinct cuticular ridges or wrinkles (). These stomata-like structures are similar to the ethereal oils cells seen on the carpel surface of Chloranthus spicatus (Thunb.) Makino (Endress & Igersheim Citation1997) and are most likely also ethereal oil cells. The cells are often broken leaving small holes scattered over the fruit wall (, , ), and in some specimens, these holes contain resinous material () corroborating the interpretation of the structures as ethereal oil cells.
There is a single orthotropous, bitegmic and endotestal seed in each fruit (, , , ). The seed is pendant and ventral inferred from the position of the subtending bract in specimen S174156 from Famalicão. A vascular bundle extends on the presumed ventral side of the fruit (opposite the stamen scars) from the base of the fruit to the level of the chalaza and stigma (, , ). There are also vascular bundles on the side bearing the stamen scars, but the fruit wall is usually poorly preserved and the bundles are difficult to trace. A single fruit from the Catefica locality (S172333) diverges from all other fruits assigned to Canrightiopsis in having two seeds. The seed wall is comparable to that of other Canrightiopsis species in general structure, but the specimen is compressed and somewhat fragmented and details on seed attachment and scars are uncertain.
The chalaza is indicated by a thickening in the exotegmen (, , ). The micropyle is placed opposite of the chalaza and marked by a narrow opening in the endotesta and a thickening of the cuticle of the exotegmen (); cells of exotegmen in the micropylar region are sinuous.
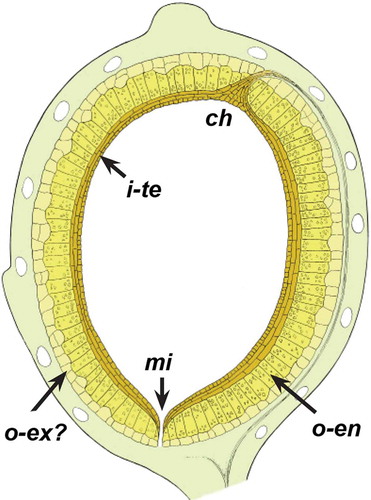
The seed wall is mainly made up by the outer integument (testa). The endotesta is composed of one layer of palisade-shaped cells, 50–120 µm high (Canrightiopsis intermedia: about 50–90 µm; C. crassitesta: about 80–120 µm; C. dinisii: about 100 µm). So far, seeds with a very thick endotesta have only been observed from specimens from the Catefica mesofossil flora assigned to C. crassitesta, but there is a considerable variation in endotesta thickness of Canrightiopsis in most mesofossil floras. The endotesta cells have fibrous infilling and crystals (, , , ). In surface view, the endotestal cells are isodiametric and polygonal (). Crystals are concentrated mainly in the inner and middle part of endotesta. The endotesta is characterised by small holes placed in longitudinal grooves that give the seed surface a ribbed appearance (, H, I, , ). A layer of larger, isodiametric cells is preserved outside the endotesta. These cells have thin cell walls and sometimes an infilling of amorphous material that is interpreted as tannin (, ). It is possible that these cells represent the epidermis of the testa (exotesta) and that the testa is only two cell layers thick, but there is not a clear delimitation between this layer and the compressed cells of the fruit wall. Above the holes in the endotesta, there may be additional thin walled cells.
The inner integument (tegmen) consists of several layers of thin-walled cells (), but these are often collapsed (). One specimen has radially elongated cells of the inner integument () that show some resemblance to the endothelium cells observed in Canrightia and extant Lactoris Phil. This specimen is probably aborted or immature. Other specimens show a particular pattern of a fragmented tegmen with elongated cells () that may also be remains of an endothelium, but none of the Canrightiopsis specimens shows a distinct endothelium. A schematic overview of the fruit and seed wall is shown in .
Figure 14. Schematic outline of ovule structure of Canrightiopsis from the Early Cretaceous of Portugal. Fruit wall is indicated by light green colour and oil cells in fruit wall not coloured. The outer integument (testa) is indicated in light yellow and separated in an outer layer of thin walled cells perhaps representing exotesta (o-ex?) and an inner endotestal layer with crystals (o-en). The inner integument (tegmen, i-te) is indicated by a brownish colour; note that the structure of tegmen is not fully understood.
The embryo is small and less than one quarter of the total length of the seed. It is rarely preserved, but may leave a hollow space in the surrounding endosperm in well-preserved, mature seeds (). Sometimes, part of the embryo may also be preserved ().
Seeds are found inside the fruits as well as isolated, sometimes with fruit wall remnants preserved (, , ). Isolated seeds, characterised by the foveolate and grooved surface, are more common in the mesofossil flora than the fruits and vary much more in size than the fruits (0.6–1.5 mm long and 0.45–1.3 mm). They may represent different developmental stages or perhaps other species. Unless other characters are available such as internal structures obtained from SRXTM analysis, the isolated seeds have been treated as Canrightiopsis sp.
