Abstract
Preliminary results of the palynoflora from the Schaßbach clay pit comprise accessorial floral elements of three Cornales and five Ericales pollen taxa that have modern living equivalents from different continents. The eight taxa are relatively rare in the palynological assemblages and are affiliated with the genera Nyssa, Mastixia, Erica, Vitellariopsis, Rehderodendron and Polyspora. Nyssa sp. resembles closely the deciduous Nyssa sylvatica of temperate America, the two Erica spp. resemble the south European tree heathers Erica arborea and Erica lusitanica, and Vitellariopsis sp. looks like evergreen Vitellariopsis marginata from subtropical southern Africa. The living equivalents of Mastixia spp., Rehderodendron sp. and Polyspora sp. are evergreen taxa from subtropical and tropical Asia.
The Miocene sedimentary rocks of the Lavanttal Basin (Carinthia, Austria) are a mixture of freshwater and brackish to marine beds deposited between c. 17 to c. 11 myr: Deposition commenced during the early Miocene (Burdigalian) and terminated around the early late Miocene (early Tortonian; Beck-Managetta Citation1952; Reischenbacher & Sachsenhofer Citation2013). The sedimentary succession was interrupted in the upper part (middle Serravallian) and probably in the lower part (around 16 myr at the Burdigalian/lower Langhian boundary) by two unconformities (see Beck-Managetta Citation1952; Tollman Citation1985; Reischenbacher & Sachsenhofer Citation2013; Meller et al. Citation2015, ). The investigated sediments were collected in the Oberaigen district, situated at the western border of the central Lavanttal, from an open pit at Schaßbach (; see Meller et al. Citation2015 for comprehensive geological overview). This pit was on and off mined for clays and is now partly re-filled and naturalised. The mined sediments are interpreted to represent a part of the Langhian age Mühldorf Formation (Beck-Managetta Citation1952; Lippolt et al. Citation1975; Reischenbacher et al. Citation2007; Reischenbacher & Sachsenhofer Citation2013; Meller et al. Citation2015). Regular field campaigns with students from Vienna University since 2010 have yielded large numbers of plant fossils (mostly leaves, diaspores and twigs) and sediment samples for palynology from Schaßbach. The very rich macrofossil spectrum is dominated by leaves of Platanus, various Fagaceae (e.g. Fagus, Quercus) and Betulaceae (Alnus, Betula), Juglans, Ulmus, Zelkova, numerous entire margined leaves, Glyptostrobus twigs and cones and many more leaves and diaspores, that all await a thorough systematic treatment. The macrofossil diversity of this locality is similar to the more-or-less contemporaneous Schönweg locality in the lower Lavanttal (see Meller et al. Citation2015, table 1). Of the macrofossils so far studied, findings of Ginkgo adiantoides (Unger) Heer from Schaßbach have been published by Meller et al. (Citation2015). Previous palynological investigations of Lavanttal material concentrated on younger, Sarmatian (Serravallian) deposits including terrestrial sediments from plant fossil-bearing localities (Klaus Citation1955, Citation1984), and phosphorite nodules that yielded excellently preserved pollen and spores (e.g. Grímsson et al. Citation2011, Citation2015, Citation2016). Only four samples of the Schaßbach sediments collected for palynology had significant amounts of reasonably well-preserved pollen that comprise accessorial pollen from the orders Cornales and Ericales.
Figure 1. A. Stratigraphy and sediments of the investigated locality in the Lavanttal. Stratigraphic chart of the middle Miocene sediments in the Lavanttal (after Reischenbacher & Sachsenhofer Citation2013; Meller et al. Citation2015). B. Part of a small sedimentary profile of the lacustrine lower part of the Mühldorf Formation at the clay pit Schaßbach, district Oberaigen. The four pollen samples are indicated with an asterisk. C. Reinhard Zetter kneeling on the banded grey-green clay. D. Photograph of the of the banded grey-green clay and overlying calcareous silty sand and sand with detrital micas.
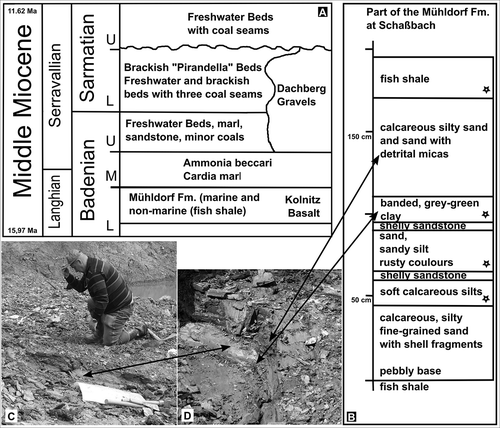
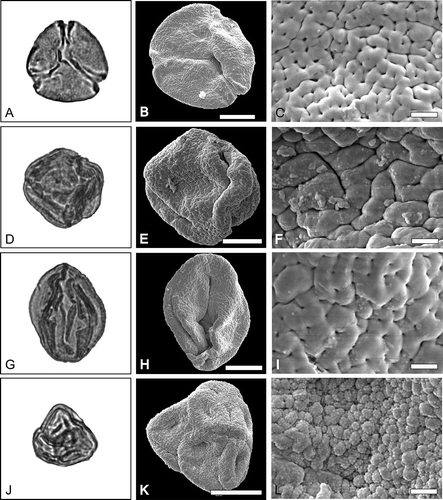
Figure 2. LM and SEM images of pollen of Cornales and Ericales. A–C. Nyssa sp. A. LM overview. B. SEM overview of the same grain as A. C. SEM detail of the same grain as A. D–F. Mastixia sp. 1. D. LM overview. E. SEM overview of the same grain as in D. F. SEM detail of the same grain as in D. G–I. Mastixia sp. 2. G. LM overview. H. SEM overview of the same grain as in G. I. SEM detail of the same grain as in G. J–L. Erica sp.1. J. LM overview. K. SEM overview of the same grain as in J. L. SEM detail of the same grain as in J. Scale bars – 10 µm (B, E, H, K), 2 µm (C, F, I, L).
