Abstract
A new Early Cretaceous flower, Kenilanthus marylandensis, is described from the fossil plant bearing sequence exposed in early-middle Albian Potomac Group sediments at the Kenilworth (Bladensburg) locality, Maryland, USA. The flower is small, actinomorphic, pentamerous, and isomerous, and contains both pistillate and staminate parts. The receptacle is slightly dome-shaped and bears one whorl of five tepals, with a possible additional whorl toward the outside, ten stamens in two whorls of five, and one whorl of five free carpels. Stamens have elongate, tetrasporangiate, dithecate anthers that arc slightly inwards towards the centre of the flower. Thecae protrude and are oriented outwards. Pollen grains are small, tricolpate and reticulate with a graded, heterobrochate reticulum and segmented, spiny muri. Carpels are elongate with abundant stomata on the outer surface, and each carpel has a broad oblique base. Ovules are numerous in each carpel and are borne on two marginal placentae that extend along either side of the ventral suture. Kenilanthus has a combination of features that occur among different early-diverging eudicot lineages, suggesting a phylogenetic position close to the base of the eudicot clade, but its precise phylogenetic position is difficult to resolve.
Fossil evidence strongly indicates that the initial phylogenetic diversification of angiosperms during the Early Cretaceous involved many kinds of plants that are now extinct, and suggests that their closest living relatives are among extant angiosperms in the ANA-grade (Amborellales, Nymphaeales, Austrobaileyales), Chloranthaceae, eumagnoliids and early lineages of monocots (Friis et al. Citation2011). Eudicots also appear early in the diversification of angiosperms, as evidenced by the presence of isolated tricolpate pollen grains in palynological assemblages from around the Barremian-Aptian boundary onwards (Penny Citation1988, Citation1991; Doyle Citation1992; Hughes Citation1994). Early tricolpate pollen is known from floral remains and in coprolites in early mesofossil floras from Portugal (Friis et al. Citation2010a, Citation2011) and there are scattered reports of eudicot leaf fossils from Aptian to mid-Albian strata (e.g. Jud Citation2015). Eudicots become more common towards the end of the Albian. The earliest pollen grains that can unequivocally be assigned to eudicots are tricolpate, like the pollen produced by many extant early-diverging lineages in this clade. Tricolporate pollen grains, which occur in some basal grade eudicots, but that are more characteristic of core eudicots (Furness et al. Citation2007), are also present in the fossil record by the end of the Early Cretaceous.
The fossil record of pollen, floral structures and leaves all indicate that eudicots had already attained considerable diversity by the end of the Early Cretaceous, albeit mainly at a level of evolution below that of extant core eudicots. However, knowledge of these early eudicots is currently poor and both leaves and flowers are rare especially from early to mid-Albian or earlier fossil assemblages. The sparse record of flowers suggests that prior to mid-Albian eudicots were a relatively minor component of Early Cretaceous vegetation compared to more abundant ANA-lineages and early eumagnoliids, and infrequent preservation of eudicot leaves may partially reflect the low fossilisation potential of what are inferred to have been predominantly herbaceous or shrubby plants (Friis et al. Citation2010b, Citation2011; Jud Citation2015). Some early eudicot leaves may also have been misidentified as ferns (Jud Citation2015).
Here we add to the diversity known among eudicots from the Early Cretaceous based on a single floral structure of early-middle Albian age, collected at the Kenilworth locality, Maryland, USA. The flower preserves remains of the perianth, stamens with tricolpate reticulate in situ pollen, and a whorl of five free carpels, each of which contains remains of ovules. The combination of characters indicates a phylogenetic position near the base of the eudicot radiation, which is consistent with its Early Cretaceous age.
Material and methods
The single flower described here is from the Kenilworth locality (sample 174), collected about 1.5 km southwest of Bladensburg, Prince Georges County, Maryland, USA (Bladensburg, Station 17; Brenner Citation1963) by PRC in 1992. The fossil-bearing sediments are part of the Early Cretaceous Patapsco Formation of the Potomac Group. Based on palynological evidence, the level yielding the plant mesofossils is tentatively assigned to the basal part of Subzone II-B, corresponding to early-middle Albian (for more information on locality and age see Brenner [Citation1963] and Friis et al. [Citation1997, Citation2011]), although a slightly younger age was suggested by Hochuli et al. (Citation2006). The Kenilworth plant remains are preserved mainly as lignitic fossils with a subordinate charcoalified component. The flora includes a diverse assemblage of angiosperm floral structures, such as fruits and seeds of Anacostia marylandensis E.M.Friis, P.R.Crane et K.R.Pedersen and several angiosperm seeds with exquisitely preserved embryos and nutritive tissue (Friis et al. Citation2015). The flora also includes a variety of non-angiosperm seed plants, such as welwitschoid seeds of Bicatia costata E.M.Friis, K.R.Pedersen et P.R.Crane and Bicatia juncalensis E.M.Friis, K.R.Pedersen et P.R.Crane (Friis et al. Citation2014b), as well as various pteridophytes.
The fossil flower described here is lignitic. It was isolated and cleaned following standard methods for Cretaceous mesofossils (Friis et al. Citation2011) and was then mounted on a brass stub for synchrotron radiation X-ray microtomography (SRXTM) at the Tomcat beamline of the Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland (Stampanoni et al. Citation2006). The specimen was measured using a 10× objective with isotopic pixel size of 0.65 μm at 10 keV using a sCMOS detector and a 20 μm thick LAG:Ce scintillator screen (for more details on the technique, see Friis et al. Citation2014a). Data derived from the SRXTM (Hintermüller et al. Citation2010) were reconstructed and imaged using Avizo (version 7.1 and 9) software for computed tomography. Following SRXTM, the fossil was remounted on an aluminium stub for scanning electron microscopy (SEM), sputter coated with gold, and studied using a Hitachi S-4300 Field Emission Scanning Electron Microscope at 2 kV.
