Abstract
Centauropsis is a genus of eight species in the family Compositae, all of which are endemic to Madagascar. There is almost no information about the pollen of this genus, with only one species having its pollen described to date, which hinders systematic studies involving this genus and closely related taxa. In this study, we comprehensively characterise the pollen of Centauropsis, with details on morphology and ultrasculpture for six of the eight species of the genus. The pollen of Centauropsis is here characterised as 3-colporate, with sublophate ornamentation and nanoreticulate sexine. The species differ from each other mostly in length of the axis, morphology of the colporus endoaperture, and spine shape and size. The correlation between palynological characters and their variation within and between species was explored using principal component analyses (PCA) and cluster analyses (unweighted pair group method with arithmetic mean [UPGMA] and Euclidean distance). Full palynological descriptions, measurements and scanning electron microscopy (SEM) and light microscopy (LM) images are provided for all examined species.
Centauropsis Bojer ex DC. is a genus belonging to the tribe Vernonieae in the sunflower family, Compositae (or Asteraceae), currently comprising eight species that are all endemic to Madagascar (Robinson Citation2007; Keeley & Robinson Citation2009). The genus is characterised by its shrubby habit; one or few capitula, subsessile to long-pedunculate; appendiculate phyllaries; paleaceous receptacles; anthers with broad tails; style without node; glabrous cypselae and pappus formed by numerous bristles (Humbert Citation1960; Robinson Citation2007).
The genus was validly published by Candolle (Citation1836), based on specimens collected by W. Bojer. Candolle described two species: Centauropsis lanuginosa Bojer ex DC. ( = Oliganthes lanuginosa (Bojer ex DC.) Humbert) and Centauropsis fruticosa Bojer ex DC. Robinson (Citation1999) revised the taxonomy of paleotropical Vernonieae Cass., proposing three new subtribes (Centrapalinae H.Rob., Erlangeinae H.Rob., and Gymantheminae H.Rob.) and several new genera, placing Centauropsis within subtribe Gymnantheminae based on the shrubby habit and sweeping hairs often blunt. Nonetheless, molecular phylogenetic studies within Vernonieae have placed Centauropsis in a clade with members of the polyphyletic Erlangeinae and Centrapalinae (Keeley et al. Citation2007, Citation2021), a clade here referred as ‘Erlangeinae/Centrapalinae combined’ clade. In those studies, the single species of Centauropsis that was sampled is sister to a clade comprising Hilliardiella H.Rob. (Centrapalinae), Crystallopollen Steetz, Parapolydora H.Rob., Cyanthillium Blume (Erlangeinae) and Vernonia subplumosa O.Hoffm. (unplaced) (Keeley et al. Citation2007, Citation2021). According to Keeley and Robinson (Citation2009), Centauropsis is actually placed in Centrapalinae.
Pollen morphology has been informative for inferring relationships at the family level in Compositae and important characters include the pollen grain shape in polar view (amb), class of endoapertures, thickness of exine layers, and size of spines (Blackmore et al. Citation2009). Pollen has also been pivotal in the new subtribal classification of paleotropical Vernonieae proposed by Robinson (Citation1999). Historically, pollen characters have been studied in Vernonieae by different authors in the last century and have been of great importance for systematic study of the tribe at the subtribal and generic level (e.g. Wodehouse Citation1928; Salgado-Labouriau Citation1973; Jones Citation1981; Bolick Citation1991; Robinson Citation1999).
The pollen of Centauropsis has been poorly documented and inconsistently characterised (Kingham Citation1976; Wortley et al. Citation2007; Blackmore et al. Citation2009). A single species of this genus (Centauropsis fruticosa Bojer ex DC.) was included in prior palynological studies (Kingham Citation1976) and its pollen was characterised as medium-sized (35.0 µm), spherical, 3-colporate, subechinolophate/echinate sexine with micropores, appearing to present a reticulation, spines length 5.0 µm, and exine thickness 4.0 µm.
Wortley et al. (Citation2007) suggested that the pollen of Centauropsis may be similar to that of the African genera Cabobanthus, Cyanthillium and Crystallopollen, and the South American Pacourina sharing the pororate aperture pattern (sensu Blackmore et al. Citation2009; = porate sensu Punt et al. Citation2007) and non-perforated tectum; however, this suggestion was based only on the sole specimen imaged by Kingham (Citation1976) and no other species of Centauropsis. In addition, there is also inconsistency in the application of the descriptive terminology in Centauropsis, leading to confusion: the pollen grain is said to be ‘porate’ when the ectoaperture is a pore and not a colpus (i.e. pororate in Wortley et al. [Citation2007] and Blackmore et al. [Citation2009]).
Blackmore et al. (Citation2009), based on the phylogeny of Keeley et al. (Citation2007), suggested that pororate pollen grains is a synapomorphy for the clade Erlangeinae/Centrapalinae combined clade and, therefore, the tricolporate pollen of Centauropsis and Parapolydora would be a reversion. However, Keeley et al. (Citation2021) have provided a different composition for this clade, in which a tricolporate genus (Hilliardiella) and the pororate Cabobanthus belong to another clade (Centrapalinae II), thus not recovering a porate clade. Further studies on pollen character evolution, with implications for finding synapomorphies for the clades, are not yet possible due to a lack of data. In this study, the pollen morphology of Centauropsis is described in a comprehensive and standardised way for the first time, as a means to support future taxonomic, palynological, and evolutionary studies of Vernonieae.