Material and methods
The four investigated sediment samples from the Schaßbach clay pit came from a c. 120 cm thick horizon consisting of intercalations of plant fossil-bearing silty clays, medium-grey, barren clays, and plant fossil-bearing slightly sandy silts and clays, and organic rich papery clays (‘Papierschiefer’ containing mostly fish remains); the sample of the latter is generally poor in palynomorphs, and palynomorph preservation in all samples is not good (, pollen samples are indicated by an asterisk). Sample preparation followed standard wet chemical procedures (e.g. Klaus Citation1987): crushed samples were boiled in hydrochloric acid (HCl) until the carbonate part was dissolved and then treated with hydrofluoric acid (HF) to dissolve the silicates (cold processing over four days). The organic remains were again boiled in HCl for 5 min, decanted and washed several times with water, but not sieved, thus retaining palynomorphs < 10 µm in size, followed by acetolysis. The remaining residues were mixed with glycerine and stored in tightly closing glass vials. For light microscopy (LM) investigation, the pollen were isolated with a micro-manipulator from the sample smears on a glass slide into a clean drop of glycerine on a new slide and photographed with a Samsung digital camera. Afterwards, the same pollen were moved with a micro-manipulator to a scanning electron microscopy (SEM) stub and rinsed in a drop of 100% alcohol to remove the glycerine. The stubs were sputtered with gold and examined with a SEM (FEI InspectS 500). Stubs and photographs are stored in the Department of Palaeontology, University of Vienna, under inventory numbers IPUWSB7841/1/1-10 to IPUWSB7841/4/1-10.
Systematic palynology
The Schaßbach pollen assemblages are generally dominated by various taxa of the wind pollinated Pinaceae, Cupressaceae, Fagaceae, Juglandaceae, Ulmaceae and Platanaceae. In contrast, fern and moss spores are rare (seven taxa). The here presented accessorial elements of Cornales pollen comprise Nyssa sp. and two Mastixia spp. (Nyssaceae), whereas the Ericales pollen are slightly more diverse; two Erica spp. (Ericaceae), one Vitellariopsis sp. (Sapotaceae), one Rehderodendron sp. (Styracaceae), and one Polyspora sp. (Theaceae). These taxa represent only a small portion of the accessory elements of the pollen and spore flora (up to now c. 60 taxa) and occur in very small numbers (N = 1–3). In the following systematic treatment the classification follows Stevens (Citation2001, and onwards) and pollen terminology follows Hesse et al. (Citation2009).
Description of the pollen grains
Order Cornales Link
Family Nyssaceae Martius
Genus Nyssa Gronov. ex. L.
Nyssa sp. ()
Description LM
Tricolporate, prolate to subspheroidal pollen grains (N = 3), rhombic to elliptic in equatorial view or triangular to circular in polar view in compressed state (); polar axes from 26.7 to 37.7 µm, equatorial axes from 21.6 to 29.2 µm; colpi 16.3 to 29.1 µm long, endoapertures slightly lalongate, rectangular to circular, c. 2.3 to 4.7 µm high and 3.1 to 6.2 µm wide, costae well visible; wall thickness c. 1–1.4 µm with the sexine as thick as to thicker than nexine; tectate, scabrate.
Description SEM
The ectexine is tectate, fossulate, perforate, in between fossulae micro-rugulate to micro-verrucate, the margo is tectate and sparsely perforate and smooth in the equator (); micro-rugulae are irregularly shaped c. 0.5 µm wide and up to 1 µm long. ().
Comparative remarks
Among extant species of Nyssa, the fossil pollen is more similar in size, apertural arrangement and ectexine ornamentation to Nyssa sylvatica Marsh. depicted in Göschl (Göschl Citation2008, plates 10–12). Nyssa is a flood tolerant genus disjunctly distributed in North and Central America and Asia; Nyssa sylvatica is a North American species. Examples of fossil Nyssa pollen resembling Nyssa sylvatica are given in Masselter (Citation2001, plate 14, figures 4–6) and Vomela (Citation2016, plate 28, figures 7–9).
Genus Mastixia Blume
Mastixia sp. 1 ()
Description LM
Tricolporate, prolate pollen grain in equatorial view elliptic to rhombic (obliquely compressed), polar axis 30.5 µm and equatorial axis 27.9 µm (N = 1, ); colpus difficult to discern c. 19.3 µm long and endoporus not properly visible but probably lalongate with visible costae (); wall thickness c. 1.3–1.5 µm with the sexine thicker than the nexine; tectate, scabrate.
Description SEM
The ectexine is tectate, rugulate to verrucate, fossulate and perforate (), whereas the rugulae/verrucae are partly more-or-less angular or rounded, rugulae and verrucae are flat and smooth with sizes ranging between c. 1.5 to 3 µm in length and 0.7 to 1 µm in width and are bordered by the fossulae, fossulae and perforation are often connected ().
Mastixia sp. 2 ()
Description LM
Tricolporate, prolate pollen grain in equatorial view elliptic, polar axis 30.5 µm and equatorial axis 27.9 µm (N = 1, ); colpus c. 29.2 µm long and endoporus lalongate to elliptic, c. 2.7 µm high and 4.4 long, costae visible; wall thickness c. 1.3–1.6 µm, with the sexine thicker than the nexine; tectate, scabrate, perforate.
Description SEM
The ectexine is tectate, perforate, fossulate and faintly rugulate, whereas the rugulae are pronounced only in the mesocolpium areas, the poles and the margo are perforate to fossulate ().