The systematic position of the fossil was assessed by scoring it in two different morphological matrices for extant plants that included relevant eudicots: the Doyle and Endress (Citation2010) matrix for magnoliids and basal eudicots, and the Wang et al. (Citation2009b) matrix for Ranunculales. Characters states were optimised on backbone constraint trees with extant taxa in fixed positions following Doyle and Endress (Citation2010) and the strict consensus tree of eight most parsimonious trees (MPTs) of Wang et al. (Citation2009b) using the program Mesquite (Maddison & Maddison Citation2011). Applying the same approach, we also assessed the relationships of Kenilanthus using the morphological dataset of Hermsen et al. (Citation2006) for Saxifragales and other basal rosids.
The specimen is housed at the Field Museum of Natural History, Chicago, USA (PP). Raw data from the SRXTM study are stored at Swedish Museum of Natural History, Stockholm, Sweden.
Systematic palaeobotany
Angiospermae (early-diverging eudicot lineage)
Kenilanthus gen. nov.
Derivation of generic name
From Kenilworth, the locality where the fossil was collected, and anthus, Greek for flower.
Generic diagnosis
Flower small, pentamerous, isomerous, and containing both stamens and carpels. Receptacle dome-shaped. Perianth of a single whorl of five tepals with a possible additional whorl toward the outside; aestivation quincuncial. Androecium of ten stamens in two whorls. Stamens differentiated into a short, stout filament and an elongated, basifixed anther. Anthers dithecate and tetrasporangiate with thecae projecting towards the outside of the flower. Pollen tricolpate and reticulate, heterobrochate with graded reticulum. Muri obliquely segmented and spinulose, supported by sturdy columellae. Gynoecium superior, consisting of one whorl of five free carpels. Carpels distinctly separate below, but converging above. Carpels sessile, elongate, and plicate, with two longitudinal placentae on either side of the ventral suture, each of which bears many ovules in a longitudinal row. Stomata are present on the carpel surface.
Type species designated here
Kenilanthus marylandensis sp. nov.
Kenilanthus marylandensis sp. nov.
–
Figure 1. SEM images of Kenilanthus marylandensis gen. et sp. nov., flower from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A–C. Flower in different angles showing broad tepals (t), stamens with elongated anthers (a), which are well preserved on one side of the flower, and carpels (c) in the centre; carpel surface with abundant stomata (arrow) and peltate trichomes (arrowhead). D. Detail of carpel surface showing peltate, probably glandular, trichomes (arrowheads). Scale bars – 1 mm (A, C), 500 µm (B), 200 µm (D).
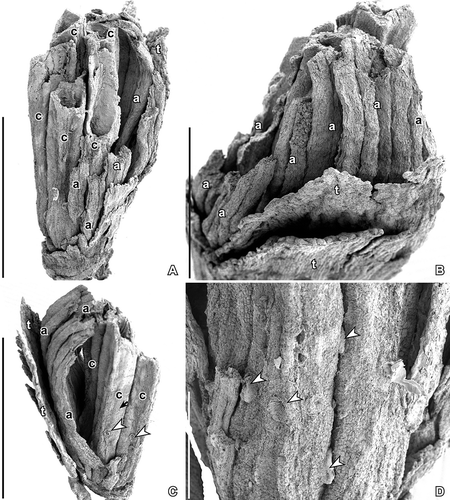
Figure 2. SEM images (A, B) and SRXTM surface renderings (C, D) of Kenilanthus marylandensis gen. et sp. nov., details of the flower from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A. Broken carpel revealing the locule and two placental flanges (pl) extending along the ventral suture; immature ovules (o) are borne along each placental flange; note abundant stomata (arrows) on surface of adjecent carpel. B. Broken stamen showing cross-section of anther with two thecae, each with two pollen sacs (ps); note the connective (co) positioned toward the inside of the flower and the broad broken tepal (t) toward the outside of the flower; note striation on surface of tepals formed by the bulging elongated cells. C. Outer surface of carpels (c) with abundant stomata (arrows). D. Basal part of flower showing one broad tepal (t) and possible scar or scars below. Scale bars – 100 µm.
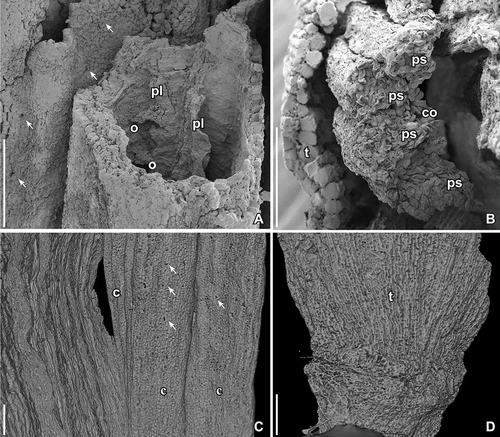
Figure 3. SRXTM volume renderings of Kenilanthus marylandensis gen. et sp. nov., sections of the flower from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A–C. Oblique transverse views of flower at different levels from middle part of flower towards base (A, cut at orthoslice xy1121; B, cut at orthoslice xy1827; C, cut at orthoslice xy2097) showing transverse sections of broad tepals (t), anthers (a) and carpels (c); eight filaments are seen in transverse section in the basalmost section (C, yellow arrows); based on the position of the eight stamens, the likely positions of the two missing stamens are indicated (red arrows). Note different dimensions of stamens at different levels; (A) shows a transverse section through the middle part of anthers; (B) shows a transverse section of anthers near the point of attachment to the filaments; (C) shows transverse sections of filaments below the point of filament-anther attachment. D. Longitudinal section of flower (orthoslices xz490–510) showing dome-shaped receptacle with attachment of tepals (t) below the elongated stamens (st), and two of the five free carpels in the centre (c); note broad, oblique bases of the carpels. E. Inner surface of androecium showing short filament (black arrow) and base of the two thecae of the corresponding anther (white arrows). Scale bars – 500 µm (A–D), 250 µm (E).