Character scoring and phylogenetic analysis
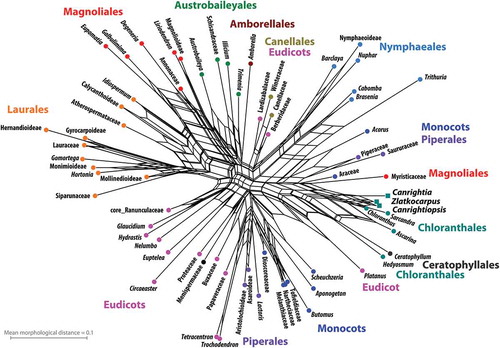
Out of the 142 characters in the D&E matrix, 48 could be scored for the new fossil genus Canrightiopsis described here compared to 57 defined characters for Canrightia and 34 for Zlatkocarpus, providing a total of 63 characters defined for at least one of the three fossil taxa. This reduced character set is sufficient to group all extant Chloranthaceae except for Hedyosmum in the resultant neighbour-net (). The three fossil taxa, Canrightia, Canrightiopsis and Zlatkocarpus were resolved as close relatives of the core Chloranthaceae (Ascarina, Chloranthus, Sarcandra), with Canrightiopsis being very similar to extant Chloranthus and Sarcandra in its preserved morphological traits.
Figure 15. Phylogenetic position of Canrightia, Canrightiopsis and Zlatkocarpus based on a data set of 63 morphological characters. Shown is a planar phylogenetic network, a neighbour-net splits graph, inferred from pairwise (Hamming) distances calculated from the character matrix. Members of commonly accepted clades are generally grouped and the reduced character set is sufficient to group extant Chloranthaceae except for Hedyosmum in the neighbour-net, with the three fossil taxa resolved as close relatives of the core Chloranthaceae (Ascarina, Chloranthus, Sacandra). Canrightiopsis is resolved as most similar to extant Chloranthus and Sarcandra in its preserved morphological traits.
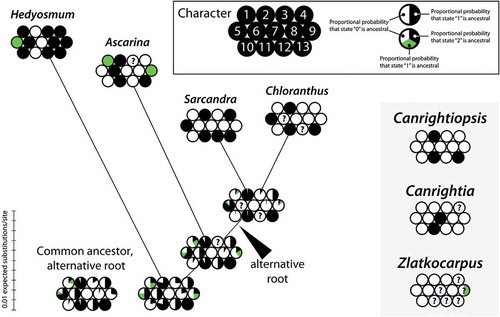
Character mapping of 13 morphological traits variable within modern Chloranthaceae unequivocally places Canrightiopsis in the core Chloranthaceae (). The character suite of Canrightiopsis brings it close to the putative common ancestor of Chloranthus and Sarcandra, independent of the root of Chloranthaceae, either with Hedyosmum as sister to the rest, as inferred from most molecular data, or with Hedyosmum as sister to Ascarina, as traditionally perceived based on morphological evidence, such as presence of unisexual flowers and monoaperturate pollen.
Figure 16. ‘Balls-and-sticks’ graph with proportional branch lengths depicting the result of the ancestral state reconstruction under maximum likelihood. Ancestral states were reconstructed for 11 characters that differ among extant members of the Chloranthaceae. States of two characters (character 7, 13), identical among extant members, are shown for comparison with the fossil taxa. Each ‘ball’ shows the relative probability of a character state at the given node. Character states observed in fossil members of Chloranthaceae are shown to the right. The branch (‘stick’) lengths reflect the amount of molecular evolution (expected substitutions per site).
Discussion
The small one-seeded berries and isolated, foveolate seeds assigned here to Canrightiopsis gen. nov. occur abundantly in the Early Cretaceous plant-bearing sequence of western Portugal. Judged from the abundance of specimens and the widespread occurrence in the mesofossil floras, extending from Catefica in the south to Vila Verde in the north, Canrightiopsis was clearly an important component of the Early Cretaceous vegetation in Portugal. Three species have been distinguished. Canrightiopsis intermedia is common in the Famalicão mesofossil flora with about 50 specimens isolated from the organic residue. This species is also recorded from the Buarcos and Vale de Água localities. Canrightiopsis crassitesta is known from the Catefica locality and C. dinisii from the Chicalhão locality. There are also many abraded fruits and isolated seeds of Canrightiopsis from these localities as well as from two other localities (Arazede and Vila Verde) that have not been assigned at the specific level due to lack of critical information. Most of them probably belong to one of the three species described here, but there may also be further diversity among the small fruits that has not yet been detected. Currently, Canrightiopsis is known only from Portugal and has not been recorded in any of the Early Cretaceous mesofossil floras from the Potomac group of eastern North America.
Only mature fruits of Canrightiopsis have been recovered, but based on the distinct stamen scars on the fruit wall, we conclude that the Canrightiopsis flowers were bisexual with an androecium of three stamens in a one-sided position on the ovary. All stamens are shed, but the three scars on the fruits are of similar size and shape indicating that all three stamens of the flower were equally developed. All associated isolated stamens in the mesofossil floras including those with pollen similar to that of Canrightiopsis have tetrasporangiate anthers and we therefore infer tetrasporangiate anthers also for Canrightiopsis.