Material and methods
Taxonomic sampling of the genus comprised six (out of eight) currently recognised species of Centauropsis: C. antanossi (Scott Elliot) Humbert, C. cuspidata Humbert, C. decaryi Humbert, C. fruticosa Bojer ex DC. var. fruticosa, C. perrieri Humbert, C. rhaponticoides Drake, plus, one variety C. fruticosa var. baronii Humbert. Unopened mature flower buds were obtained from 13 specimens from K and P herbaria (acronyms following Thiers, [continuously updated]); all specimens examined are listed in the Specimens Investigated section. Samples were labelled with an abbreviation of the specific epithet plus the last two numbers of the herbarium voucher or collection number (see , , Specimen column).
Table I. Centauropsis dimensions (μm) pollen grains in equatorial and polar view using light microscopy.
Table II. Dimensions (μm) pollen grains aperture, spine, reticulum and exine layers in equatorial view using light microscopy.
Pollen grains were treated with acetolysis (Erdtman Citation1960), as modified by Melhem et al. (Citation2003). Measurements and photomicrographs were performed under a light microscope Leica LMD7 microdissection microscope, and photomicrographs were taken with a video camera Leica DFC 7000 T, supported by LAS software. Permanent slides for light microscopy (LM) were deposited in the pollen reference collection of the Bioimaging Laboratory, Royal Botanic Gardens, Kew, United Kingdom. For scanning electron microscopy (SEM), acetolysed pollen grains were washed and placed on a metal stub with carbon cement and sputter coated with platinum (10 nm) using a quorum-Q150t es Series. Samples were imaged under a Hitachi 8230 scanning electron microscope, with a 5 kV electron beam, at the Bioimaging Laboratory, Royal Botanic Gardens, Kew.
Measurements were taken under LM, using a Leica LMD7 microdissection microscope, on 25 randomly selected pollen grains from each specimen. The polar and equatorial axes were measured in equatorial view, and the equatorial axis in polar view, excluding spines. Ten measurements of the main pollen morphometric parameters were also made: length and width of the colpus, length and width of the endoaperture, the thickness of the nexine and sexine layers (excluding spines, Kingham Citation1976) and the spine length. Exine measurements were made in the mesocolpium region.
The measurements of the polar and equatorial axes were statistically analysed for the arithmetic mean (x), average standard deviation (Sx), sample standard deviation (s), coefficient of variability (V%), and 95% confidence interval (CI) (Zar Citation2010; Vieira Citation2011). For exine characters, the arithmetic mean and range were calculated for each pollen grain size class and exine (sexine + nexine). The CI and Sx are present in descriptions in the following order: (EA × PA) × (PV).
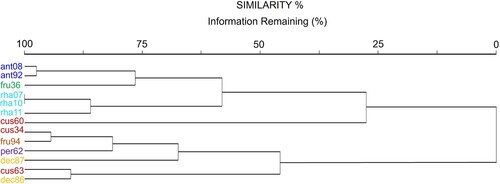
A principal component analysis (PCA) was performed to explore whether pollen grain characters corroborate species and specimen grouping. Eleven metric variables (polar axis [PA], equatorial axis [EA], equatorial axis in polar view [PV], colpus length [CL], colpus width [CW], endoaperture length [EL], endoaperture width [EW], nexine thickness [Ne], sexine thickness [Se = infratectum + tectum], exine thickness [Ex = N + S], spine length [Sp]) and one classes/index (endoaperture class [Ecl]) were analysed for ordination using FITOPAC (Shepherd Citation1996) and PC-ORD version 7 (McCune & Mefford Citation2016). To understand how Centauropsis specimens relate to each other, based on pollen morphology, a cluster analysis (CA) was performed using the software PC-ORD version 7 (McCune & Mefford Citation2016). We produced a similarity dendrogram by calculating the unweighted pair group method with arithmetic mean (UPGMA) using the Euclidean distance based on the same PCA metric variables and three classes/indexes.
Pollen terminology follows Punt et al. (Citation2007) and Halbritter et al. (Citation2018). Pollen shape classes and amb types follow Erdtman (Citation1952), colpus length categories follow Mark et al. (Citation2012) and specific sculpturing and apertures type to Vernonieae follow Robinson et al. (Citation2016). The endoaperture classes and sexine/nexine thickness index follow Antonio-Domingues et al. (Citation2022a).
Results
General description
Centauropsis Bojer ex DC
Pollen grains are monads, medium to large-sized (43.0 [50.9 ± 4.0] 58.9 × 38.3 [46.5 ± 4.1] 54.6) × (41.5 [50.2 ± 4.4] 58.8) (), radially symmetrical, isopolar (, , , ), oblate spheroidal to prolate spheroidal (), amb circular or subtriangular, circular to ellipsoidal in equatorial view (, ), angulaperturate, polar area large to small (, ). Apertures 3-zonocolporate, colporus narrow, long to short, and non-constricted, (, , , ) apices acute or rounded (, , , , ), margo prominent, membrane granulate, protruding aperture absent; operculum not observed (, , , ); endoaperture lalongate to very lalongate, terminals acute or rounded (, , ). Exine thick, 5.0‒10.0 μm, sexine 1.1‒3.3 times thicker than nexine (), sculpture sublophate-echinolophate (sublophate) (, , ); spine 1.3–8.3 μm (), base constricted () or non-constricted (, , ), apices acute (, , ) or obtuse (, ); lopha (ridge) nanoreticulate (, , ) or nanoreticulate-microechinate (, ), sublacuna nanoreticulate; muri perforate, lumina rounded. Tectum tectate perforate (, ), infratectum with thick digitate columella supporting the spines in a concordant pattern (C, I, ) and thin simple columellae supporting the pluricolumellate lophae ().