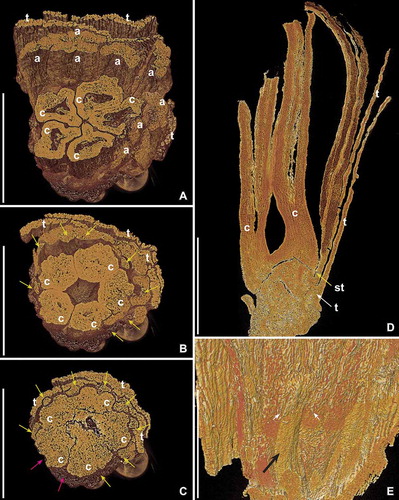
Figure 4. SRXTM reconstructions (orthoslices) of Kenilanthus marylandensis gen. et sp. nov., sections of the flower from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A–D. Transverse section of flower from about the middle (A, B) to base of stamens and carpels (C) and floral base (D) showing remains of tepals (t), stamens (anthers [a], filaments [arrows]) and carpels (c); note the two placental flanges visible inside the carpels in (A) and (B) (A, orthoslice xy1365; B, orthoslice xy1400; C, orthoslice xy1850; D, orthoslice xy2150). Perianth and stamens coloured in (B) and (D); one perianth part green and two perianth parts blue, connective tissue and filaments in orange, pollen sacs in yellow. Scale bar – 500 µm (A), for all figures.
![Figure 4. SRXTM reconstructions (orthoslices) of Kenilanthus marylandensis gen. et sp. nov., sections of the flower from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A–D. Transverse section of flower from about the middle (A, B) to base of stamens and carpels (C) and floral base (D) showing remains of tepals (t), stamens (anthers [a], filaments [arrows]) and carpels (c); note the two placental flanges visible inside the carpels in (A) and (B) (A, orthoslice xy1365; B, orthoslice xy1400; C, orthoslice xy1850; D, orthoslice xy2150). Perianth and stamens coloured in (B) and (D); one perianth part green and two perianth parts blue, connective tissue and filaments in orange, pollen sacs in yellow. Scale bar – 500 µm (A), for all figures.](/cms/asset/803e77b1-a141-4016-b9ae-6a9c2d1d55df/sgra_a_1158863_f0004_c.jpg)
Figure 5. SEM images of Kenilanthus marylandensis gen. et sp. nov., in situ pollen in anthers from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A. Longitudinal view of broken pollen sac showing many pollen grains and abundant orbicules lining the inner side of the theca wall. B. Pollen grains and densely packed orbicules inside an anther. C–E. Pollen grains in different views showing the colpi (two of the three on each grain in [C, D]), graded reticulum, and sculptured colpus membrane (on the single colpus seen in [E]). F. Detail of pollen and orbicules showing the segmented, spiny muri, the columellae supporting the muri and irregular, spheroidal orbicules. Scale bars – 25 µm (A), 20 µm (B), 5 µm (C–E), 2 µm (F).
![Figure 5. SEM images of Kenilanthus marylandensis gen. et sp. nov., in situ pollen in anthers from the Early Cretaceous (early–middle Albian) Kenilworth locality, Maryland, USA; holotype and only specimen (PP54087; sample Kenilworth 174). A. Longitudinal view of broken pollen sac showing many pollen grains and abundant orbicules lining the inner side of the theca wall. B. Pollen grains and densely packed orbicules inside an anther. C–E. Pollen grains in different views showing the colpi (two of the three on each grain in [C, D]), graded reticulum, and sculptured colpus membrane (on the single colpus seen in [E]). F. Detail of pollen and orbicules showing the segmented, spiny muri, the columellae supporting the muri and irregular, spheroidal orbicules. Scale bars – 25 µm (A), 20 µm (B), 5 µm (C–E), 2 µm (F).](/cms/asset/282ffe93-c864-47e0-95f4-30a3f4fac399/sgra_a_1158863_f0005_b.gif)
Derivation of specific epithet
From the State of Maryland, USA, where the fossil was recovered.
Specific diagnosis
As for the genus.
Holotype designated here
PP54087 (sample Kenilworth 174), figured in –.
Type locality
Kenilworth (Bladensburg), west facing exposure overlooking Washington, DC, Prince Georges County, Maryland, USA (38° 55ʹ 53ʺ N; 76° 55ʹ 45ʺ W).
Type horizon and age
Early Cretaceous (Patapsco Formation; basal part of pollen subzone II-B; early-middle Albian).
Description
The material includes a single fragmentary flower with the floral parts abraded on one side as well as in the apical region (–). No pedicel is preserved and it is uncertain how the flower was borne on the plant. The small size of the ovules and the undehisced pollen sacs indicate that the flower is preserved in a pre-anthetic or early anthetic stage. The floral orgnisation is summarised in .
Figure 6. Floral diagram of Kenilanthus marylandensis. Note that the exact position of the one abraded tepal is uncertain. It is also uncertain whether the outer whorl of stamens alternates with the tepals as indicated here, or is opposite to them.

The flower has both stamens and carpels. Pollen grains in the stamens are fully developed as apparently also the carpels. Therefore, we interpret the flower as bisexual, although functional unisexuality (functionally male) cannot be ruled out. The flower is radially symmetrical, pentamerous and isomerous with an apocarpous gynoecium. Floral parts are inserted on a dome-shaped receptacle. The perianth consists of a single whorl of broad and thin tepals with overlapping margins (, , , –). Indistinct scars outside the tepals () indicate that there were bracts or a further perianth whorl outside the five clearly visible tepals, but the preservation is too poor to be definitive. Therefore, it is uncertain whether the perianth consisted of one or two whorls of tepals. Aestivation of the preserved whorl of tepals is quincuncial. Each tepal is broad where it is attached to the receptacle and has fine longitudinal striations on the internal and external surfaces (, ). Two of the tepals are almost complete while two have only the basal most parts preserved. The fifth tepal is completely abraded, but its former presence is inferred from the position of the other four, and it is clear that originally there were five tepals in the whorl. There are no other perianth scars or floral parts between the tepals and the stamens ().
The remains of eight stamens are preserved in the most complete part of the flower (–, –) and their position shows that the androecium was originally comprised of ten stamens. Five stamens are opposite the tepals (and also the carpels) and five stamens alternate with the tepals (and also the carpels). Whether the outer or inner whorl of stamens alternates with the tepals is difficult to establish given the slightly compressed nature of the specimen and the relatively mature stage at which it is preserved.