Comparison of Canrightiopsis with other Early Cretaceous fossils
Three Early and mid-Cretaceous genera with small resinous fruits and monocolpate-reticulate pollen show some similarity with the new fossils described here: Canrightia from the Early Cretaceous of Portugal (Friis & Pedersen Citation2011), Zlatkocarpus from the mid-Cretaceous of the Czech Republic (Kvaček & Friis Citation2010), and Couperites from the Early and mid-Cretaceous of eastern North America (Pedersen et al. Citation1991). As indicated by the name, the fossil fruits of Canrightiopsis show similarity with those of Canrightia described from the same plant bearing horizons in Portugal. Fruits of the two genera are small, elliptical to oval or almost spherical berries with ethereal oil cells (or resin bodies) in the fruit wall, and often wrinkled surface that may obscure details on the fruit wall. Fruits of both genera also have distinct scars from stamens on the fruit wall indicating a semi-inferior ovary. Pollen grains observed on the stigmatic surfaces and fruit wall are monocolpate, semitectate and reticulate. Seeds are pendant, orthotropous and endotestal with endotesta consisting of a single layer of palisade cells with crystals and fibrous infilling. In other features, Canrightiopsis is clearly distinct from Canrightia. Most notably, in Canrightiopsis, there are three stamen scars on one side of the ovary in contrast to the radial arrangement of the four or five stamens in Canrightia; Canrightiopsis also lacks a clearly delimited hypanthium, while in Canrightia the hypanthium is distinct. Further, Canrightiopsis has one-seeded berries, while Canrightia has two to five seeds per fruit, and the beaded supratectal ornamentation of the muri in the Clavatipollenites-type pollen of Canrightiopsis is clearly distinct from the smooth, unsculptured muri of the Retimonocolpites-type pollen of Canrightia.
Zlatkocarpus from the Cenomanian of the Bohemian Basin includes two species, Z. brnikianus J.Kvaček et E.M.Friis and Z. pragensis (J.Kvaček et H.Eklund) J.Kvaček et E.M.Friis, both of which have small resinous fruits comparable to those of Canrightiopsis in general morphology, but as in Canrightia, there is a distinct hypanthium (Kvaček & Eklund Citation2003; Kvaček & Friis Citation2010). The fruits of Zlatkocarpus are apparently one-seeded, but none of the specimens is sufficiently well-preserved to reveal details of seed organisation or internal structures. Associated pollen is of the Retimonocolpites-type; monocolpate and semitectate-reticulate with a long colpus and smooth muri. A recent phylogenetic analysis by Doyle and Endress (Citation2014) resolved Zlatkocarpus as closely related to Chloranthaceae, at the stem or as member of the crown group.
Couperites from the mid-Cretaceous Mauldin Mountain mesofossil flora (Pedersen et al. Citation1991) is similar to Canrightiopsis in having small, one-seeded fruits with ethereal oil cells (or resin bodies) in the fruit wall and associated monocolpate, semitectate-reticulate pollen with beaded muri. A close relationship of Couperites with extant Chloranthaceae was initially suggested by Pedersen et al. (Citation1991), although the authors concluded that particularly seed characters excluded an assignment of Couperites to this family. Seed characters also distinguish Couperites from Canrightiopsis. The seeds of Couperites are anatropous and exotestal in contrast to the orthotropous and endotestal seeds of Canrightiopsis and extant Chloranthaceae (Ascarina, Chloranthus, Sarcandra). The pollen of Couperites was originally referred to as Clavatipollenites-type based on the monocolpate-reticulate nature of the grains and the beaded ornamentation of the muri. However, Couperites pollen is distinct from typical Clavatipollenites pollen and pollen of extant Chloranthaceae with distal apertures (Ascarina and Hedyosmum) in having sharply delimited colpus margins, while pollen of Clavatipollenites, Canrightiopsis, Ascarina and Hedyosmum has a gradual transition from colpus membrane to the reticulum of the main body (see also Friis et al. Citation2011). Although both seed and pollen features suggest a position distinct from Chloranthaceae, a possible chloranthaceous affinity of Couperites has been addressed in several subsequent phylogenetic analyses (Doyle & Endress Citation2014, and references cited therein).
Clavatipollenites was first described based on dispersed grains from the Early Cretaceous (Barremian–Aptian) of southern England (Couper Citation1958). Originally, a single species, Clavatipollenites hughesii Couper, was included and studied using light microscopy only. SEM studies of Clavatipollenites-type pollen from the type horizon by Hughes et al. (Citation1979) demonstrated a much higher diversity than is discernible in light microscopy (LM) studies, particularly in the supratectal ornamentation. Current practice is, therefore, to use the circumscription of the genus indicated by Couper (Citation1960) and, later, by Walker and Walker (Citation1984) for monoaperturate, reticulate grains with aperture configuration and supratectal ornamentation closely comparable to that of extant Ascarina (see also discussion in Friis et al. Citation2011). Clavatipollenites is very common in Early Cretaceous palynofloras with the earliest record from the Hauterivian of Israel (Brenner & Bickoff Citation1992). It is also common in the Early Cretaceous palynofloras of Portugal represented by several different species (e.g. Pais & Reyre Citation1981; Trincão Citation1990; Heimhofer Citation2004; Heimhofer et al. Citation2007; Mendes et al. Citation2014a). None of the dispersed grains from Portugal are figured at high resolution and detailed comparison with the Canrightiopsis pollen is not possible.