Figure 1. Light microscopy of Centauropsis Bojer ex DC. pollen grains. A‒C. Centauropsis antanossi (Scott Elliot) Humbert. A. Detail of the colporus, endoaperture and surface, equatorial view. B. Apertures 3-colporate, long and acute apices and apocolpium surface. C. Optical section, equatorial view. D‒F. Centauropsis cuspidata Humbert. D. Detail of the colporus, endoaperture and surface, equatorial view. E. Apertures 3-colporate, long and acute apices and apocolpium surface. F. Optical section, equatorial view. G‒H. Centauropsis decaryi Humbert. G. Detail of the colporus, endoaperture and surface, equatorial view. H. Apertures 3-colporate, long and acute apices and apocolpium surface. I. Optical section, equatorial view. J‒L. Centauropsis fruticosa Bojer ex DC. J. Detail of the colporus, endoaperture and surface, equatorial view. K. Apertures 3-colporate, long and acute apices and apocolpium surface. L. Optical section, equatorial view. Scale bars – 10 μm (A‒C, G‒L), 8 μm (D‒F).

Figure 2. Scanning electron microscopy of Centauropsis Bojer ex DC. pollen grains. A‒C. Centauropsis antanossi (Scott Elliot) Humbert. A. General view of the mesocolpium, equatorial view. B. Detail of apertural membrane of the colporus, margo, and mesocolpium. C. Fractured pollen grain, structure of exine layers. D‒F. Centauropsis cuspidata Humbert. D. General view of the apertural area and mesocolpium, equatorial view. E. Detail of apocolpium, polar view. F. Detail of the spines and lophae on mesocolpium, equatorial view. G–I. Centauropsis decaryi Humbert. G. General view of the mesocolpium, equatorial view. H. General view of the apocolpium, polar view. I. Fractured pollen grain, structure of exine layers. J‒L. Centauropsis fruticosa Bojer ex DC. J. General view of the apertural area and mesocolpium, equatorial view. K. Detail of apocolpium, polar view L. Detail of apertural membrane of the colporus, margo, and mesocolpium. Scale bars – 10 μm (A, D, G, H, J), 1 μm (B, C, E, F, I, K, L).
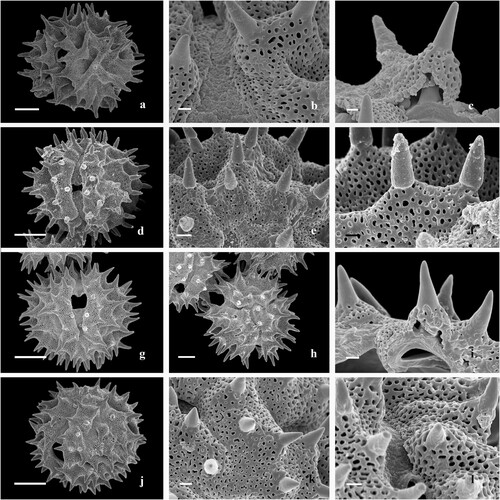
Figure 3. Light microscopy of Centauropsis Bojer ex DC. pollen grains. A‒C. Centauropsis fruticosa var. baronii Humbert. A. Detail of the colporus, endoaperture and surface, equatorial view. B. Apertures 3-colporate, long and acute apices and apocolpium surface. C. Optical section, equatorial view. D‒F. Centauropsis perrieri Humbert. D. Detail of the colporus, endoaperture and surface, equatorial view. E. Apertures 3-colporate, long and acute apices and apocolpium surface. F. Optical section, equatorial view. G‒I. Centauropsis rhaponticoides Drake. G. Detail of the colporus, endoaperture and surface, equatorial view. H. Apertures 3-colporate, long and acute apices and apocolpium surface. I. Optical section, equatorial view. Scale bars – 10 μm (A‒C), 8 μm (D‒I).
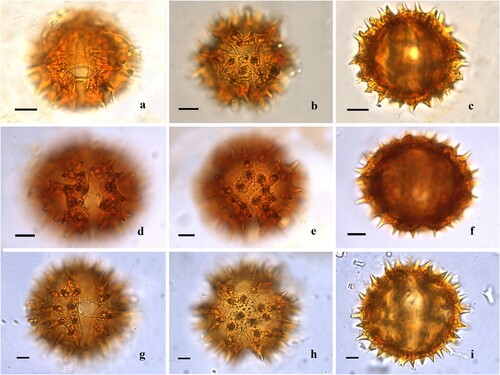
Figure 4. Scanning electron microscopy of Centauropsis Bojer ex DC. pollen grains. A‒C. Centauropsis fruticosa var. baronii Humbert. A. General view of the apertural area, mesocolpium, and apocolpium, inclined equatorial view. B. Detail of the spines, lophae and microspines on mesocolpium, equatorial view. C. Fractured pollen grain, structure of exine layers. D‒F. Centauropsis perrieri Humbert. D. General view of the apertural area, mesocolpium, equatorial view. E. Detail of the apocolpium, polar view. F. Detail of apertural membrane of the colporus, margo, and mesocolpium. G‒I. Centauropsis rhaponticoides Drake. G. General view of the apertural area, mesocolpium, equatorial view. H. Detail of the apocolpium, polar view. I. Detail of the spines on mesocolpium, equatorial view. J, K. Fractured pollen grain, structure of exine layers of Centauropsis fruticosa var. baronii Humbert. L. Fractured pollen grain, structure of spine of Centauropsis perrieri Humbert. Scale bars ‒ 10 μm (A, D, G), 1 μm (B, C, E, F, H‒L).
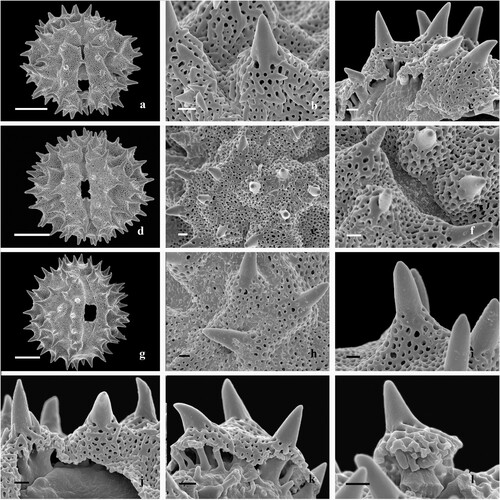
Table III. Morphology and ultrasculpture of Centauropsis pollen grains using light and scanning electron microscopy.