Comparative remarks
The two Mastixia fossil species strongly resemble a number of extant Mastixia taxa described and depicted by Ferguson (Citation1977, figures 9a–f). Mastixia sp. 1 is reminiscent of Mastixia taxa of probably early Miocene age from Wiesa (Germany) depicted in Vomela (Citation2016, plate 29, figures 1–6), of middle Miocene Mastixia sp. from Kreuzau (Germany) depicted in Ferguson et al. (Citation1997, plate 5, –), and of Mastixia sp. from the middle Miocene in the Hausruck area (Austria) in Masselter and Hofmann (Citation2005, figures 5, 1–3); all these dispersed pollen grains resemble the extant Mastixia cuspidata Blume (Ferguson Citation1977, figure 9a, h) and Mastixia tetrandra (Thwaites) C.B. Clarke in Premathilike and Nilsson (Citation2001, figure 6D, E). Mastixia sp. 2 is reminiscent of Mastixiaceae gen indet. from Wiesa (Germany) depicted in Vomela (Citation2016, plate 29, figures 10–12) and the LM image resembles more extant Mastixia montana (Wight ex Thwaites) C.B. Clarke Bedd. (Premathilike & Nilsson Citation2001, figure 6A). Manchester et al. (Citation2009) suggested that the European fossils affiliated with Mastixia or ‘mastixioids’ might actually belong to the sister genus Diplopanax Handel-Mazzetti.
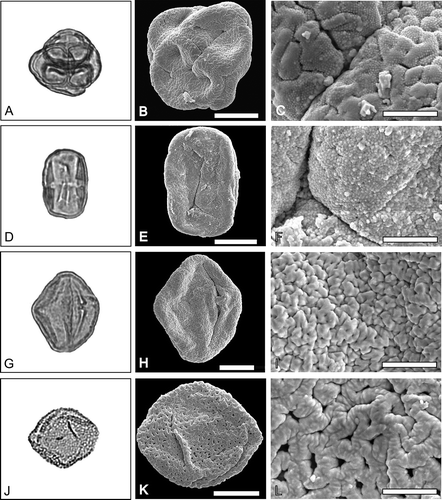
Figure 3. A–C. LM and SEM images of pollen of Ericales. Erica sp. 2. A. LM overview. B. SEM overview of the same grain as A. C. SEM detail of the same grain as A. D–F. Vitellariopsis sp. D. LM overview. E. SEM overview of the same grain as in D. F. SEM detail of the same grain as in D. G–I. Rehderodendron sp. G. LM overview. H. SEM overview of the same grain as in G. I. SEM detail of the same grain as in G. J–L. Polyspora sp. J. LM overview. K. SEM overview of the same grain as in J. L. SEM detail of the same grain as in J. Scale bars – 10 µm (B, E, H, K), 2 µm (C, F, I, L).
Ericales Dumort.
Ericaceae Juss.
Ericoideae
Ericeae DC ex Duby
Erica L.
Erica sp. 1 ()
Description LM
Tetrahedral tetrad, triangular to slightly rounded outline in apical view and quadrangular to circular in basal view (); tetrad size 20 µm × 21 µm to 23 µm × 24 µm in diameter (N = 2); pollen tricolporoidate/tricolporate; wall thickness c. 1–1.2 µm with sexine as thick as to probably slightly thicker than nexine; tectate, scabrate.
Description SEM
The ectexine is tectate, micro-verrucate to micro-rugulate/rugulate, fossulate (), the mesocolpium and polar areas are micro-verrucate (c. 0.2–0.6 µm in diameter) and the areas at the margo micro-rugulate to rugulate (rugulae c. 0.7–1.3 µm long and 0.8 µm wide), all micro-rugulae and micro-verrucae are densely covered by regularly spaced micro-echini ().
Erica sp. 2 ()
Description LM
Tetrahedral tetrad, elliptic to slightly quadrangular in outline in basal view (); tetrad size 26 µm × 27.5 µm in diameter (N = 1); pollen tricolporoidate/tricolporate (); wall thickness c. 0.9–1.3 µm with the sexine slightly thicker than the nexine; tectate, scabrate.
Description SEM
The ectexine in the polar areas and margos is rugulate (rugulae up to 2 µm in length and up to 1.3 µm in width; ) and conspicuously fossulate (), the mesocolpium areas are more micro-verrucate to micro-rugulate, the micro-verrucae and micro-rugulae are more flat-topped than in Erica sp. 1; the entire tectum is covered densely by regularly spaced micro-echini ().
Comparative remarks
Many modern Erica species display this distinctive ectexine pattern and are covered by regularly spaced micro-echini. But generally the tetrads of these species are double the size of the fossil tetrads described here. The only extant Erica taxon comparable in size is Erica arborea L. in Halbritter (Citation2016, 10–25 µm in diameter) and in Sarwar and Takahashi (Citation2014, SEM figures 1A, 2A, 2E; 27–32 µm in diameter), but the ornamentation of the micro-echini is not as pronounced as in our fossil Erica specimen. Erica sp. 1 also slightly resembles Erica bergiana L. (heather from the Cape flora in South Africa) in Halbritter and Buchner (Citation2016, LM and SEM images), which also has pronounced micro-echini, and Erica arborea (near east, Mediterranean, north African tree heather) in Halbritter (Citation2016, LM and SEM images) that has not so pronounced micro-echini. Eocene pollen affiliated with Erica arborea has been described by Hofmann (Citation2018, figure 2A–C). Erica sp. 2 resembles more Erica lusitanica Rudolphi (Spanish and Portuguese tree heather) in Halbritter (Citation2017) and middle Miocene Erica pollen from Germany (Ferguson et al. Citation1997, plate 4, figures 10–12).
Sapotaceae Juss.
Sapotoideae Eaton
Mimusopeae Hartog
Vitellariopsis (N.E. Br.) Aubrév.
Vitellariopsis sp. ()
Description LM
Tetracolporate, prolate pollen grain, rounded rectangular in equatorial view; polar axis c. 25.5 µm and equatorial axis c. 16.7 µm (N = 1); colpus c. 14.8 µm long, endoaperture lalongate to circular c. 1.8 µm high and c. 2.5 µm wide (); wall thickness c. 0.9–1 µm; tectate, psilate.
Description SEM
The extexine is tectate, micro-verrucate and sparsely, but regularly perforate (); micro-verrucae are regularly distributed, dense and evenly sized (1–1.2 µm in diameter, ).