Stamens are elongate, about 1.5 mm long. Anthers are very long (–, –) and are attached basally to a relatively short, stout filament (, ). Because the fossil is probably preserved in a pre-anthetic or early anthetic stage, the filaments may not be fully elongated. Filaments are free from each other and are triangular to elliptical in transverse section at the base (, , ). Anthers are dithecate and tetrasporangiate, and arc slightly inwards toward the centre of the flower (, , ). The two pairs of pollen sacs that comprise the two thecae are borne laterally on a thick and broad connective (, , , ). The pollen sacs protrude and are oriented towards the outside of the flower. Dehiscence is unknown, but was probably extrorse by longitudinal slits.
Pollen grains are minute, 9.0–11.7 µm in diameter, and abundant in the pollen sacs (, –). Pollen is tricolpate and more or less spheroidal (), although the grains are typically folded inwards along the colpi resulting in a more prolate shape (–). Pollen ornamentation is reticulate with densely spaced, sturdy columellae. Colpi are short, about 6.5 µm long, with the colpus membrane covered by finely microrugulate elements (). The reticulum is heterobrochate and graded with smaller lumina concentrated in a zone around the colpi and in the polar regions (–). Larger lumina are concentrated in the intercolpal regions. Muri are segmented with the segments oriented obliquely and sometimes ending in a small spinule resulting in spinulate supratectal ornamentation (). Orbicules are abundant on the inside of the theca wall (–) and are indicative of a secretory tapetum. They are tiny, spherical, about 0.75 µm in diameter and have an irregular segmented surface ().
There are five free carpels (, , , –, –) arranged in a ring around a space at the apex of the receptacle. Near the base of the carpels the ventral faces do not meet, but at this level their lateral surfaces closely adhere (, ). Above, the carpels converge so that both their ventral and lateral surfaces are in close contact (, , ). None of the carpels have the apex preserved and there are no remains of stigma or style. Carpels are sessile, elongated and narrow, about 1.5 mm long and 0.25 mm wide with a broad, oblique base (). They are almost the same width for the full length that is preserved. Comparison with extant angiosperms suggests that the carpels are plicate. In each carpel numerous ovules are borne on two linear, slightly raised, placentae that extend for almost the whole preserved length of the carpels on either side of the ventral suture (, , ). Ovules are tiny and do not fill out the ovary cavity. They appear campylotropous or anatropous, but insufficient details are preserved for definitive characterisation.
The carpel wall is about 35 µm thick along the lateral flanks, but thicker along the ventral suture and dorsal line. The outer and inner surfaces of the carpels are smooth. The outer epidermis is composed of tiny, equiaxial cells and abundant stomata (, ). There are also scattered, rounded, flattened structures that are probably glandular trichomes near the bases of the carpels ().
Discussion
Comparison of Kenilanthus marylandensis with flowers of other Early Cretaceous eudicots
Two other flowers known from the Early Cretaceous based on lignitic mesofossils share many features with Kenilanthus. Both are from the Lusitanian Basin of western Portugal: Teixeiraea lusitanica von Balthazar, K.R.Pedersen et E.M.Friis (2005) from the Vale de Água locality (late Aptian–early Albian) and Kajanthus lusitanicus Mendes, G.W.Grimm, Pais et E.M.Friis (2014) from the Chicalhão locality (late Aptian–early Albian). Like Kenilanthus, both are known only from a single flower with tricolpate in situ pollen. Teixeiraea was interpreted as an extinct lineage, on the stem lineage or within the crown group of Ranunculales. Kajanthus was assigned to the ranunculalean family Lardizabalaceae (Mendes et al. Citation2014).
Teixeiraea is similar to Kenilanthus in having very long anthers with strongly protruding thecae. Each anther is borne laterally on a broad connective that is attached to a short sturdy filament. However, Teixeiraea has multiple parts and spiral phyllotaxis and is apparently a unisexual flower. The corresponding pistillate structures are not known. Teixeiraea also differs from Kenilanthus in having the protruding thecae oriented towards the inside of the flower, rather than outwards, and in having foveolate, rather than reticulate pollen grains.
Kajanthus is similar to Kenilanthus in having staminate and pistillate organs in the same flower, thecae orientated outwards, an apocarpous gynoecium and plicate carpels with many ovules borne in two rows along the ventral suture. However, Kajanthus is trimerous rather than pentamerous. The anthers are also shorter than in Kenilanthus and the pollen of Kajanthus is foveolate rather than reticulate.
Comparison of the Kenilanthus pollen with pollen from dispersed palynological assemblages from the Early Cretaceous
We have not been able to match the Kenilanthus pollen grains to any dispersed pollen species formally described from the Early Cretaceous Potomac Group. Grains assigned to the dispersed pollen genus Rousea are tricolpate and foveolate or reticulate with a graded reticulum comparable to that of the Kenilanthus pollen. According to J.A. Doyle (personal communication, January 2016), Rousea prosimilis (Norris) Srivastava is common in Zone II of the Potomac Group. However, according to the original descriptions, much dispersed Rousea pollen, including Rousea prosimilis, is typically much larger than the grains of Kenilanthus (Srivastava Citation1975). Further, these grains are known mainly from light microscopy and Rousea species figured using SEM have smooth muri (e.g. Srivastava Citation1981; Ward Citation1986) very different from the segmented muri of Kenilanthus pollen. Grains from the Potomac Group sediments assigned by Brenner (Citation1963) to Retitricolpites are also larger than the pollen of Kenilanthus.
Systematic assessment
Tricolpate pollen preserved in the anthers unequivocally places Kenilanthus among the eudicots and its presumed plesiomorphic floral features (see later) strongly suggest a phylogenetic position close to extant taxa that diverged near the base of the eudicot clade. The basal grade of early-diverging eudicots includes Ranuculales, which are often resolved as the sister group to all other eudicots, as well as the Proteales (Nelumbonaceae, Platanaceae, Proteaceae, Sabiaceae), Trochodendrales (Trochodendraceae) and Buxales (Buxaceae sensu lato), which are successively closer outgroups to the core eudicots (Stevens Citation2001 onwards). While Kenilanthus is similar to flowers in some core eudicots, especially some Saxifragales (e.g. Crassulaceae, Paeoniaceae), in its regularly pentamerous, isomerous organisation, and apocarpous gynoecium, the combination of presumed plesiomorphic features seen in Kenilanthus, especially of the stamens, pollen and carpels, makes a relationship within core eudicots less likely.