Isolated stamens with Clavatipollenites-type pollen in situ are also common in the Early Cretaceous mesofossil floras of Portugal. A summary of early angiosperm diversification in Portugal (Friis et al. Citation1999) listed ten different kinds of monoaperturate, semitectate-reticulate pollen types with beaded muri, most of them are Clavatipollentites-type and a few are Asteropollis-type. Since then new material has been added. Based on stamen size and shape, there are clearly several different species (E. M. Friis and K. R. Pedersen, work in progress), but currently none of the isolated stamens can be linked to Canrightiopsis.
Comparison with extant Chloranthaceae
A suite of characters unequivocally links Canrightiopsis to members of the Chloranthaceae (, ). Of particular importance is the unusual androecium in the two bisexual members of Chloranthaceae (Chloranthus and Sarcandra) and in Canrightiopsis with stamens borne on one side of the ovary, one to two thirds up on the ovary, without preceding a perianth or distinguishable hypanthium (Endress Citation1987). A similar arrangement is not known for any other extant angiosperm. Based on the protruding scars on the fruit wall Canrightiopsis has three stamens: one borne centrally and two borne laterally with one on each side of the central stamen, about 300 µm from the central stamen. In Chloranthus, the androecium is tripartite typically with a broad base bearing a central, dithecate and tetrasporangiate lobe and two lateral, monothecate and bisporangiate lobes with the sporangia of the lateral lobes borne on the outer margin (facing away from the central lobe). In Sarcandra, there is a single, central, dithecate and tetrasporangiate, stamen. As in extant Chloranthaceae, the subtending bracts are not attached to the fruits.
Pollen in all extant members of the Chloranthaceae is semitectate-reticulate with beaded muri in Ascarina, Hedyosmum and occasionally in Chloranthus, while pollen of Chloranthus and Sarcandra typically has smooth muri. The Clavatipollenites-type pollen of Canrightiopsis is particularly similar to that of extant Ascarina. Resemblance of isolated Clavatipollenites to Ascarina was already pointed out earlier by Couper (Citation1960) and Walker and Walker (Citation1984). Pollen of Ascarina is the only extant Chloranthaceae pollen with a distal colpus that is simple or sometimes three-armed. Pollen of Hedyosmum also has a single distal aperture, but the aperture is diffusely five-armed. Pollen of Sarcandra is polyporate and that of Chloranthus variable, spiraperturate to polycolpate. None of them has distal apertures. A distal aperture is, however, probably present in the peculiar pollen of Chloranthistemon alatus H. Eklund, K.R.Pedersen et E. M. Friis.
As in Canrightiopsis, fruits of Ascarina, Chloranthus and Sarcandra are berries with a single orthotropous, pendant and endotestal seed. The testa in Canrightiopsis may consists of only two layers of cells, an outer layer of large, thin-walled cells (exotestal) and an inner layer (endotesta) of palisade-shaped cells with fibrous infilling and scattered crystals. The same condition is known for Ascarina, while the testa in Chloranthus and Sarcandra consists of several layers of cells (Endress & Igersheim Citation1997). Because the delimitation of the outer testa and fruit wall is not distinct in the fossil material, we have not included this character in the analysis.
Ethereal oil cells are present in the surface of the fruit wall of Canrightiopsis as well as in Chloranthus and Sarcandra, while in Ascarina the oil cells are deeper in the fruit wall (Endress & Igersheim Citation1999). The embryo in mature seeds is small surrounded by copious endosperm in Canrightiopsis and in all extant Chloranthaceae.
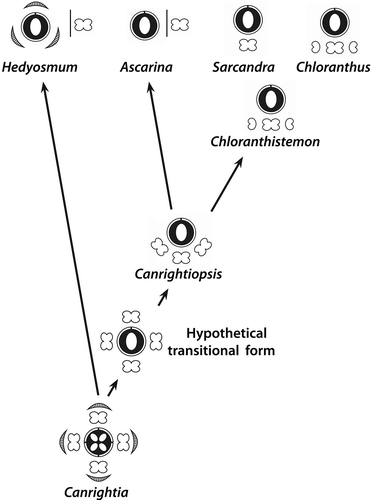
Canrightiopsis, an evolutionary link between Canrightia and Chloranthus–Sarcandra?
