Abstract
Hemoglobin vesicles (HbVs), cellular-type artificial oxygen carriers containing human hemoglobin, were assessed for their biocompatibility by mixing with human plasma in vitro. Among three kinds of HbVs (PEG-DPEA-HbV, PEG-DPPG-HbV and DPPG-HbV), PEG-DPEA-HbV did not affect the extrinsic or intrinsic coagulation activities of the plasma, while PEG-DPPG-HbV and DPPG-HbV tended to shorten the intrinsic coagulation time. The kallikrein-kinin cascade of the plasma was slightly activated by PEG-DPPG-HbV and DPPG-HbV, but not by PEG-DPEA-HbV. The complement consumption of the plasma was observed by incubation with DPPG-HbV, but not with PEG-DPEA-HbV or PEG-DPPG-HbV. These results indicate that PEG-DPEA-HbV has a higher biocompatibility with human plasma.
INTRODUCTION
Hemoglobin vesicles (HbVs) are human hemoglobin encapsulated into a lipid bilayer (i.e. liposome) with a polyethylene glycol (PEG) surface modification and have been developed as an artificial oxygen carrier. We have been evaluating the biocompatibility of the HbVs in vitro using human blood. So far, we have revealed that HbVs hardly activated platelets in terms of the aggregation response, p-selectine expression and the release of RANTES [Citation[1], Citation[2]]. For neutrophils, HbVs have the least effect on the chemotactic activity, gelatinase B release, up-regulation of Mac-1 expression and superoxide production triggered by f-MLP [Citation[3]]. On the basis of these experiments, HbVs appeared to have an acceptable biocompatibility to human blood.
In general, liposomes interact with various kinds of biological components in vitro and in vivo. The interaction depends on the liposome characteristics such as particle size, surface charge, lipid composition and surface modification. It is widely recognized that negatively charged liposomes activate complement in the rat, guinea pigs and humans Citation[4-6], resulting in rapid removal from the blood circulation as opsonized liposomes by the reticuloendothelial system. A negatively charged surface triggers the intrinsic coagulation pathway and kallikrein-kinin cascade by activating coagulation factor XII (FXII) [Citation[7], Citation[8]]. In addition, the cholesterol contents affects the complement activation [Citation[5], Citation[9]], which is thought to be mediated by natural antibodies [Citation[10]].
Incorporation of the PEG-conjugate lipid into liposomes (PEGylation) has been reported to be efficacious to avoid these biological responses Citation[11-13]. Indeed, liposome-encapsulated hemoglobin (LEH), which was not PEGylated, has induced complement activation via both the classical and alternative pathways in human serum containing natural anti-phospholipid antibodies [Citation[14]]. These phenomena become of great concern as a pseudoallergic reaction [Citation[15], Citation[16]], which was already observed in a pig model [Citation[17]]. Recently, however, it has been reported that not only LEH but also PEGylated-liposomes induce hypotension, flushing, respiratory distress, decrease of mean arterola pressure and chest pain [Citation[18], Citation[19]].
In this study, we have used three modified HbVs and assessed their effects on plasma coagulation activity, the kallikrein-kinin cascade and the complement system using human blood. The results obtained here have reinforced the higher biocompatibility of HbVs.
MATERIALS AND METHODS
Liposomes
HbVs were prepared as previously described [Citation[20], Citation[21]]. Briefly, hemoglobin solution prepared from outdated red blood cells for transfusion was heated under a CO gas atmosphere to inactivate possibly contaminated viruses and to remove the stroma and non-hemoglobin proteins [Citation[22]]. After the removal of impurities by centrifugation and filtration, hemoglobin solution was encapsulated into liposomes by mixing with lipids. The liposomes were then extruded through membrane filters with a pore size of 0.22 µm. Three kinds of HbVs were prepared in this study and the lipid composition (mol%) was as follows: dipalmitoyl phosphatidylcholine (DPPC):cholesterol (CHOL):dipalmitoyl phosphatidylglycerol (DPPG):polyethylene glycol-conjugated distearoyl phosphatidylethanolamine (PEG5000-DSPE) = 5:5:1:0.033 (designated PEG-DPPG-HbVs); DPPC:CHOL:DPPG = 5:5:1 (DPPG-HbVs); DPPC:CHOL:dipalmitoyl-L-glutamate-N-succinic acid (DPEA):PEG5000-DSPE = 5:5:1:0.033 (PEG-DPEA-HbVs). All lipids were purchased from Nippon Fine Chemical Co. (Osaka, Japan) except PEG5000-DSPE, which was from NOF Co. (Tokyo, Japan). HbVs were suspended in saline and each of them contains 10 g of Hb/dL, 5.7 g lipids/dL and < 0.1 endotoxin unit of lipopolysaccharide/mL. Empty liposome (Coatsome EL-A, 3.1 g lipids/dL) was purchased from NOF Co. and the lipid composition (mol%) was DPPC:CHOL:DPPG = 30:40:30. The particle size and surface charge as the zeta potential of HbVs suspended in saline were measured by Photal ELS-8000HO (OTSUKA Electronics, Tokyo, Japan).