Comparative remarks
The apertural configuration visible under LM and the ectexine pattern visible under SEM resemble extant Mimusopeae pollen depicted in Harley (Citation1991, figure 6D–F, LM images of e.g. Mimusops angel Chiov; figure 10C–F, LM and SEM images of Vitellariopsis marginata (N.E, Br.) Aubrév.). Fossil pollen comparable to our Vitellariopsis pollen are known from the lower Eocene: e.g. Mimusopeae-type pollen from Krappfeld (Austria) and middle Eocene Mimusopeae-type pollen from Borken (Germany), both in Hofmann (Citation2018, figure 6D–F and G–I, respectively), from the lower Miocene of Germany in Kmenta (Citation2011, plate 15, figures 4–14 as Sapotaceae gen. indet.; resembling Manilkara), in Kottik (Citation2002, plate 10, figures 8–15 resembling Manilkara and Mimusops), from Austria, in Meller et al. (Citation1999, plate 5, figure 1–3 as Sapotaceae gen. indet.; resembling Manilkara), from the middle Miocene of Austria (Draxler & Zetter Citation1991, plate 3, figure 1–3 as Sapotaceae-Habitus; resembling Vitellariopsis and Mimusops), from Turkey (Bouchal et al. Citation2017, plate 11, figures 8–10; as tribe Sapoteae with suggestions to Mimusops, Payena and Madhuca).
Styracaceae Dumort.
Rehderodendron Hu
Rehderodendron sp. ()
Description LM
Tricolporate prolate to subprolate pollen grain, rhombic to elliptical in equatorial outline; polar axes range from c. 30.6 to 34.8 µm and equatorial axes from c. 24.6 to 25.4 µm (N = 2, ); colpus length between c. 26.3 and 29.6 µm, colpus protrudes conspicuously at the endoapertures, endoapertures lalongate, more-or-less rectangular c. 1.4–1.7 µm high and c. 2.3–3.3 µm long; wall thickness c. 1–1.3 µm; tectate, scabrate.
Description SEM
The ectexine is micro-rugulate to rugulate, fossulate and perforate (), whereas the irregularly, angular shaped micro-rugulae and rugulae (c. 0.5–0.8 µm wide and up to 2.5 µm long) are bordered by fossulae and perforations, the ornamentation by micro-verrucae give the impression that the micro-rugulae and rugulae are composed of even smaller tectal elements ().
Comparative remarks
LM images of this pollen strongly resemble extant Styracaceae pollen described and depicted by Morton and Dickison (Citation1992). SEM images resemble particularly Rehderodendron macrocarpum Hu and to a lesser extent Sinojackia xylocarpa Hu (Morton & Dickison Citation1992, plate 1, figures 2K, 4N). Lower Miocene Rehderodendron pollen from Austria also look similar, e.g. in Hofmann et al. (Citation2002, plate 4, figures 7–9) and from Germany in Kottik (Citation2002, plate 10, figures 4–7) and in Vomela (Citation2016, plate 31, figures 1–9).
Theaceae Ker Gawler
Theeae Szyszylowicz
Polyspora Sweet
Polyspora sp. ()
Description LM
Tricolporate, subprolate pollen grain, rhombic to elliptical in equatorial outline; polar axis 22.9 µm and equatorial axis 26.5 µm (N = 1, ); colpus length c. 11.5 µm, endoaperture most likely lalongate c. 2.3 µm high and c. 3.8 µm wide; wall thickness c. 0.7–0.9 µm with the sexine thicker than the nexine; reticulate.
Description SEM
The ectexine is semitectate, reticulate to micro-reticulate, heterobrochate; the brochi are either slit-shaped, roughly triangular or more-or-less rectangular (diameters or lengths of brochi range from 0.3 to 1.3 µm, ); muri are c. 0.5–0.7 µm wide, flat and ornamented with grooves running perpendicular to the muri displaying a sort of coarse annelid pattern (); visible footlayer is smooth.
Comparative remarks
This pollen type resembles well Polyspora chrysandra (Cowan) Hu ex B. Bartholomew & T.L. Ming depicted in Wei (Citation2003, plate 27, figures 7, 8 as Gordonia chrysandra Cowan) and extant Polyspora axillaris (Roxb. ex Ker Gawl.) Sweet ex G. Don in Li et al. (Citation2011, p. 1093 as Gordonia axilliaris [Roxb. ex Ker Gawl.] Endl.) and in Fendt (Citation1996, plate 10, figures 1–7 as Gordonia axilliaris). However, the extant Gordonia pollen depicted are nearly twice the size of the fossil one. The Asian species of Gordonia have been transferred to the genus Polyspora by Bartholomew and Ming (Citation2005), based on molecular phylogenetic work by Yang et al. (Citation2004) and Zhang et al. (Citation2014).
Discussion
Cornales
Today, Nyssa (Nyssaceae) has a disjunct distribution in the Americas (four species in the southern United States and Costa Rica) and East Asia (three species; Wang et al. Citation2012). However, since the late Palaeogene Nyssa was distributed across the entire northern hemisphere (Eyde Citation1997). The fossil record from this time onwards is dominated by diaspores and pollen (e.g. Kuprianova Citation1960, in Kazakhstan; Mai & Walther Citation1978, in Europe; Manchester Citation1994, in North America). Our Nyssa pollen resembles pollen of the extant North American Nyssa sylvatica Marsh., a tall deciduous tree with variable climatic requirements for growth. It occurs in the entire eastern part of North America (to northern Mexico in the south and to southern Ontario to the north) and inhabits moist soils of valleys and uplands up to c. 1200 m above sea level (a.s.l.) (Little Citation1996). Miocene aged diaspores of Nyssa disseminata (Ludw.) Kirchh. from Europe are also affiliated with Nyssa sylvatica, in contrast to Paleogene Palaeonyssa and Protonyssa, both erected by Reid and Chandler, that have been associated with Nyssa sinensis Oliv., and the same is true for Eocene Nyssa pollen from Europe and China (Hofmann et al. Citation2019).