In the character analysis, Canrightia differs from the reconstructed common ancestor of extant Chloranthaceae only by the number of carpels and ovules () and it may represent a plesiomorphic ancestral Chloranthaceae (see also Doyle & Endress Citation2014), while Zlatkocarpus may represent an extinct side lineage, not directly related to any of the modern Chloranthaceae. The analysis further shows close resemblance between the character suites of Canrightiopsis and the hypothetical common ancestors of Chloranthus–Sarcandra and Ascarina–Chloranthus–Sarcandra. This configuration suggests an evolutionary pathway in the Chloranthaceae clade that links Canrightia and Canrightiopsis to the extant members of the Chloranthaceae and provides a simple explanation for the derivation of the unusual floral structure and androecium of Chloranthus and Sarcandra ().
Figure 17. Trait evolution in Chloranthaceae. The synopsis is based on reconstructed states for hypothetical ancestors of extant genera (cf. ) and empirical evidence from fossil members of the lineage. The only conflict between reconstruction and empirical evidence is highlighted by red colouring: According to the ancestral state reconstruction, tepals would have been already reduced in the common ancestor of all extant taxa. Hedyosmun would then have de-evolved to an ancestral state (reversal), which is unlikely. In contrast, the situation in Canrightiopsis indicates that tepals were subsequently reduced in the stem lineage of core Chloranthaceae Ascarina, Chloranthus and Sarcandra.
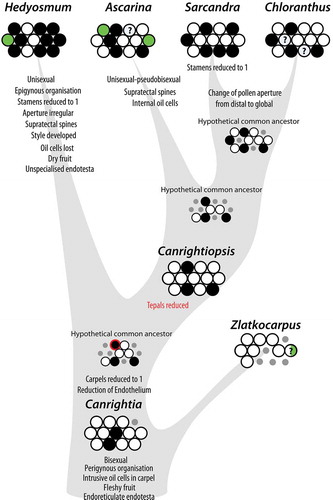
Figure 18. Proposed evolutionary sequence from the bisexual, radial symmetrical and partly epigynous flowers of Canrightia to the bisexual, monosymmetrical and naked flowers of Canrightiopsis, Chloranthus and Sarcandra, illustrating also the three-stamen scenario for the derivation of the tripartite androecium of Chloranthus.
Extant Hedyosmum deviates markedly from Canrightia and the common ancestor of all other Chloranthaceae in having unisexual flowers with unistaminate male flowers, pollen grains with irregular aperture, epigynous pistillate flowers with well-developed style, lack of oil cells in the fruit wall, dry fruit and unspecialised endotesta. The derived position of Hedyosmum is also corroborated by the much longer root to tip distance of its terminal branch compared to the other three branches in the molecular-based trees ().
Under the currently accepted rooting scenario (Qiu et al. Citation1999; Zhang & Renner Citation2003; Doyle & Endress Citation2014), loss of tepals, reduction of stamen number and displacement of the androecium to one side would represent putative synapomorphies (or more precise: shared apomorphies) of the lineage leading to Ascarina, Chloranthus and Sarcandra. Thus, Canrightiopsis may be considered as an early, yet relatively plesiomorphic representative of the crown group of Chloranthaceae, probably predating the split between Ascarina and Chloranthus–Sarcandra. Under this model, the change from one to many (non-distal) apertures is a shared apomorphy of Chloranthus–Sarcandra. Ascarina is distinguished from the common ancestor/Canrightiopsis by its unisexual flowers and apparent superior ovary.
In Canrightia and Zlatkocarpus, the perianth is strongly reduced, represented by short tips on the rim of the hypanthium. In Canrightiopsis as well as in extant Ascarina, Chloranthus and Sarcandra, the perianth parts are completely lost and there is no obvious hypanthium. However, stamens/stamen scars are borne at the same level as in Canrightia suggesting that the hypanthium is completely absorbed by the ovary wall in Canrightiopsis, Chloranthus and Sarcandra. The androecium of Canrightiopsis has a reduced number of stamens compared to that of Canrightia that could have developed by loss of one or two ventral stamens and a displacement of the remaining three stamens to one side. Only few evolutionary steps further are required for deriving the tripartite (three-lobed) androecium of Chloranthus and the unistaminate androecium of Sarcandra from the three stamens of Canrightiopsis: further displacement of the two lateral stamens towards the centre; concrescence of the three stamen bases; loss of one set of sporangia from the lateral stamens in Chloranthus; and loss of both lateral stamens in Sarcandra. Concrescence of stamens could have happened before or after loss of sporangia. The latter scenario is in accordance with one of the interpretation of the Chloranthus androecium presented by Swamy (Citation1953). In both extant Chloranthus and in fossil Chloranthistemon, there are species in which the basal parts of the individual lobes are free. The three-stamen model contrasts the single-stamen models by Endress (Citation1987). In these models, the tripartite androecium is derived from a single stamen either by polymerisation, concrescence and reduction or by lobation and fractionation. In both single stamen scenarios, the thecae of the lateral lobes are borne on the outer margin. In the Chloranthistemon-like fossils from mid-Cretaceous of Germany, the thecae of the lateral lobes appear to be borne on the inner margins (Friis et al. Citation2011; Hartkopf-Fröder et al. Citation2011), which may also have implications for understanding the derivation of the tripartite androecium. However, the apparent diverging pattern in the German taxon may be an artefact caused by compression (Peter K. Endress, personal communication, April 2015), and further studies are needed to establish the nature of this mid-Cretaceous fossil.