Among basal eudicots, the precise phylogenetic position of Kenilanthus is difficult to resolve. Analysing the phylogenetic position of Kenilanthus using the morphological data sets of Doyle and Endress (Citation2010) and Wang et al. (Citation2009b) for early-diverging lineages of eudicots and extant Ranunculales failed to provide a clear or convincing phylogenetic signal. Using the Wang et al.-matrix, Kenilanthus was resolved in three alternative equally parsimonious positions; near the base of the Ranunculales above Euptelea, as sister to Papaveraceae, or between Papaveraceae and the Lardizabalaceae-Circaeasteraceae clade. Placing Kenilanthus within any extant family of Ranunculales or outside the order, resulted in less parsimonious trees, although a position as sister to Paeonia was only one step longer. We also examined possible relationships of Kenilanthus using the morphological dataset of Hermsen et al. (Citation2006) for Saxifragales and other basal rosids, but again the results were equivocal. The weak phylogenetic signal provided by these formal phylogenetic analyses highlights the need for more comprehensive matrices that include thorough consideration of potential homologies for the flowers of a greater range of extant and fossil taxa.
Sexual system
The flower of Kenilanthus has pistillate and staminate organs in the same flower. Pollen grains in situ are fully developed and although the ovules are tiny the carpels also appear fully formed. We therefore interpret the flower as bisexual, but the possibility that the flower of Kenilanthus was functionally unisexual (male because of its fully developed pollen) cannot be completely ruled out. In some Lardizabalaceae (Sinofranchetia and Decaisnea), flowers appear bisexual, but are functionally unisexual (Zhang et al. Citation2005; Wang et al. Citation2009a). In Ranunculales, bisexual flowers appear plesiomorphic in most families, although unisexual flowers occur scattered through the order as in Lardizabalaceae and Menispermaceae. Among basal grade eudicots outside Ranuculales, unisexual flowers are more common, and occur in both Buxaceae and Platanaceae. In extant Platanaceae, staminate flowers have vestiges of the carpels (Von Balthazar & Schönenberger Citation2009).
Phyllotaxis
Whorled floral phyllotaxis, as in Kenilanthus, is widespread among eudicot angiosperms as a whole and predominates among basal grade eudicots (Endress Citation2010, Citation2011). Exceptions occur in Circaeasteraceae and certain core Ranunculaceae that have spiral phyllotaxis of both perianth parts and stamens. A few other basal grade eudicots (e.g. Nelumbo) have spiral phyllotaxis of either perianth parts or stamens (Endress & Doyle Citation2007; Doyle & Endress Citation2010). Phyllotaxis of the carpels is also mainly whorled in the Ranunculales, although in Ranunculaceae and a few other ranunculalean taxa carpels are spiral or irregularly arranged (Endress & Igersheim Citation1999).
Merism
Pentamerous flowers predominate among core eudicots, but are relatively rare among Ranunculales and other basal grade eudicots. Pentamery, in at least the perianth, does, however, occur in many Ranunculaceae, Circaeasteraceae (Kingdonia) and some Berberidaceae, Menispermaceae, Sabiaceae and Buxaceae. Most other basal eudicots have trimerous or dimerous flowers (Kubitzki Citation1987; Drinnan et al. Citation1991, Citation1994; Wang et al. Citation2009b; Endress Citation2010, Citation2011), but variable patterns of merism may also occur at this level of angiosperm evolution. For example, most flowers in Berberidaceae are trimerous, but in Berberis vulgaris L., terminal flowers are often pentamerous (Kubitzki Citation1987). Flowers of extant Platanaceae are trimerous or tetramerous (Von Balthazar & Schönenberger Citation2009; Endress Citation2010), but pentamerous flowers are widespread among related Cretaceous and Early Cenozoic platanoids (Manchester Citation1986; Friis et al. Citation1988, Citation2011).
Flowers with equal number of parts in all floral whorls, as seen in Kenilanthus, are not common among basal eudicots, but do occur in some groups such as Platanaceae. Isomerous flowers are more common among core eudicots and occur for example in Crassulaceae (Saxifragales) and some rosids (e.g. Matthews & Endress Citation2002).
Perianth
The number of perianth whorls in Kenilanthus cannot be established with certainty. There is clearly one whorl of sepal-like perianth parts each with a very thin lamina and broad base. Faint scars outside this whorl indicate that the fossil flower may have had two perianth whorls. In Ranunculales, and many other eudicots, especially core eudicots, flowers typically have both sepals and petals (Endress Citation2010). The occurrence of a single whorl in certain lineages is generally thought to reflect secondary loss (Endress Citation2010). Among the taxa included in the ranuculalean matrices of Doyle and Endress (Citation2010), a single perianth whorl occurs only in Hydrastis and Circaeaster (Doyle & Endress Citation2010), as well as scattered in other taxa that occupy more derived phyogenetic positions, while Euptelea lacks a perianth completely. However, in the grade of basal eudicots above the level, at which Ranunculales diverged (Proteales, Trochodendrales, Buxales), perianth parts are predominantly sepaloid and there is variation in whether there are one or two perianth whorls.
Androecium
The two whorls of free stamens seen in Kenilanthus are comparable to the stamen whorls in Lardizabalaceae and several other families of Ranunculales, as well as many other eudicots. Ten or fewer stamens were suggested by Damerval and Nadot (Citation2007) as the plesiomorphic state in Ranunculales and Doyle and Endress (Citation2011) suggest that the common ancestor of the eudicots had two whorls of stamens. Flowers of Ranunculaceae typically have many stamens, and higher stamen numbers also occur in other ranuculalean families, such as Eupteleaceae and Papaveraceae. Among core eudicots, two stamen whorls are characteristic of many rosids, while a single whorl is more typical of core asterids (Endress Citation2010).