A summary of the measurements is shown in and and additional observations and descriptions are provided in .
Centauropsis antanossi (Scott Elliot) Humbert (, )
Pollen grains are large-sized, rarely medium (52.1 [53.3 ± 0.6] 54.6 × 47.2 [48.8 ± 0.8] 50.3) × (53.0 [54.2 ± 0.6] 55.3), oblate spheroidal (), amb circular to subtriangular (A), ellipsoidal in equatorial view (, ), angulaperturate, polar area medium (). Colporus medium, apices acute (), membrane granulate (); endoaperture lalongate terminals rounded (). Exine 6.8‒10.0 μm, sexine 1.6‒2.9 times thicker than nexine; spines 3.5 μm (), base non-constricted, apices acute (); lophae and sublacunae nanoreticulate.
Centauropsis cuspidata Humbert (, )
Pollen grains are medium-sized (42.5 [43.9 ± 0.6] 45.3 × 39.2 [40.2 ± 0.4] 42.9) × (41.4 [42.4 ± 0.4] 43.4), oblate spheroidal to prolate spheroidal (), amb circular to subtriangular (), circular to ellipsoidal in equatorial view (, ), angulaperturate, polar area medium (). Colporus medium, apices acute (, ), rarely rounded, membrane granulate; endoaperture very lalongate, terminals rounded (), lalongate only in acus60. Exine 5.0–7.7 μm, sexine from 1.3–2.9 times thicker than nexine; spines 3.5 μm (), base constricted, apices acute (, ); lophae and sublacunae nanoreticulate ().
Centauropsis decaryi Humbert (, )
Pollen grains are medium-sized (46.3 [47.8 ± 0.7] 49.2 × 41.1 [42.5 ± 0.7] 43.9) × (44.9 [46.2 ± 0.6] 47.5), oblate spheroidal (), amb subtriangular to triangular (), ellipsoidal in equatorial view (, ), angulaperturate, polar area small (, ). Colporus long, apices rounded (, ), membrane granulate; endoaperture very lalongate, terminals acute (, ). Exine 5.2‒8.4 μm, sexine from 1.3‒2.6 times thicker than nexine; spines 5.0 μm (), base non-constricted, apices acute (); lophae and sublacunae nanoreticulate ().
Centauropsis fruticosa Bojer ex DC. var. fruticosa (, )
Pollen grains are medium to large-sized (49.8 [50.9 ± 0.6] 52.1 × 43.5 [46.0 ± 0.3] 45.3) × (49.0 [50.5 ± 0.7] 52.0), oblate spheroidal (), amb subtriangular, circular in equatorial view (), angulaperturate, polar area small (). Colporus long, apices rounded (, ), membrane granulate (); endoaperture very lalongate, terminals rounded (). Exine 2.3‒7.1 μm, sexine from 1.8–3.2 times thicker than nexine; spines 4.1 μm (), base non-constricted, apices acute (); lophae and sublacunae nanoreticulate ().
Centauropsis fruticosa var. baronii Humbert (, )
Pollen grains are medium-sized (46.9 [47.6 ± 0.4] 48.4 × 42.3 [43.0 ± 0.3] 43.6) × (45.4 [46.3 ± 0.4] 47.3), oblate spheroidal (), amb subtriangular, ellipsoidal in equatorial view (), angulaperturate, polar area small (). Colporus long, apices rounded, rarely acute (), membrane granulate (); endoaperture very lalongate, terminals acute (). Exine 5.0‒7.5 μm, sexine from 1.8‒3.1 times thicker than nexine; spines 4.3 μm (), base non-constricted, apices acute (); lophae nanoreticulate-microechinate () and sublacunae nanoreticulate ().
Centauropsis perrieri Humbert (, )
Pollen grains are medium-sized (46.8 [47.3 ± 0.3] 47.8 × 41.1 [41.8 ± 0.3] 42.4) × (45.1 [45.8 ± 0.4] 46.6), oblate spheroidal (), amb subtriangular (), ellipsoidal in equatorial view (), angulaperturate, polar area medium (, ). Colporus medium, apices rounded (, ) membrane granulate (); endoaperture very lalongate, terminals rounded (, ). Exine 5.4‒7.8 μm, sexine from 1.8‒3.3 times thicker than nexine; spines 3.2 μm (), base non-constricted, apices acute (); lophae and sublacunae nanoreticulate ().
Centauropsis rhaponticoides Drake (, )
Pollen grains are large-sized (56.4 [58.4 ± 1.0] 60.4 × 53.2 [54.8 ± 0.7] 56.3) × (56.1 [58.3 ± 0.9] 59.8), oblate spheroidal (), amb triangular (), circular to ellipsoidal in equatorial view (, ), angulaperturate, polar area small (). Colporus long, apices rounded (, ), membrane granulate; endoaperture lalongate to very lalongate, terminals rounded (). Exine 7.4‒9.8 μm, sexine from 1.1‒2.0 times thicker than nexine; spines 5.4 μm (), base non-constricted, apices acute (); lophae and sublacunae nanoreticulate ().
Principal component analysis (PCA)
The PCA explored the correlation between a suite of selected palynological characters, in a total of 11 metric variables and one class/index (, ). Correlation coefficients are shown in . The first two axes accounted for 88.76% of the total variance of the quantitative data analysed. The first axis explained 69.41% of the variance and was mainly associated with EL, CW, CL, PV, PA, EA and Se. The second axis summarised 19.35% of the total variance, with Sp, Ecl, EW, Ne, and Ex being the variables that contributed most to this axis.