Conclusion
The new fossil genus, Canrightiopsis, is important in several respects. It represents a new extinct angiosperm and further documents the extensive diversity among basal angiosperms in the Early Cretaceous mesofossil floras. It is also significant in corroborating the importance of Chloranthaceae in early angiosperm diversification and provides a key for interpreting the unusual floral structure and androecium in extant Chloranthus and Sarcandra. An evolutionary sequence from the bisexual, radial symmetrical and partly epigynous flowers of Canrightia to the bisexual, monosymmetrical and naked flowers of Canrightiopsis, Chloranthus and Sarcandra is proposed. Based on the new fossils, we also propose that the tripartite androecium of Chloranthus is derived from closing up of three full stamens towards the centre of the dorsal side followed by concrescence and loss of sporangia, contrasting the prevailing single stamen hypotheses where the tripartite androecium is thought to have developed from a single stamen. Canrightiopsis occurs abundantly in the Portuguese mesofossil floras from the Early Cretaceous and was clearly an important element in the Early Cretaceous vegetation of the region. So far, it has not been recorded from the more or less contemporary mesofossil floras of eastern North America.
Supplemental data
Supplemental data for this article can be accessed here.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary_information_SGRA-2015-0018.zip
Download Zip (151.1 KB)Acknowledgements
The authors thank Marco Stampanoni, Federica Marone (PSI) and Anna Lindström (NRM) for help with the SRXTM analyses performed at the Swiss Light Source, Paul Scherrer Institute (PSI), Villigen, Switzerland. Pollyanna von Knorring (NRM) is thanked for help with the illustration in . The authors also thank Peter K. Endress, University of Zürich, Switzerland, and Patrick Herendeen, Chicago Botanical Garden, Chicago, U.S.A., for helpful comments and suggestions. Financial support by the Swiss Light Source (European Union FP6 projects 20110963 and 20130185), by the Swedish Research Council (621-2011-5431) and by CretaCarbo project (PTDC/CTE-GIX/113983/2009) is gratefully acknowledged.
References
- Angiosperm Phylogeny Group (APG). 2009. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society 161: 105–121. doi:10.1111/(ISSN)1095-8339.
- Brenner GJ, Bickoff IS. 1992. Palynology and age of the Lower Cretaceous basal Kurnub group from the coastal plain to the northern Negev of Israel. Palynology 16: 137–185. doi:10.1080/01916122.1992.9989411.
- Bryant D, Moulton V. 2002. NeighborNet: An agglomerative method for the construction of planar phylogenetic networks. In: Guigó R, Gusfield O, eds. Second Workshop on Bioinformatics (WABI) 2002. LNCS 2452, 375–391. Berlin, Heidelberg: Springer.
- Bryant D, Moulton V. 2004. NeighborNet: An agglomerative method for the construction of phylogenetic networks. Molecular Biology and Evolution 21: 255–265. doi:10.1093/molbev/msh018.
- Couper RA. 1958. British Mesozoic microspores and pollen grains. A systematic and stratigraphic study. Palaeontographica Abteilung B 103: 75–179.
- Couper RA. 1960. New Zealand Mesozoic and Cainozoic plant microfossils. New Zealand Geological Survey. Palaeontological Bulletin 32: 1–87.
- Crane PR, Friis EM, Pedersen KR. 1989. Reproductive structure and function in Cretaceous Chloranthaceae. Plant Systematics and Evolution 165: 211–226. doi:10.1007/BF00936003.
- Denk T, Grimm GW. 2009. The biogeographic history of beech trees. Review of Palaeobotany and Palynology 158: 83–100. doi:10.1016/j.revpalbo.2009.08.007.
- Dinis JL 2001. Definição da Formação da Figueira da Foz - Aptiano a Cenomaniano do sector central da margem oeste ibérica /Definition of the Figueira da Foz Formation - Aptian to Cenomanian of the central sector of the western Iberian margin. Comunicações do Instituto Geológico e Mineiro 88: 127–160.
- Dinis JL, Rey J, Cunha PP, Callapez P, Pena dos Reis R. 2008. Stratigraphy and allogenic controls of the western Portugal Cretaceous: An updated synthesis. Cretaceous Research 29: 772–780. doi:10.1016/j.cretres.2008.05.027.
- Doyle JA, Endress PK. 2010. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: Magnoliidae and eudicots. Journal of Systematics and Evolution 48: 1–35. doi:10.1111/jse.2010.48.issue-1.
- Doyle JA, Endress PK. 2014. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: ANITA lines and relatives of Chloranthaceae. International Journal of Plant Sciences 175: 555–600. doi:10.1086/675935.