Mastixia is a genus consisting of c. 20 species of evergreen trees inhabiting the subtropics and tropics in southeast Asia (Eyde & Qiuyun Citation1990). Fossil occurrences are chiefly diaspores (e.g. Mastixiocarpum, Eomastixia) and to a lesser degree pollen. First occurrences of Mastixia diaspores are known from the late Cretaceous (Knobloch & Mai Citation1986). However, the majority of finds are from the Eocene (e.g. Chandler Citation1926; Reid & Chandler Citation1933; Stockey et al. Citation1998, diaspores) and Miocene (e.g. Kirchheimer Citation1957; Mai Citation1995, diaspores); these all occur in the western part of the northern hemisphere. Pollen assigned to Mastixia were less commonly encountered during the Eocene (e.g. Thiele-Pfeiffer Citation1988: under Tricolporopollenites satzveyensis Pflug and Tricolporopollenites edmundi [R. Pot.] Thomson & Pflug), but have been more frequently described from Miocene deposits (Ferguson et al. Citation1997; Masselter & Hofmann Citation2005; Vomela Citation2016). Abundant occurrences of Mastixia in Miocene strata of Europe have been always associated with warmer periods and called ‘Mastixiodeen-Floras’ according to Mai (Citation1970). Up to recently, mastixioid fossils had not been reported from Asia, however, leaves and diaspores assigned to Mastixia recently have been described from Miocene to Pleistocene Siwalik strata in eastern India (Khan et al. Citation2017).
Ericales
Erica of the Ericaceae comprises around 800 taxa occurring in Europe, North Africa and the Middle East and tropical Africa and the Cape Flora (South Africa) the latter area is known for having the highest diversity (McGuire & Kron Citation2005). Our two Erica taxa described here can be affiliated with southern European tree heathers: Erica sp. 1 resembles the tree heather Erica arborea, a taxon distributed from the Mediterranean biome in Europe and North Africa to the Ethiopian highlands and Erica sp. 2 resembles more closely Erica lusitanica (Spanish or Portuguese tree heather). However, Erica sp. 1 is also slightly reminiscent of the shrubby heather Erica bergiana from the Cape Flora. Molecular phylogenetic studies by McGuire and Kron (Citation2005) proposed that Erica arborea is the sister to all the African Erica taxa and that the European ancestor of all African Erica species must have been as widespread as the extant Erica arborea today. We suggest that the Schaßbach Erica sp. 1, plus similar Erica pollen records from the middle Miocene in western Anatolia (Bouchal et al. Citation2016), lower Eocene Erica-type pollen from the Cobham lignite, England (Hofmann Citation2018), and fossil diaspores of Erica palaeoarborea Van der Burgh from upper Miocene strata of Germany (Van der Burgh Citation1987) are from the same stock as the ancestor lineage for the African Erica taxa and support the hypothesis of McGuire and Kron (Citation2005) and Pirie et al. (Citation2016) that migration and radiation through Africa to South Africa could have taken place since the middle Miocene (around 15 Ma). More information on Eocene Erica and Ericaceae pollen are given in Hofmann (Citation2018).
Vitellariopsis (tribe Mimusopeae)
The Sapotaceae are a tropical to subtropical family consisting of five tribes (Pennington Citation1991; Swenson & Anderberg Citation2005). Our taxon can be affiliated with Vitellariopsis, which is a small genus of small trees living in southern Africa. The Mimusopeae comprise amongst others also Mimusops (Africa and southern Asia) and pantropical Manilkara (Pennington Citation1991). One of the oldest fossils of Sapotaceae are pollen: Sapotaceoidaepollenites rotundus Harris of the Upper Cretaceous (Australia; Harris Citation1972; Stoian Citation2002) might be a member of the Mimusopeae according to Harley in Armstrong (Citation2010). After the Cretaceous, the diversity of Sapotaceae pollen during the Eocene increased tremendously. All five tribes are present in the fossil record in Europe (e.g. Harley Citation1991; Armstrong Citation2010; Hofmann Citation2018). The tribe Mimusopeae is known since the lower Eocene in Europe: Lower Eocene Krappfeld in Austria (Hofmann Citation2018), mid-Eocene from the Isle of Wight, England (Harley Citation1991, affiliated with the African Tieghemella heckelii [A. Chev] Pierre ex Dubard), and mid-Eocene Borken, Germany (Hofmann Citation2018, affiliation with South American Manilkara). However, after the global Oligocene cooling event, all Sapotaceae tribes were probably wiped out and only members of the Mimusopeae tribe (e.g. Vitellariopsis, Mimusops and Manilkara) were present in Europe during Miocene times. These genera were either more adaptable to the successively cooling temperatures or the result of long distance dispersal from, for example, Africa (Armstrong et al. Citation2014).
Rehderodendron of the Styracaceae is an Asian genus, with five species, of small deciduous trees that are distributed today in China, Vietnam and Myanmar (Fritsch et al. Citation2001). The fossil record of this genus is based mainly on diaspores and starts in the early Eocene in Europe and ends in the Pliocene (see summaries in Mai Citation1970; Manchester et al. Citation2009). Unambiguous assignation of fossil pollen to different Styracaceae genera can be only achieved by using the SEM: Styrax is known since the Eocene (Hofmann Citation2018) and Rehderodendron occurs relatively frequently as an accessorial element in Miocene palynofloras (e.g. Hofmann et al. Citation2002; Kottik Citation2002; Vomela Citation2016).
Polyspora is a monophyletic small evergreen genus of the Theaceae (five species: Bartholomew & Ming Citation2005); these species were previously included in Gordonia. However, it has been demonstrated by molecular phylogenetic analyses that the Asian members of Gordonia should be incorporated into the Asian monophyletic genus Polyspora and this genus is sister to the remaining tribe Theeae (Yang et al. Citation2004; Zhang et al. Citation2014). Additionally, the Polyspora pollen from Schaßbach does not show any similarities to pollen of the American Gordonia lasianthus. Fossil occurrences of Polyspora are rare: Diaspores identified as ‘Gordonia’ truncata Chandler are known from Bartonian strata in England (Chandler Citation1926; Mai Citation1971), diaspores of Polyspora kilpperi Gregor have been described from Miocene browncoals in Germany (Gregor Citation1978), and middle Miocene ‘?Gordonia oberdorfensis’ Kovar-Eder (affiliation with Gordonia including Polyspora, e.g. Polyspora axilliaris) leaf fossils from a seam parting of a browncoal mine in Austria have been described by Kovar-Eder and Meller (Citation2001). However, this is the first time that pollen of Polyspora has been described and it fits well with the affiliation with Polyspora axilliaris of the fossil leaves from Oberndorf in Austria.