Figure 5. Principal component analysis biplot of the pollen grain metric variables and classes/indices of Centauropsis specimens.
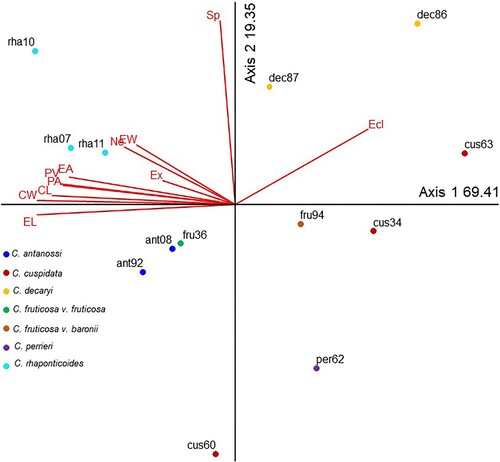
Table IV. Pearson and Kendall coefficients of pollen grain metric variables and classes/indices from the first two ordination axes of the principal component analysis (PCA) of Centoropsis species.
Three individuals of Centauropsis rhaponticoides (rah07, rah10, rah11) represent the largest pollen grains, and the highest values relative to colporus and endoaperture dimensions were located on the upper left side of the PCA associated with high values of EA, PA, CL, CW, EL, EW. Centauropsis antanossi (and08, ant92), C. fruticosa var. fruticosa (fru36) and C. cuspidata (cus60) showed high Ex and the lowest Ecl representing the lalongate endoaperture, were located on the bottom left side of the PCA.
Due to their high Ecl values, Centauropsis decaryi (dec86, dec87) and C. cuspidata (cus63) were placed on the upper right side of the PCA with very lalongate endoaperture. Specimens with the smallest pollen grains and spines were located mainly on the bottom right side of the graph. These specimens also had the lowest EA, PA, PV, and Sp values: C. fruticosa var. fruticosa (fru94), C. cuspidata (cus34) and C. perrieri (per62).
Cluster analysis (UPGMA and Euclidean distance)
We identified two groups of specimens with 0% similarity (). One group had similarity greater than 30% and comprised the species with the smallest pollen grains, colporus and endoaperture dimensions and the highest endoaperture class (Centauropsis cuspidata [cus34, cus63] C. decaryi [dec86, dec87], C. fruticosa var. fruticosa [fru94], C. perrieri [per32]). The other group had similarity greater than 45% and comprised the remaining species with the largest pollen grains, colporus and the endoaperture values and the smallest endoaperture class (C. antanossi [ant08, ant92], C. fruticosa [fru36], C. rhaponticoides [rah07, eah10, rah11] and C. cuspidata [cus60]). Two C. rhaponticoides specimens [rah07, rah70] showed 100% similarity and two C. antanossi almost 95% (ant08, ant92).
Discussion
Pollen morphology of Centauropsis
The pollen of Centauropsis is characterised by 3-colporate aperture pattern, sublophate ornamentation, and nanoreticulate sexine. Our results also corroborate previous observations made for Centauropsis fruticosa by Kingham (Citation1976), with exception of the spine length, which we have documented as slightly shorter (4.1 µm in our study versus 5.5‒6.5 µm in Kingham’s work). Pollen grain axis, colporus size, shape and size of spines, shape, and terminal of the endoaperture were useful characters to distinguish the species of Centauropsis ().
In Compositae (and notably, several other genera in tribe Vernonieae), pollen grain size and morphology of the apertures (colporus and endoaperture), as well as of the spines, have been of important systematic value, such as in the genera Chresta Vell. ex DC., Eremanthus Less., Graphistylis B. Nord. Paralychnophora MacLeish, Piptolepis Sch.Bip. and Verbesina L. (Loeuille et al. Citation2012; Souza-Souza et al. Citation2015; Souza 2016; Siniscalchi et al. Citation2017; Moreira et al. Citation2019; Souza-Souza et al. Citation2022). Beyond these characters, other pollen morphological characters have been of importance for recognising species and groups of species, such as the shape of pollen grains (spheroidal, oblate spheroidal, prolate spheroidal), aperture number and position (3-colporate, 3-colpate, pantoporate), sexine ornamentation and reticulation (echinolophate, psilolophate, subechinolophate, echinate), muri ornamentation (psilate, perforate, reticulate, echinate), lacunae shape (hexagonal, pentagonal, circular) and ridge of the lacunae (interrupted, non-interrupted); these should be considered in future palynological and evolutionary studies in Vernonieae (Keeley & Jones Citation1979; Stanski et al. Citation2013; Souza et al. 2016; Siniscalchi et al. Citation2017; Souza-Souza et al. Citation2022).
Centauropsis fruticosa var. baronii showed acute endoaperture terminals (A) (versus rounded endoaperture terminals in C. fruticosa var. fruticosa) (J) and nanoreticulate-microechinate lophae (B, C, J) (versus nanoreticulate lophae in C. fruticosa var. fruticosa) (K) (). These varieties also discretely differ in length of the pollen grain axes (i.e. they are different in shape), and in length of the colporus and endoaperture (, ). These morphometrical differences are confirmed by results from the PCA, in which C. fruticosa var. baronii is positioned on the bottom left corner of the plot (versus bottom right in C. fruticosa var. fruticosa) (), and each specimen is represented in a different group, with 0% similarity (). More studies are ongoing to re-assess their delimitation, based on other characters.