- Eklund H, Friis EM, Pedersen KR. 1997. Chloranthaceous floral structures from the Late Cretaceous of Sweden. Plant Systematics and Evolution 207: 13–42. doi:10.1007/BF00985207.
- Endress PK. 1987. The Chloranthaceae: Reproductive structures and phylogenetic position. Botanische Jahrbücher für Systematik. Pflanzengeschichte und Pflanzengeographie 109: 153–226.
- Endress PK, Igersheim A. 1997. Gynoecium diversity and systematics of the Laurales. Botanical Journal of the Linnean Society 125: 93–168. doi:10.1111/boj.1997.125.issue-2.
- Endress PK, Igersheim A. 1999. Gynoecium diversity and systematics of the basal eudicots. Botanical Journal of the Linnean Society 130: 305–393. doi:10.1111/boj.1999.130.issue-4.
- Friis EM, Crane PR, Pedersen KR. 1997. Fossil history of magnoliid angiosperms. In: Iwatsuki K, Raven PH, eds. Evolution and diversification of land plants, 121–156. Tokyo, Berlin, Heidelberg, New York: Springer-Verlag.
- Friis EM, Crane PR, Pedersen KR. 2011. Early flowers and angiosperm evolution. Cambridge: Cambridge University Press.
- Friis EM, Marone F, Pedersen KR, Crane PR, Stampanoni M. 2014. Three-dimensional visualization of fossil flowers, fruits, seeds, and other plant remains using synchrotron radiation X-ray tomographic microscopy (SRXTM): New insights into Cretaceous plant diversity. Journal of Paleontology 88: 684–701. doi:10.1666/13-099.
- Friis EM, Pedersen KR. 2011. Canrightia resinifera gen. et sp. nov., a new extinct angiosperm with Retimonocolpites-type pollen from the Early Cretaceous of Portugal: Missing link in the eumagnoliid tree? Grana 50: 3–29. doi:10.1080/00173134.2011.559728.
- Friis EM, Pedersen KR, Crane PR. 1999. Early angiosperm diversification: The diversity of pollen associated with angiosperm reproductive structures in Early Cretaceous floras from Portugal. Annals of the Missouri Botanical Garden 86: 259–296.
- Friis EM, Pedersen KR, von Balthazar M, Grimm GW, Crane PR. 2009. Monetianthus mirus gen. et sp. nov., a nymphaealean flower from the Early Cretaceous of Portugal. International Journal of Plant Sciences 170: 1086–1101. doi:10.1086/592013.
- Göker M, García-Blázquez G, Voglmayr H, Tellería MT, Martín MP. 2009. Molecular taxonomy of phytopathogenic fungi: A case study in Peronospora. PLoS ONE 4: e6319.
- Göker M, Grimm GW. 2008. General functions to transform associate data to host data, and their use in phylogenetic inference from sequences with intra-individual variability. BMC Evolutionary Biology 8: 86.
- Grimm GW, Renner SS. 2013. Harvesting GenBank for a Betulaceae supermatrix, and a new chronogram for the family. Botanical Journal of the Linnean Society 172: 465–477. doi:10.1111/boj.12065.
- Hartkopf-Fröder C, Rust J, Wappler T, Friis EM, Viehofen A. 2011. Mid-Cretaceous charred fossil flowers reveal direct observation of arthropod feeding strategies. Biological Letters doi:10.1098/rsbl.2011.0696.
- Hedlund RW, Norris G. 1968. Spores and pollen grains from Fredericksburgian (Albian) strata, Marshall County, Oklahoma. Pollen et Spores 10: 129–159.
- Heimhofer U. 2004. Response of terrestrial palaeoenvironemnts to past changes in climate and carbon-cycling: Insights from palynology and stable isotope geochemistry. PhD Thesis, ETH, Zurich, Switzerland.
- Heimhofer U, Hochuli PA, Burla S, Weissert H. 2007. New records of Early Cretaceous angiosperm pollen from Portuguese coastal deposits: Implications for the timing of the early angiosperm radiation. Review of Palaeobotany and Palynology 144: 39–76. doi:10.1016/j.revpalbo.2005.09.006.
- Herendeen PS, Crepet WL, Nixon KC. 1993. Chloranthus-like stamens from the Upper Cretaceous of New Jersey. American Journal of Botany 80: 865–871.
- Hintermüller C, Marone F, Isenegger A, Stampanoni M. 2010. Image processing pipeline for synchrotron-radiation-based tomographic microscopy. Journal of Synchrotron Radiation 17: 550–559. doi:10.1107/S0909049510011830.
- Hughes NF. 1994. The enigma of angiosperm origins. Cambridge: Cambridge University Press.
- Hughes NF, Drewry G, Laing JF. 1979. Barremian earliest angiosperm pollen. Palaeontology 22: 513–536.
- Huson DH, Bryant D. 2006. Application of phylogenetic networks in evolutionary studies. Molecular Biology and Evolution 23: 254–267. doi:10.1093/molbev/msj030.
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. doi:10.1093/molbev/mst010.