Stamens with a sturdy filament and basifixed anthers, as in Kenilanthus, are characteristic of many extant basal grade eudicots (Endress & Hufford Citation1989; Hufford & Endress Citation1989), as well as some early-diverging rosids such as Hamamelidaceae and Cercidiphyllaceae (Hufford & Endress Citation1989; Endress & Stumpf Citation1991). Long anthers with longitudinal dehiscence also occur in many basal eudicots including Euptelea, many Ranunculaceae, some Berberidaceae (e.g. Nandina, Podophyllum) and Papaveraceae (Sanguinaria). In some of these extant taxa the filaments extend considerably at anthesis. This may also have been the case in Kenilanthus. The stamens of most core eudicots differ from those of Kenilanthus in having filaments that are thin and attached to the anthers in only a small area of attachment, often on the dorsal side. Extrorse dehiscence, as inferred for Kenilanthus, is rare among basal grade eudicots, but does occur in Papaveraceae and Lardizabalaceae. In Lardizabalaceae, anthers are curved toward the centre of the flower and have protruding pollen sacs, all features that are similar to Kenilanthus.
Anthers with a broad connective between the thecae, as in Kenilanthus, occur in several families of Ranunculales, such as Berberidaceae (Berberis, Mahonia, Caulophyllum, Epimedium, Jeffersonia, Podophyllum), Papaveraceae (Sanguinaria) and Ranunculaceae (Ranunculus, Trautvetteria) (Endress & Hufford Citation1989). In most ranunculalean taxa, anther dehiscence is by simple longitudinal slits as inferred for Kenilanthus, although in most Berberidaceae anther dehiscence is valvate. Similar broad connectives are not common among more derived eudicots.
Pollen
Tricolpate pollen predominates among basal grade eudicots, while tricolporate pollen mainly occurs among core eudicots, including Paeonia and certain Crassulaceae. Pollen of Lardizabalaceae and Ranunculaceae is typically tectate-foveolate, tectate-spinulate or sometimes tectate-striate, and thus differs from the reticulate pollen of Kenilanthus. Reticulate pollen does occur in other families of Ranunculales, including Berberidaceae (Nowicke & Skvarla Citation1981) and Menispermaceae (Ferguson Citation1975). The tricolpate pollen of Podophyllum (Berberidaceae) is particularly similar to that of Kenilanthus in having a reticulate tectum with coarsely and obliquely segmented muri with spinulose ornamentation. Reticulate pollen is widespread among Platanaceae and certain other early-diverging lineages of non-ranunculid eudicots, but in these taxa the muri are typically smooth without the ridges seen in Kenilanthus. Based on geological-stratigraphic appearance as well as phylogenetic analyses (Doyle Citation2005), reticulate pollen, or variants with striate ornamentation, seems most likely to be basic for eudicots as a whole.
Gynoecium
Free carpels are common among Ranunculales, and completely, or largely, plicate carpels occur in Lardizabalaceae, Ranunculaceae and Platanaceae (Endress & Igersheim Citation1999). Elongate marginal placentae, with many ovules that do not fill the ovary cavity as in Kenilanthus, also occur in Lardizabalaceae and some Ranunculaceae. Carpels of Platanaceae are distinct from those of Kenilanthus in having only one to two ovules, a feature that was established early in the history of the group (Friis et al. Citation1988; Crane et al. Citation1993; Pedersen et al. Citation1994). Apocarpous gynoecia also occur in several groups of core eudicots, such as Paeonia (Saxifragales) and Crassulaceae. The carpel surface of Kenilanthus has scattered stomata, which is typical for most members of Ranunculales and Proteales, but stomata are generally lacking in Saxifragales (Endress & Igersheim Citation1999).
Summary
The systematic relationships of Kenilanthus cannot be conclusively established. The fossil flower appears to possess a constellation of features that are plesiomorphic among eudicots as a whole, such as sepaloid perianth parts, two whorls of stamens, stamens that are basifixed with a stout connective, tricolpate reticulate pollen and apocarpous, plicate carpels with many ovules on marginal placentae and stomata on the outer surface of the carpels. The combined features indicate a position close to the base of the eudicots although a position either in, or close to, core eudicots cannot be ruled out.
Several features of Kenilanthus are unusual among basal eudicots and especially intriguing is the unusual ornamentation of Kenilanthus pollen, which is particularly similar to that of Podophyllum (Berberidaceae). Flowers of Podophyllum are very different from Kenilanthus, but if found isolated the pollen might easily have been assigned to the extant genus. Also uncommon in the context of basal eudicots is the pentamerous and isomerous arrangement of the floral parts and perhaps the presence of a single perianth whorl. Although these features do occur among basal eudicots they could be interpreted as pointing to a relationship with certain taxa among core eudicots. However, the occurrence of these features is scattered, and a close relationship to core eudicots is contradicted by other features. For example, while isomerous, pentamerous flowers with an apocarpous gynoecium occur in Crassulaceae (Saxifragales), they differ from those of Kenilanthus in their small anthers and thin filaments, as well as their tricolporate pollen. Basifixed anthers occur in Cercidiphyllaceae, Hamamelidaceae and other Saxifragales, but the gynoecia in these groups differ from that of Kenilanthus. Tricolpate reticulate pollen with graded tectum and segmented muri is also not reported for any of these groups. Similarly, while flowers of Paeonia (Saxifragales) also have apocarpous gynoecia, they are distinguished from Kenilanthus in their spiral floral phyllotaxis, polystemonous androecium and tricolporate pollen. If currently accepted patterns of relationships among extant angiosperms are correct, positioning Kenilanthus among core eudicots would likely result in considerable homoplasy.
Conclusion
A phylogenetic position of Kenilanthus close to the base of the eudicot tree is consistent with the relationships inferred for all other Early Cretaceous floral structures that have tricolpate pollen within the anthers or on stigmatic surfaces. Two of these early eudicot flowers have been assigned to Ranuculales: Kajanthus, which can be placed in the extant family Lardizabalaceae as it is currently circumscribed (Mendes et al. Citation2014), and Teixeiraea, which may represent an extinct lineage within the order. Other Early Cretaceous eudicots, have been assigned to Buxales (Lusicarpus, Lusistemon, Silucarpus, Valecarpus, Aguacarpus and Spanomera) and Proteales (e.g. platanoids such as Friisicarpus, Aquia, Hamatia), or they possess a mosaic of plesiomorphic features found in several separate lineages (e.g. Sinocarpus, Hyrcantha, Ternariocarpites and other impression fossil summarised in Friis et al. Citation2011). Kenilanthus fits this pattern, in showing features that point to a relationship to Lardizabalaceae and other Ranunculales, or perhaps core eudicots. Fossil leaves of Fairlingtonia thyrsopteroides (Fontaine) Jud, described from Early Cretaceous localities in the Potomac Group that are of similar or somewhat older age to the Kenilworth site (Jud Citation2015), show a mosaic of features found in extant Papaveraceae and Proteaceae. Unequivocal fossil flowers of core eudicots have not yet been recorded from the Early Cretaceous, the earliest such flower currently known is the Rose Creek flower, which dates from around the Early-Late Cretaceous boundary (Basinger & Dilcher Citation1984).