Palynotaxonomy
The 3-colporate pattern, presence of circular endoapertures, and smooth to perforate ornamentation are shared with the genera Cabobanthus, Crystallopollen, and Cyanthillium, belonging in the subtribes Erlangeinae and Centrapalinae, as well as with the South American genus Pacourina (subtribe Pacourininae) (Wortley et al. Citation2007; Blackmore et al. Citation2009).
Phylogenetic analyses of the total nuclear internal transcribed spacer (ITS) region, the chloroplast gene ndhF, and the non-coding spacer region trnL-F have placed Centauropsis in a combined Centrapalinae/Erlangeinae clade (Keeley et al. Citation2021). The palynological data available for this clade showed variation in the type of aperture (3-colporate, 3-porate, pantoporate) and type of ornamentation (lophate, sublophate) (Wodehouse Citation1928; Erdtman Citation1952; Jones Citation1981; Robinson Citation2007; Bunwong & Chantaranothai Citation2008; Robinson et al. 2014; Robinson et al. Citation2016). Both these characters have shown systematic value in different groups of angiosperms, including Compositae (Walker Citation1974; Feuer Citation1990; Banks Citation2003; Robinson et al. Citation2016; Oliveira et al. Citation2019). According to our observations and information from the literature, the 3-colporate pollen pattern (Parapolydora, Hilliardella, and Centauropsis) co-occurs with a sublophate exine pattern. Thus, all the porate pollen patterns (3-porate and pantoporate; Crystallopollen, Cyanthillium, Pacourina and Cabobanthus) share a lophate exine pattern.
However, palynological data from the remaining genera and species in the Centrapalinae/Erlangeinae clade is still lacking that would allow us to infer synapomorphic characters beyond Centauropsis. Thus, to date, only 15 of the 32 species (< 50%) in the clade that is sister to Centauropsis have had their pollen described (Wodehouse Citation1928; Erdtman Citation1952; Jones Citation1981; Robinson Citation2007; Bunwong & Chantaranothai Citation2008; Robinson et al. 2014; Robinson et al. Citation2016). In addition to a low number of species analysed, a complete, comprehensive, and standardised description of other pollen characters (i.e. pollen grain axes, colporus and endoaperture shape and size, spine shape and size) are not provided at species level, which would also help differentiate the species within the genera or perform a more robust pollen evolution analysis in future studies.
Previous studies have suggested the sublophate 3-colporate pattern, (‘Pollen Type A’ of Keeley and Jones (Citation1979), found mostly in Africa, south-eastern Asia and some Neotropical taxa) is the ancestral condition (Keeley & Jones Citation1979) in Vernonieae but not all authors support this hypothesis (Robinson Citation1999). However, the model of pollen evolution in Vernonieae has not been tested in a molecular phylogenetic context at the tribal level, in part due to insufficient sampling breadth in previous studies especially for taxa from Africa and south-eastern Asia. The sampling limitations of previous studies are not surprising, as this is one of the most species-rich groups in Compositae, with c. 1500 species and a wide distribution (Keeley et al. Citation2021; Siniscalchi et al. Citation2017). Thanks to improved data accessibility from collections, as well as increasing scalability of molecular methods, these sampling limitations can be alleviated during the next decade, to help cast a broader picture of palynological diversity and pollen evolution in Vernonieae. As with the Centrapalinae-Erlangeinae combined clade, more extensive sampling at the species level (both molecular and palynological), and standardisation of pollen terminology, will be key to developing these resources in the future and more accurately test these hypotheses.
Conclusions
Centauropsis is a stenopalynous genus, with the 3-colporate aperture being conserved in all representatives of the genus studied to date. Further study that includes more comprehensive sampling of species in Vernonieae will be necessary to understand the diversity and evolution of pollen within this tribe, and to more confidently phylogenetically place Centauropsis, in relation to other genera in this tribe. Phylogenomic analyses are ongoing that will help understand the classification of the species of Centauropsis and elucidate the relationships within the genera of Vernonieae, for which the integration of these palynological data will be important.
Specimens investigated
Centauropsis Bojer ex DC. [superscript letter a in herbarium voucher represents a species affinis].
Centauropsis antanossi (Scott Elliot) Humbert. Madagascar: Toamasina/Fivondronana. R. Rabevohitra et al. 5005, 18 February 2004 (K000662008). Madagascar, Toamasina/Fivondronana. R. Rabevohitra et al. 4365, 1 February 2003 (K000614892).
Centauropsis cuspidata Humbert. Madagascar: Diego-Suarez/Antsiranana. L. Gautier, T. Rakotomamonjy, LG 3736, 02 June 2000 (K002069634a). Madagascar: Antsiranana, J. Bosser 5641, 1 July 1953 (P0611963). Madagascar: Antsiranana. C. Rakotovao, T. Jaovazaha 2574, 23 November 2005 (P00611960a).
Centauropsis decaryi Humbert. Madagascar: Antananarivo. J.L. Zarucchi et al. 7361, 8 May 1991 (K002069587). Madagascar: Tamatave. D.J. Mabberley 817, 29‒30 March 1971 (K002069586).
Centauropsis fruticosa Bojer ex DC. var. fruticosa Madagascar: Fianarantsoa. R. Rakoto 133, 6 July 1992 (K000662036).
Centauropsis fruticosa var. baronii Humbert. Madagascar: Fianarantsoa/Vatovavy-Fitovinany region. R. Rakoto 133, 6 July 1992 (K002069594).
Centauropsis perrieri Humbert. Madagascar: Mangindrano/Androranga. R. Capuron 24902, 1951 (P00611962).
Centauropsis rhaponticoides Drake. Madagascar: Diego-Suarez/Antsiranana. L. Gautier, S.D. Ramandimbimanana, LG 5128, 4 November 2007 (K002069610). Madagascar: Manangotry. J.H. Mc Whirter 216, 12 September 1968 (K002069611). Madagascar: Antsiranana/SAVA region. D. Randriambolomamonjy et al. 268, 7 November 2007 (K002069607).