- Kvaček J, Eklund H. 2003. A report on newly recovered reproductive structures from the Cenomanian of Bohemia (Central Europe). International Journal of Plant Sciences 164: 1021–1039. doi:10.1086/ijps.2003.164.issue-6.
- Kvaček J, Friis EM. 2010. Zlatkocarpus gen. nov., a new angiosperm reproductive structure with monocolpate-reticulate pollen from the Late Cretaceous (Cenomanian) of the Czech Republic. Grana 49: 115–127. doi:10.1080/00173134.2010.481845.
- Lewis PO. 2001. A likelihood approach to estimating phylogeny from discrete morphological character data. Systematic Biology 50: 913–925. doi:10.1080/106351501753462876.
- Maddison WP, Maddison DR. 2011. Mesquite: A modular system for evolutionary analysis. Version 2.75. http://mesquiteproject.org.
- Martínez C, Madriñán S, Zavada M, Alberto Jaramillo C. 2013. Tracing the fossil pollen record of Hedyosmum (Chloranthaceae), an old lineage with recent Neotropical diversification. Grana 52: 161–180. doi:10.1080/00173134.2012.760646.
- Mendes MM, Dinis J, Pais J, Friis EM. 2014a. Vegetational composition of the Early Cretaceous Chicalhão flora (Lusitanian Basin, western Portugal) based on palynological and mesofossil assemblages. Review of Palaeobotany and Palynology 200: 65–81. doi:10.1016/j.revpalbo.2013.08.003.
- Mendes MM, Grimm GW, Pais J, Friis EM. 2014b. Fossil Kajanthus lusitanicus gen. et sp. nov. from Portugal: Floral evidence for Early Cretaceous Lardizabalaceae (Ranunculales, basal eudicot). Grana 53: 283–301. doi:10.1080/00173134.2014.932431.
- Nichols DJ, Matsukawa M, Ito M. 2006. Palynology and age of some Cretaceous non-marine deposits in Mongolia and China. Cretaceous Research 27: 241–251. doi:10.1016/j.cretres.2005.11.004.
- Pais J, Reyre Y. 1981. Problèmes posés par la population sporo-pollinique d’un niveau à plantes de la série de Buarcos (Portugal). Boletim da Sociedade Geológica de Portugal 22: 35–40.
- Pattengale ND, Masoud A, Bininda-Emonds ORP, Moret BME, Stamatakis A. 2009. How many bootstrap replicates are necessary? In: Batzoglou S, ed. RECOMB 2009, 184–200. Berlin, Heidelberg: Springer.
- Pedersen KR, Crane PR, Drinnan AN, Friis EM. 1991. Fruits from the mid-Cretaceous of North America with pollen grains of the Clavatipollenites type. Grana 30: 577–590. doi:10.1080/00173139109427816.
- Qiu Y-L, Lee JH, Bernasconi-Quadroni F, Soltis DE, Soltis PS, Zanis M, Zimmer EA, Chen ZD, Savolainen V, Chase MW. 1999. The earliest angiosperms: Evidence from mitochondrial, plastid and nuclear genomes. Nature 402: 404–407. doi:10.1038/46536
- Rey J, Dinis JL, Callapez P, Cunha PP. 2006. Da rotura continental à margem passiva. Composição e evolução do Cretácico de Portugal. Lisbon: Ministério da Economia e da Inovação.
- Stamatakis A. 2006. RAxML-VI-HPC: Maximum-Likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22: 2688–2690. doi:10.1093/bioinformatics/btl446.
- Stamatakis A, Hoover P, Rougemont J. 2008. A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology 57: 758–771. doi:10.1080/10635150802429642.
- Stampanoni M, Groso A, Isenegger A, Mikuljan G, Chen Q, Bertrand A, Henein S, Betemps R, Frommherz U, Bohler P, Meister D, Lange M, Abela R. 2006. Trends in synchrotron-based tomographic imaging: The SLS experience. In: Bonse U, ed. Developments in X-ray tomography V, Vol. 6318. San Diego: Proceedings of SPIE-The International Society for Optical Engineering.
- Swamy BGL. 1953. The morphology and relationships of the Chloranthaceae. Journal of the Arnold Arboretum 34: 375–411.
- Swofford DL. 2002. PAUP*: Phylogenetic analysis using parsimony (* and other methods). Champaign: National Illinois History Survey.
- Trincão PRP. 1990. Esporos e pólenes do Cretácio inferior (Berriasiano-Aptiano) de Portugal: Paleontologia e biostratigrafia. PhD Thesis, Universidade Nova de Lisboa, Lisbon.
- Walker JW, Walker AG. 1984. Ultrastructure of Lower Cretaceous angiosperm pollen and the origin and early evolution of flowering plants. Annals of the Missouri Botanical Garden 71: 464–521.
- Zhang L-B, Renner SS. 2003. The deepest splits in chloranthaceae as resolved by chloroplast sequences. International Journal of Plant Sciences 164: S383–S392. doi:10.1086/ijps.2003.164.issue-s5.