Conclusions
Palynological studies on macrofossil-bearing sediments complement the floral spectrum and add to the understanding of the ancient vegetation. Despite the prevalence of deciduous leaf fossils, such as Platanus, Quercus, Fagus, Alnus, Betula, and unidentified entire margined leaves at the Schaßbach clay pit (Austria) of Langhian age, the pollen record provides evidence of small evergreen undergrowth trees and shrubs that are no longer present in Austria. Eight accessorial pollen taxa were described and affiliated with mostly evergreen extant genera from different continents: Nyssa sp. (resembling deciduous American Nyssa sylvatica), two Mastixia spp. from Asia, two Erica spp. (one resembling the tree heather Erica arborea and one the tree heather Erica lusitanica) from southern Europe, Vitellariopsis sp. from southern Africa, Rehderodendron sp. and Polyspora sp. (resembling Polyspora axilliaris) from Asia. The last is interesting, because leaves of Gordonia/Polyspora have been previously reported from middle Miocene strata of Styria (Austria). Except for the two tree heather species, which both nowadays inhabit the Mediterranean biome, the evergreen taxa (Mastixia spp., Polyspora and Vitellariopsis) and the deciduous Rehderodendron and Nyssa disappeared from Austria, probably due to the subsequent cooling during the Pliocene and Pleistocene.
Acknowledgements
Numerous students of Vienna University are thanked for digging, collecting, preparing and fixating the macrofossils and pollen samples whilst partaking the summer semester field and laboratory courses of the Palaeontology Department since 2010. These field and laboratory courses were always taught by Reinhard Zetter and Ch.-Ch.H. All knowledge in palynology of Ch.-Ch.H. is based on the patient teaching of Reinhard Zetter since 1994 and she is immensely grateful for that.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Armstrong KE 2010. Systematics and biogeography of the pantropical genus Manilkara Adans. (Sapotaceae). PhD Thesis, Institute of Evolutionary, School of Biological Sciences of the University of Edinburgh.
- Armstrong KE, Stone GN, Nicholls JA, Valderrama E, Anderberg AA, Smedmark J, Goutier L, Naciri Y, Milne R, Richardson JE. 2014. Patterns of diversification amongst tropical regions compared: A case study in Sapotaceae. Frontiers in Genetics 5: 362. doi:10.3389/fgene.2014.00362.
- Bartholomew B, Ming TL. 2005. New combinations in Chinese Polyspora (Theaceae). Novon 15: 264–266.
- Beck-Managetta P. 1952. Zur Geologie und Paläontologie des Tertiärs des unteren Lavanttales. Jahrbuch der Geologischen Bundesanstalt Wien 95: 1–102.
- Bouchal JM, Mayda S, Zetter R, Grímsson F, Akgün F, Denk T. 2017. Miocene palynofloras of the Tinaz lignite mine, Mugla, southwest Anatolia: Taxonomy, palaeoecology and local vegetation change. Review of Palaeobotany and Palynology 243: 1–36. doi:10.1016/j.revpalbo.2017.02.010.
- Bouchal JM, Zetter R, Grímsson F, Denk T. 2016. The middle Miocene palynoflora and palaeoenvironments of Eskihisar (Yaragan basin, south-western anatolia): A combined LM and SEM investigation. Botanical Journal of the Linnean Society 182: 14–79. doi:10.1111/boj.12446.
- Chandler MEJ. 1926. The Upper Eocene flora of Hordle Hants. London: Monographs of the Palaeontological Association.
- Draxler I, Zetter R 1991. Palynologische Untersuchungen in den mittel-miozänen Hochriegelschichten (Süßwasserschichten) von Weingraben (Gemeinde Kaisersdorf, Burgenland, Österreich). In: Jubiläumsschrift 20 Jahre Geologische Zusammenarbeit Österreich-Ungarn, Jahrbuch der Geologischen Bundesanstalt. Vienna: Geologische Bundesanstalt Wein; 71–91.
- Eyde RH. 1997. The fossil record and ecology of Nyssa (Cornaceae). The Botanical Review 63: 97–123. doi:10.1007/BF02935928.
- Eyde RH, Qiuyun X. 1990. Fossil mastixioid (Cornaceae) alive in eastern Asia. American Journal of Botany 77: 689–692. doi:10.1002/j.1537-2197.1990.tb13553.x.
- Fendt A 1996. Beiträge zur Palynologie rezenter und fossiler Theaceae. Master Thesis, University Vienna.
- Ferguson DK, Pingen M, Zetter R, Hofmann Ch-Ch. 1997. Advances in our knowledge of the Miocene plant assemblage of Kreuzau. Review of Palaeobotany and Palynology 101: 147–177. doi:10.1016/S0034-6667(97)00074-2.
- Ferguson IK. 1977. World Pollen and Spore Flora 6 – Cornaceae Dum. Stockholm: Almqvist & Wiksell.
- Fritsch PW, Morton CM, Chen T, Meldrum C. 2001. Phylogeny and biogeography of the Styracaceae. International Journal of Plant Sciences 162: 95–116. doi:10.1086/323418.
- Göschl W 2008. Beiträge zur Pollenmorphologie ausgewählter rezenter und fossiler Vertreter der Davidiaceae und Nyssaceae. Master Thesis, University of Vienna.
- Gregor HJ. 1978. Neue Pflanzenfossilien aus der niederrheinischen Braunkohle. II. Polyspora kilpperi nova. spec. (Theaceae) aus dem Obermiozän des Tagebaus Zukunft-West bei Eschweiler/Rhld. Paläontologische Zeitschrift 52: 198–204.