Acknowledgements
The authors would like to thank Dr Jehova Lourenco Jr and Manoj Kumar Mahto for assisting with the use of the SEM. The authors are grateful to curators of the herbaria K and P who provided specimens for pollen sampling.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alcantara C, Santos FAR, Alves M. 2022. Pollen morphology and a new combination in tribe Justicieae (Acanthaceae), with emphasis on Brazilian species. Systematic Botany 47: 171–183. doi:10.1600/036364422X16442668423482.
- Antonio-Domingues H, Fortuna Perez AP, Rossi ML, de Almeida RF, Martinelli AP, Luz CFP. 2023. The study of pollen grains as a contribution to the taxonomy of Nissolia (Leguminosae – Papilionoideae – Dalbergieae). Grana 62: 149–171. doi:10.1080/00173134.2023.2227190.
- Antonio-Domingues H, Fortuna Perez AP, Rossi ML, Martinelli AP, Luz CFP. 2022a. Pollen morphology, ultrasculpture and ultrastructure of Poiretia Vent. (Leguminosae – Papilionoideae – Dalbergieae – Adesmia informal clade). Grana 62: 1–19. doi:10.1080/00173134.2021.1984574.
- Antonio-Domingues H, Fortuna Perez AP, Rossi ML, Martinelli AP, Luz CFP. 2022b. Palynology of Amicia Kunth. (Leguminosae – Papilionoideae – Dalbergieae – Informal Adesmia clade) set in a systematic and phylogenetic context. Grana 61: 421–435. doi:10.1080/00173134.2022.2130011.
- Banks H. 2003. Structure of pollen apertures in the Detarieae sensu stricto (Leguminosae: Caesalpinioideae), with particular reference to underlying structures (Zwischenkorper). Annals of Botany 92: 425–435. doi:10.1093/aob/mcg155.
- Blackmore S, Wortley AH, Skvarla JJ, Robinson H. 2009. Evolution of pollen in Compositae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, eds. Systematic, evolution and biogeography of the Compositae, 101–130. Austria: Vienna, University of Vienna, IAPT.
- Bolick MR. 1991. Pollen diameter, exine thickness, and ultrastructure type in the tribes of the Compositae. Compositae Newsletter 19: 17–21.
- Bunwong S, Chantaranothai T. 2008. Pollen morphology of the Tribe Vernonieae (Compositae) in Thailand. The Natural History Journal of Chulalongkorn University 8: 45–55.
- Candolle AP. 1836. Prodromus Systematis Naturalis Regni Vegetabilis, vol. 5. Paris: Treutel & Würtz.
- Erdtman G. 1952. Pollen morphology and plant taxonomy: Angiosperms. Stockholm: Alquist & Wiksell.
- Erdtman G. 1960. The acetolysis method. A revised description. Svensk Botanisk Tidskrift 54: 561–564.
- Feuer S. 1990. Pollen aperture evolution among the subfamilies Persoonioideae, Sphalmioideae, and Carnarvonioideae (Proteaceae). American Journal of Botany 77: 783–794. doi:10.1002/j.1537-2197.1990.tb14468.x.
- Halbritter H, Ulrich S, Grímsson F, Weber M, Zetter R, Hesse M, Buchner R, Svojtka M, Frosch-Radivo A. 2018. Illustrated pollen terminology. Cham: Springer.
- Humbert H. 1960. Flore de Madagascar et des Comores. Paris: Muséum National d'Histoire Naturelle.
- Jones SJ. 1981. Synoptic classification and pollen morphology of “Vernonia” (Compositae: Vernonieae) in the old world. Rhodora 83: 59–75.
- Kingham DL. 1976. A study of the pollen morphology of tropical African and certain other Vernonieae (Compositae). Kew Bulletin 31: 9–26. doi:10.2307/4108992.
- Keeley SC, Forsman ZH, Chan R. 2007. A phylogeny of the “evil tribe” (Vernonieae: Compositae) reveals old/new world long distance dispersal: Support from separate and combined congruent datasets (trnL-F, ndhF, ITS). Molecular Phylogenetics and Evolution 44: 89–103. doi:10.1016/j.ympev.2006.12.024.
- Keeley SC, Jones SB. 1979. Distribution of pollen types in Vernonia (Vernonieae: Compositae). Systematic Botany 4: 195–202. doi:10.2307/2418418.
- Keeley SC, Cantley JT, Gallaher TJ. 2021. The “evil tribe” spreads across the land: A dated molecular phylogeny provides insight into dispersal, expansion, and biogeographic relationships within one of the largest tribes of the sunflower family (Vernonieae: Compositae). American Journal of Botany 8: 505–519. doi:10.1002/ajb2.1614.
- Keeley SC, Robinson H. 2009. Vernonieae. In: Funk VA, Susanna A, Stuessy TF, Bayer RJ, eds. Systematic, evolution and biogeography of the Compositae, 439–469. Austria: Vienna, University of Vienna, IAPT.
- Loeuille B, Souza-Souza R, Abreu VHR, Mendonça CBF, Gonçalves-Esteves VL. 2012. Pollen morphology of the genus Eremanthus Less. (Vernonieae, Asteraceae). Acta Botanica Brasilica 26: 46–57. doi:10.1590/S0102-33062012000100006.
- LPWG. 2017. A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon 66: 44–77. doi:10.12705/661.3.
- Lu L, Wortley AH, Li D, Wang H, Blackmore S. 2015. Evolution of angiosperm pollen. 2. The basal angiosperms. Annals of the Missouri Botanical Garden 100: 227–269. doi:10.3417/2012048.