Acknowledgements
The authors thank Marco Stampanoni and Federica Marone, Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland, and Anna Lindström, NRM, Stockholm, Sweden, for help with the SRXTM analyses performed at the Swiss Light Source, Paul Scherrer Institute, Villigen, Switzerland. The authors also thank J.A. Doyle for valuable comments on the paper.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Basinger JF, Dilcher DL. 1984. Ancient bisexual flowers. Science 224: 511–513. doi:10.1126/science.224.4648.511.
- Brenner GJ. 1963. The spores and pollen of the Potomac Group of Maryland. Maryland Department of Geology, Mines and Water Resources, Bulletin 27: 1–215.
- Crane PR, Pedersen KR, Friis EM, Drinnan AN. 1993. Early Cretaceous (early to middle Albian) platanoid inflorescences associated with Sapindopsis leaves from the Potomac Group of eastern North America. Systematic Botany 18: 328–344. doi:10.2307/2419407.
- Damerval C, Nadot S. 2007. Evolution of perianth and stamen characteristics with respect to floral symmetry in Ranunculales. Annals of Botany 100: 631–640.
- Doyle JA. 1992. Revised palynological correlations of the lower Potomac Group (USA) and the Cocobeach sequence of Gabon (Barremian–Aptian). Cretaceous Research 13: 337–349. doi:10.1016/0195-6671(92)90039-S.
- Doyle JA. 2005. Early evolution of angiosperm pollen as inferred from molecular and morphological phylogenetic analyses. Grana 44: 227–251. doi:10.1080/00173130500424557.
- Doyle JA, Endress PK. 2010. Integrating Early Cretaceous fossils into the phylogeny of living angiosperms: Magnoliidae and eudicots. Journal of Systematics and Evolution 48: 1–35. doi:10.1111/jse.2010.48.issue-1.
- Doyle JA, Endress PK. 2011. Tracing the early evolutionary diversification of the angiosperm flower. In: Wanntorp L, Ronse De Craene LP, eds. Flowers on the tree of life, 88–119. Cambridge: Cambridge University Press.
- Drinnan AN, Crane PR, Hoot SB. 1994. Patterns of floral evolution in the early diversification of non-magnoliid dicotyledons (eudicots). Plant Systematics and Evolution [Suppl.] 8: 93–122.
- Drinnan AN, Crane PR, Pedersen KR, Friis EM. 1991. Angiosperm flowers and tricolpate pollen of buxaceous affinity from the Potomac Group (mid-Cretaceous) of eastern North America. American Journal of Botany 78: 153–176. doi:10.2307/2445239.
- Endress PK. 2010. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden 97: 541–583. doi:10.3417/2009139.
- Endress PK. 2011. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany 98: 370–396. doi:10.3732/ajb.1000299.
- Endress PK, Doyle JA. 2007. Floral phyllotaxis in basal angiosperms: Development and evolution. Current Opinion in Plant Biology 10: 52–57. doi:10.1016/j.pbi.2006.11.007.
- Endress PK, Hufford LD. 1989. The diversity of stamen structures and dehiscence patterns among Magnoliidae. Botanical Journal of the Linnean Society 100: 45–85. doi:10.1111/boj.1989.100.issue-1.
- Endress PK, Igersheim A. 1999. Gynoecium diversity and systematics of the basal eudicots. Botanical Journal of the Linnean Society 130: 305–393. doi:10.1111/boj.1999.130.issue-4.
- Endress PK, Stumpf S. 1991. The diversity of stamen structures in ‘Lower’ Rosidae (Rosales, Fabales, Proteales, Sapindales). Botanical Journal of the Linnean Society 107: 217–293. doi:10.1111/boj.1991.107.issue-3.
- Ferguson IK. 1975. Pollen morphology of the tribe Triclisieae of the Menispermaceae in relation to its taxonomy. Kew Bulletins 30: 49–75. doi:10.2307/4102875.
- Friis EM, Crane PR, Pedersen KR. 1988. Reproductive structures of Cretaceous Platanaceae. Biologiske Skrifter, Det Kongelige Danske Videnskabernes Selskab 31: 1–55.
- Friis EM, Crane PR, Pedersen KR. 1997. Anacostia, a new basal angiosperm from the Early Cretaceous of North America and Portugal with trichotomocolpate/monocolpate pollen. Grana 36: 225–244. doi:10.1080/00173139709362611.
- Friis EM, Crane PR, Pedersen KR. 2011. Early flowers and angiosperm evolution. Cambridge: Cambridge University Press.
- Friis EM, Crane PR, Pedersen KR, Stampanoni M, Marone F. 2015. Exceptional preservation of tiny embryos documents seed dormancy in early angiosperms. Nature 528: 551–554. doi:10.1038/nature16441.
- Friis EM, Marone F, Pedersen KR, Crane PR, Stampanoni M. 2014a. Three-dimensional visualization of fossil flowers, fruits, seeds and other plant remains using synchrotron radiation X-ray tomographic microscopy (SRXTM): New insights into Cretaceous plant diversity. Journal of Paleontology 88: 684–701. doi:10.1666/13-099.
- Friis EM, Pedersen KR, Crane PR. 2010a. Cretaceous diversification of angiosperms in the western part of the Iberian Peninsula. Review of Palaeobotany and Palynology 162: 341–361. doi:10.1016/j.revpalbo.2009.11.009.
- Friis EM, Pedersen KR, Crane PR. 2010b. Diversity in obscurity: Fossil flowers and the early history of angiosperms. Philosophical Transactions of the Royal Society B: Biological Sciences 365: 369–382. doi:10.1098/rstb.2009.0227.