- Grímsson F, Baal C, Zetter R. 2011. Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora of the Lavanttal Basin, Austria: Part I. Bryophyta, Lycopodiophyta, Pteridophyta, and Gnetophyta. Grana 50: 102–128. doi:10.1080/00173134.2011.585804.
- Grímsson F, Grimm GW, Meller B, Bouchal JM, Zetter R. 2016. Combined LM and SEM study of the Middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part IV. Magnoliophyta 2 – Fagales to Rosales. Grana 55: 101–163. doi:10.1080/00173134.2015.1096566.
- Grímsson F, Meller B, Bouchal JM, Zetter R. 2015. Combined LM and SEM study of the middle Miocene (Sarmatian) palynoflora from the Lavanttal Basin, Austria: Part III. Magnoliophyta1-Magnoliales to Fabales. Grana 54: 85–128. doi:10.1080/00173134.2015.1007081.
- Halbritter H 2016. Erica arborea. In: PalDat – A palynological database. https://www.paldat.org/pub/Erica_arborea/302157; accessed 30 March 2017.
- Halbritter H 2017. Erica lusitanica. In: PalDat – A palynological database. https://www.paldat.org/pub/Erica_lusitanica/302924; accessed 27 February 2017.
- Halbritter H, Buchner R 2016. Erica bergiana. In: PalDat – A palynological database. https://www.paldat.org/pub/Erica_bergiana/302649; accessed 10 January 2019.
- Harley MM. 1991. The pollen morphology of the Sapotaceae. Kew Bulletin 46: 397–491. doi:10.2307/4110538.
- Harris WK. 1972. New form species from southern Australia early Tertiary sediments. Transaction of the Royal Society of Australia 96: 53–65.
- Hesse M, Halbritter H, Zetter R, Weber M, Buchner R, Frosch-Radivo A, Ulrich S. 2009. Pollen terminology – An illustrated handbook. Vienna: Springer.
- Hofmann Ch-Ch. 2018. Light and scanning electron microscopic investigations of pollen of Ericales (Ericaceae, Sapotaceae, Ebenaceae, Styracaceae and Theaceae) from five lower and mid-Eocene localities. Botanical Journal of the Linnean Society 187: 550–578. doi:10.1093/botlinnean/boy035.
- Hofmann Ch-Ch, Kodrul TM, Liu XY, Jin JH. 2019. Scanning electron microscopy investigations of middle to late Eocene pollen from the Changchang Basin (Hainan Island, south China) – insights into the palaeobiogeography and fossil history of Juglans, Fagus, Lagerstroemia, Mortoniodendron, Cornus, Nyssa, Symplocos and some Icacinaceae in SE Asia. Review of Palaeobotany and Palynology 265: 41–61.
- Hofmann Ch-Ch, Zetter R, Draxler I. 2002. Pollen- und Sporenvergesellschaften aus dem Karpatium des Korneuburger Beckens (Niederösterreich). Beiträge Paläontologie 27: 17–43.
- Khan MA, Bera M, Spicer RA, Spicer TEV, Bera S. 2017. First occurrence of mastixioid (Cornaceae) fossil in India and its biogeographic implications. Review of Palaeobotany and Palynology 247: 83–96. doi:10.1016/j.revpalbo.2017.08.006.
- Kirchheimer F. 1957. Die Laubgewächse der Braunkohlezwit. Halle: W. Knapp Verlag.
- Klaus W. 1955. Pollenanalytisch- stratigraphische Betrachtungen zur Alterstellung einer Blattfossilien führenden Schicht aus Wolkersdorf im unteren Lavanttal (Ostkärnten). Verhandlungen der Geologischen Bundesanstalt Wien 1955: 239–242.
- Klaus W. 1984. Zur Mikroflora des Unter-Sarmats am Alpen-Südostrand. Beiträge zur Paläontologie 11: 289–419.
- Klaus W. 1987. Einführung in die Paläobotanik Band I. Wien: Deuticke Verlag.
- Kmenta M 2011. Die Mikroflora der untermiozänen Fundstelle Altmittweida, Deutschland. Master Thesis, University of Vienna.
- Knobloch E, Mai DH. 1986. Monographie der Früchte und Samen in der Kreide von Mitteleuropa. Rozpravy Ustredniho Ustavu Geologickeho Praha 47: 1–219.
- Kottik S 2002. Die Palynologie des Randecker Maars (Miozän, Baden Württemberg). Master Thesis, University of Vienna.
- Kovar-Eder J, Meller B. 2001. Plant assemblages from the hanging wall sequence of the opencast mine Oberdorf N. Voitsberg, Styria (Austria, Early Miocene, Ottnangian). Palaeontographica B 259: 65–112.
- Kuprianova AL. 1960. Palynological data contributing to the history of Liquidambar. Pollen Et Spores 2: 71–88.
- Li TQ, Cao HJ, Kang MS, Zhang ZX, Zhao N, Zhang H. 2011. Pollen flora of China woody plants by SEM. Beijing: Science Press.
- Lippolt HJ, Baranyi I, Todt W. 1975. Das Kalium-Argon Alter des Basaltes vom Lavanttal in Kärnten. Der Aufschluß 26: 238–242.
- Little EL. 1996. National Audubon Society field guide to North American trees. New York: Alfred A. Knopf, Incorporation.
- Mai DH. 1970. Subtropische Elemente im europäischen Tertiär I. Die Gattungen Gironniera, Sarcococca, Illicium, Evodia, Ilex, Mastixia, Alangium, Symplocos and Rehderodendron. Pläontologische Abhandlungen Abteilung B 3: 441–503.
- Mai DH. 1971. Über fossile Lauraceae und Theaceae in Mitteleuropa. Feddes Repertorium 82: 313–341. doi:10.1002/fedr.19710820502.
- Mai DH. 1995. Tertiäre Vegetationsgeschichte Europas. Jena: Gustav Fischer Verlag.