- Luz CFP, Santos VL, Guedes JS, Silva-Cobra GO, Wanderley MGL. Pollen morphology of some Brazilian Xyris Gronov. ex L. (Xyridaceae) species. Brazilian Journal of Botany 38: 937–950. doi:10.1007/s40415-015-0192-4.
- Maddison WP, Maddison DR. 2006. Mesquite: A modular system for evolutionary analysis, version 3.61. http://mesquiteproject.org/mesquite/mesquite.html; accessed 22 September 2023.
- Mark PJL, Wortley AH, Furness CA. 2012. Not a shrinking violet: Pollen morphology of Violaceae (Malpighiales). Grana 51: 181–193. doi:10.1080/00173134.2012.679310.
- McCune B, Mefford MJ. 2016. PC-ORD multivariate analysis of ecological data. In: Version 7. Gleneden Beach, OR: MJM Software Design.
- Melhem TS’A, Cruz-Barros MAV, Corrêa AMS, Makino-Watanabe H, Silvestre-Capelato MSF, Gonçalves-Esteves VL. 2003. Variabilidade polínica em plantas de Campos do Jordão. IBot. São Paulo: Boletim do Instituto de Botânica.
- Moreira GL, Cavalcanti TB, Mendonça CBF, Gonçalves-Esteves VL. 2019. Pollen morphology of Brazilian species of Verbesina L. (Heliantheae – Asteraceae). Acta Botanica Brasilica 33: 128–134. doi:10.1590/0102-33062018abb0395.
- Oliveira ACS, de Borges RLB, Fortuna Perez AP, Lewis GP, Silva JS. 2019. Characteristics of the exine and aperture of pollen grains of Eriosema and Rhynchosia (Leguminosae – Papilionoideae – Phaseoleae). Grana 58: 292–307. doi:10.1080/00173134.2019.1600576.
- POWO. 2023. Plants of the World Online. Royal Botanic Gardens, Kew. http://www.plantsoftheworldonline.org/; accessed 20 September 2023.
- Punt W, Hoen PP, Blackmore S, Nilsson L, Thomas A. 2007. Glossary of pollen and spore terminology. Review of Palaeobotany and Palynology 143: 1–81. doi:10.1016/j.revpalbo.2006.06.008.
- Robinson H. 1999. Revisions in Paleotropical Vernonieae (Asteraceae). Proceedings of the Biological Society of Washington 112: 220–247.
- Robinson H. 2007. VI. Tribe Vernonieae Cass. (1819). In: Kubitzki K. ed. The families and Genera of vascular plants, vol. 8, Flowering Plants. Eudicots: Asterales, 149–174. New York: Springer.
- Robinson H, Skvarla JJ, Funk VA. 2016. Vernonieae (Asteraceae) of Southern Africa: A generic disposition of the species and a study of their pollen. PhytoKeys 60: 49–126. doi:10.3897/phytokeys.60.6734.
- Salgado-Labouriau ML. 1973. Contribuição a palinologia dos Cerrados. Rio de Janeiro: Academia Brasileira de Ciências.
- Shepherd GJ. 1996. Fitopac 1: manual do usuário. Campinas: Departamento Universidade Estadual de Campinas.
- Siniscalchi CM, Souza-Souza RMB, Loeuille B, Pirani JR, Gonçalves-Esteves V. 2017. The systematic value of pollen morphology in Chresta Vell. ex DC. (Vernonieae, Asteraceae). Review of Palaeobotany and Palynology 244: 182–191. doi:10.1016/j.revpalbo.2017.05.003.
- Souza-Souza R, Loeuille B, Mendonça CBF, Esteves RL, Gonçalves-Esteves VL. 2015. Pollen morphology of the genus Paralychnophora (Vernonieae–Asteraceae). Palynology 40: 280–288. doi:10.1080/01916122.2015.1034380.
- Souza-Souza RMB, Sousa GKR, Esteves RL, Mendonça CBF, Gonçalves-Esteves V. 2022. Importance of palynology in the taxonomy of Piptolepis Sch.Bip. (Asteraceae: Lychnophorinae), a genus endemic to Brazil. Anais da Academia Brasileira de Ciência 94: 1590/0001–37652. doi:10.1590/0001-3765202220201244.
- Stanski C, Luz CFP, Nogueira A, Nogueira MKFS. 2013. Palynology of species in the Astereae and Heliantheae tribes occurring in the region of Campos Gerais, Paraná State, Brazil. Iheringia 68: 203–214.
- Su JXI, Wang W, Zhang LB, Chen ZD. 2018. Phylogenetic placement of two enigmatic genera, Borthwickia and Stixis, based on molecular and pollen data, and the description of a new family of Brassicales, Borthwickiaceae. Taxon 61: 601–611.
- Thiers B. continuously updated. Index herbariorum: A global directory of public herbaria and associated staff. New York: Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/science/ih/; accessed 20 September 2023.
- Vieira S. 2011. Introdução à Bioestatística. 4th edition. Rio de Janeiro: Elsevier Brazil.
- Walker JW. 1974. Aperture evolution in the pollen of primitive angiosperms. American Journal of Botany 61: 1112–1137. doi:10.1002/j.1537-2197.1974.tb12329.x.
- Wodehouse RP. 1928. The phylogenetic value of pollen-grain characters. Annals of Botany 42: 891–934. doi:10.1093/oxfordjournals.aob.a090149.
- Wortley AH, Funk VA, Robinson H, Skvarla JJ, Blackmore S. 2007. A search for pollen morphological synapomorphies to classify rogue genera in Compositae (Asteraceae). Review of Palaeobotany and Palynology 146: 169–181. doi:10.1016/j.revpalbo.2007.03.003.
- Zar JH. 2010. Biostatistical analysis. 5th edition. Upper Saddle River, NJ: Prentice-Hall.