- Friis EM, Pedersen KR, Crane PR. 2014b. Welwitschioid diversity in the Early Cretaceous: Evidence from fossil seeds with pollen from Portugal and eastern North America. Grana 53: 175–196. doi:10.1080/00173134.2014.915980.
- Furness CA, Magallón S, Rudall PJ. 2007. Evolution of endoapertures in early-divergent eudicots, with particular reference to pollen morphology in Sabiaceae. Plant Systematics and Evolution 263: 77–92. doi:10.1007/s00606-006-0477-y.
- Hermsen EJ, Nixon KC, Crepet WL. 2006. The impact of extinct taxa on understanding the early evolution of angiosperm clades: An example incorporating fossil reproductive structures of Saxifragales. Plant Systematics and Evolution 260: 141–169.
- Hintermüller C, Marone F, Isenegger A, Stampanoni M. 2010. Image processing pipeline for synchrotron-radiation-based tomographic microscopy. Journal of Synchrotron Radiation 17: 550–559. doi:10.1107/S0909049510011830.
- Hochuli PA, Heimhofer U, Weissert H. 2006. Timing of early angiosperm radiation: Recalibrating the classical succession. Journal of the Geological Society 163: 587–594. doi:10.1144/0016-764905-135.
- Hufford LD, Endress PK. 1989. The diversity of anther structures and dehiscence patterns among Hamamelididae. Botanical Journal of the Linnean Society 99: 301–346. doi:10.1111/boj.1989.99.issue-4.
- Hughes NF. 1994. The enigma of angiosperm origins. Cambridge: Cambridge University Press.
- Jud NA. 2015. Fossil evidence for an herbaceous diversification of early eudicot angiosperms during the Early Cretaceous. Proceedings B 282, in press. doi:10.1098/rspb.2015.1045.
- Kubitzki K. 1987. Origin and significance of trimerous flowers. Taxon 36: 21–28. doi:10.2307/1221346.
- Maddison WP, Maddison DR. 2011. Mesquite: A modular system for evolutionary analysis. Version 2.75; http://mesquiteproject.org.
- Manchester SR. 1986. Vegetative and reproductive morphology of an extinct plane tree (Platanaceae) from the Eocene of Western North America. Botanical Gazette 147: 200–226. doi:10.1086/337587.
- Matthews ML, Endress PK. 2002. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae). Botanical Journal of the Linnean Society 140: 321–381. doi:10.1046/j.1095-8339.2002.00105.x.
- Mendes MM, Grimm GW, Pais J, Friis EM. 2014. Fossil Kajanthus lusitanicus gen. et sp. nov. from Portugal: Floral evidence for Early Cretaceous Lardizabalaceae (Ranunculales, basal eudicot). Grana 53: 283–301. doi:10.1080/00173134.2014.932431.
- Nowicke JW, Skvarla JJ. 1981. Pollen morphology and phylogenetic relationships of the Berberidaceae. Smithsonian Contributions to Botany 50: 1–83. doi:10.5479/si.0081024X.50.
- Pedersen KR, Friis EM, Crane PR, Drinnan AN. 1994. Reproductive structures of an extinct platanoid from the Early Cretaceous (latest Albian) of eastern North America. Review of Palaeobotany and Palynology 80: 291–303. doi:10.1016/0034-6667(94)90006-X.
- Penny JHJ. 1988. Early Cretaceous striate tricolpate pollen from the Borehole Mersa Matruh 1, North West Desert, Egypt. Journal of Micropalaeontology 7: 201–215. doi:10.1144/jm.7.2.201.
- Penny JHJ. 1991. Early Cretaceous angiosperm pollen from the borehole Mersa Matruh 1, North West Desert, Egypt. Palaeontographica B 222: 31–88.
- Srivastava SK. 1975. Microspores from the Fredericksburg Group (Albian) of the Southern United States. Paléobiologie continentale 62: 1–119.
- Srivastava SK. 1981. Stratigraphic ranges of selected spores and pollen from the Fredericksburg group (Albian) of the southern United States. Palynology 5: 1–27. doi:10.1080/01916122.1981.9989215.
- Stampanoni M, Groso A, Isenegger A, Mikuljan G, Chen Q, Bertrand A, Henein S, Betemps R, Frommherz U, Bohler P, Meister D, Lange M, Abela R. 2006. Trends in synchrotron-based tomographic imaging: The SLS experience. In: Bonse U, ed. Developments in X-Ray tomography V. Proceedings of SPIE 6318, San Diego.
- Stevens PF. 2001 onwards. Angiosperm Phylogeny Website. Version 12, July 2012. “https://protect-us.mimecast.com/s/Md69B0u04W5gsz” http://www.mobot.org/MOBOT/research/APweb/; accessed October 2015.
- Von Balthazar M, Pedersen KR, Friis EM. 2005. Teixeiraea lusitanica, a new fossil flower from the Early Cretaceous of Portugal with affinities to Ranunculales. Plant Systematics and Evolution 255: 55–75. doi:10.1007/s00606-005-0347-z.
- Von Balthazar M, Schönenberger J. 2009. Floral structure and organization in Platanaceae. International Journal of Plant Sciences 170: 210–225. doi:10.1086/595288.
- Wang H-F, Ross Friedman C, Zhu Z-X, Qin H-N. 2009a. Early reproductive developmental anatomy in Decaisnea (Lardizabalaceae) and its systematic implications. Annals of Botany 104: 1243–1253. doi:10.1093/aob/mcp232.
- Wang W, Lu A-M, Ren Y, Endress ME, Che Z-D. 2009b. Phylogeny and classification of Ranunculales: Evidence from four molecular loci and morphological data. Perspectives in Plant Ecology, Evolution and Systematics 11: 81–110. doi:10.1016/j.ppees.2009.01.001.
- Ward JV. 1986. Early Cretaceous angiosperm pollen from the Cheyenne and Kiowa Formations (Albian) of Kansas, USA. Palaeontographica B 202: 1–81.
- Zhang X-H, Ren Y, Tian X-H, Pan L-Z. 2005. Anatomical studies on Sinofranchetia chinensis (Lardizabalaceae) and their systematic significance. Botanical Journal of the Linnean Society 149: 271–281. doi:10.1111/boj.2005.149.issue-3.