- Mai DH, Walther H. 1978. Die Floren der Haselbacher Serie im Weißelster Becken (Bezirk Leipzig, DDR). Abhandlungen des Staatlichen Museums Mineralogie und Geologie 28: 1–200.
- Manchester SR. 1994. Fruits and seeds of the middle Eocene Nut flora, Clarno Formation, north central Oregon. Palaeontographica Americana 58: 1–205.
- Manchester SR, Chen ZD, Lu AM, Uemura K. 2009. Eastern Asian endemic seed plant genera and their paleogeographic history throughout the northern hemisphere. Journal of Systematics and Evolution 47: 1–42. doi:10.1111/j.1759-6831.2009.00001.x.
- Masselter T 2001. Palynologie und organische Fazies kohleführender klastischer Sedimente des Hausruckgebietes. Master Thesis, University of Vienna.
- Masselter T, Hofmann Ch-Ch. 2005. Palynology and palynofacies of Miocene coal-bearing (clastic) sediments of the Hausruck area (Austria). Geobios 38: 127–138. doi:10.1016/j.geobios.2003.08.004.
- McGuire AF, Kron KA. 2005. Phylogenetic relationships of European and African Ericas. International Journal of Plant Sciences 166: 311–318. doi:10.1086/427478.
- Meller B, Kovar-Eder J, Zetter R. 1999. Lower Miocene leaf, palynomorph, and diaspore assemblages from the base of the lignite-bearing sequence in the opencast mine Oberdorf, N Voitsberg (Styria, Austria) as indication of the “Younger Mastixioid” vegetation. Palaeontographica B 252: 123–179. doi:10.1127/palb/252/1999/123.
- Meller B, Zetter R, Hassler A, Bouchal JM, Hofmann Ch-Ch GF. 2015. Middle Miocene macrofloral elements from the Lavanttal basin, Austria, Part 1. Ginkgo adiantoides (Unger) Heer. Austrian Journal of Earth Sciences 108: 185–198. doi:10.17738/ajes.2015.0020.
- Morton CM, Dickison WC. 1992. Comparative pollen morphology of the Styracaceae. Grana 31: 1–15. doi:10.1080/00173139209427822.
- Pennington TD. 1991. The genera of Sapotaceae. Kew: Royal Botanical Gardens.
- Pirie MD, Oliver EGH, Mugrabi de Kuppler A, Gehrke B, Le Maitre NC, Kandziora M. 2016. The biodiversity hotspot as evolutionary hot-bed: Spectacular radiation of Erica in the Cape Floristic region. Evolutionary Biology 16: 190.
- Premathilike R, Nilsson S. 2001. Pollen morphology of endemic species of the Horton Plains National Park, Sri Lanka. Grana 40: 256–279. doi:10.1080/00173130152987508.
- Reid EM, Chandler MEJ. 1933. The London clay flora. Bulletin British Museum (National History) Geology, London.
- Reischenbacher D, Rifelj H, Sachsenhofer F, Jelen B, Coric S, Gross M. 2007. Early Badenian palaeoenvironment in the Lavanttal basin (Mühldorf Formation; Austria): Evidence from geochemistry and palaeontology. Austrian Journal of Earth Sciences 100: 202–229.
- Reischenbacher D, Sachsenhofer F. 2013. Basin formation during the post-collisional evolution of the eastern Alps: The example of the Lavanttal basin. International Journal of Earth Sciences 2102: 517–543. doi:10.1007/s00531-012-0807-y.
- Sarwar AKMG, Takahashi H. 2014. Pollen morphology of Erica L. and related genera. Grana 53: 221–231. doi:10.1080/00173134.2014.912346.
- Stevens PE. 2001. Angiosperm phylogeny website version 2017 (continuously updated). http://www.mobot.org/MOBOT/research/APweb/
- Stockey RA, LePage BA, Pigg KB. 1998. Permineralized fruits of Diplopanax (Cornaceae, Mastixioideae) from middle Eocene Princeton chert of British Columbia. Review of Palaeobotany and Palynology 103: 223–234. doi:10.1016/S0034-6667(98)00038-4.
- Stoian LM 2002. Late Cretaceous - Late Eocene palynofloras from drillhole Troas 1, offshore Otway basin, South Australia. Report book 2002/010. Adelaide: Department of Primary Industries and Resources.
- Swenson U, Anderberg AA. 2005. Phylogeny, character evolution, and classification of Sapotaceae (Ericales). Cladistics 21: 101–130.
- Thiele-Pfeiffer HM. 1988. Die Mikroflora aus dem mitteleozänen Ölschiefer von Messel bei Darmstadt. Palaeontographica B 211: 1–86.
- Tollman A. 1985. Geologie von Österreich II. Vienna: Franz Deuticke.
- Van der Burgh J. 1987. Miocene floras in the lower Rhenish basin and their ecological interpretation. Review of Palaeobotany and Palynology 52: 299–366. doi:10.1016/0034-6667(87)90064-9.
- Vomela S. 2016. Die Mikroflora der untermiozänen Fundstelle Wiesa bei Kamenz, Deutschland. Master Thesis, University of Vienna.
- Wang N, Milne I, Jacques FMB, Sun BL, Zhang CQ. 2012. Phylogeny and a revised classification of the Chinese species of Nyssa (Nyssaceae) based on morphological and molecular data. Taxon 61: 344–354. doi:10.1002/tax.612006.
- Wei Z. 2003. Pollen flora of seed plants. Yunnan: Science and Technology Press.
- Yang SX, Yang JB, Lei LG, Li DZ, Yoshino H, Ikeda T. 2004. Reassessing the relationships between Gordonia and Polyspora (Theaceae) based on the combined analyses of molecular data from the nuclear, plastid and mitochondrial genomes. Plant Systematics and Evolution 248: 45–55. doi:10.1007/s00606-004-0178-3.
- Zhang W, Kan SL, Zhao H, Li ZY, Wang XQ. 2014. Molecular phylogeny of tribe Theeae (Theaceae s.s.) and its implications for generic delimination. PloS ONE 9(5): e98133. doi:10.1371/journal.pome.0098133.