Measurement of Coagulation Activity
Human plasma was prepared from voluntary donated whole blood at Japanese Red Cross blood centers and was stored at −80°C until use. The prothrombin time (PT) and activated partial thromboplastin time (APTT) as an extrinsic and intrinsic coagulation system, respectively, were determined using a physical clotting assay on a coagulation analyzer (KC10; Amelung, Lehbrinksweg, Germany) at several mixing ratios with plasma and liposomes. Reagents for these assays were purchased from Dade International Inc. (Miami, FL). PT and APTT were measured according to the manufacturer's instructions. Briefly, the mixtures at ratios of 20:80, 40:60 or 60:40 (v/v) of plasma:HbVs or saline were dispensed into a sample cup in duplicate. After the addition of reagents, time to coagulation was measured automatically.
Detection of Kallikrein Activation
The presence of kallikrein activation was evaluated as the degradation of high-molecular-weight kininogen (HMWK), which is substrate of kallikrein naturally existing in the plasma. HMWK was purchased from Enzyme Research Laboratories Inc. (South Bend, IN); anti-human HMWK light chain antiserum from Nordic Immunological Laboratories (Capistrano Beach, CA); plasma kallikrein from Sigma Chemical Co. (St Louis, MO). Plasma was incubated with HbVs or saline at ratios of 80:20, 60:40 or 40:60 (v/v) at 37°C for 24 h. After centrifugation at 15,000 g for 45 min at 4°C, supernatants were treated with 1% sodium dodecyl sulfate (SDS) and 1% 2-mercaptoethanol at 95°C for 10 min and then electrophoresed on a 5–20% gradient polyacrylamide gel containing 0.1% SDS. After proteins were transferred from the gel to nylon membrane, the membrane was treated primarily with anti-HMWK serum, and secondly with anti-goat IgG antibody labeled with horseradish peroxidase. HMWK was detected with a chemiluminescence detection system (ECL; Amersham, Buckinghamshire, UK). Single chain HMWK (S-HMWK) (33 µg) was digested with plasma kallikrein (6 mU) at 37°C for 2 h. Both digested and undigested S-HMWK were used as standards for Western blot analysis.
Measurement of Complement Titer
Human serum was prepared from whole blood of voluntary donors and stored at −80°C until use. Serum was incubated with HbVs, EL-A or saline at ratio of 80:20 or 60:40 (v/v) at 37°C for 1 h. After centrifugation at 15,000 g for 45 min at 4°C, supernatants were stored at −80°C until assay. The complement titer was measured using a 50% hemolysis assay based on Mayer's method with a commercial kit (New One point CH50 (KW); Japan BCG Supply Co., Tokyo, Japan). Therefore, the units of the complement titer were expressed as CH50.
Statistical Analysis
Data were analyzed with parametric Repeated Measures ANOVA following Dunnett post hock test. Statistical significance against saline in each group was established at p < 0.05.
RESULTS
Characteristics of Liposomes
The characteristics of HbVs and EL-A are shown in . The particle sizes of HbVs were 210–240 nm in diameter. The surface charge of PEG-DPPG-HbV and PEG-DPEA-HbV were −3.4 mV and −2.6 mV, respectively, in saline, and were considered to be neutral rather than negative. PEGylation of DPPG-HbV reduced the surface charge from −14.5 mV to −3.4 mV. EL-A, the negatively charged liposome used as a control, had a surface charge of −47.2 mV.
Table 1. Characteristics of liposomes
Coagulation Activity
Prolongation of both PT and APTT was observed as the increase of HbVs or the saline ratio to plasma (). Plasma dilution with HbVs or saline at 20% slightly prolonged or shortened the coagulation time in PT and APTT. Although a significant difference in PT was observed in three HbVs compared to saline at plasma ratios of 20% and 60%, the difference of the mean values was less than 1 second. No significant difference in APTT was observed in PEG-DPEA-HbV at any plasma ratio, but PEG-DPPG-HbV and DPPG-HbV significantly shortened the APTT. However, the shortened time was less than 2 seconds for the mean values.
Table 2. Plasma coagulation activity as measured by PT and APTT
Kallikrein-Kinin Cascade
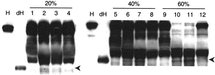
Degradation of HMWK was observed in the plasma incubated with DPPG-HbV and PEG-DPPG-HbV, but not with PEG-DPEA-HbV (). The decrease of intact HMWK and the increase of S-HMWK were evident at a ratio of 60% DPPG-HbV and PEG-DPPG-HbV.
Figure 1 Activation of kallikrein-kinin cascade by HbVs. HbVs or saline were mixed with plasma as indicated ratio (v/v) at 37°C for 24 h. Appearance of digested S-HMWK was detected using western blot analysis as a result of kallikrein activation. Arrows indicate digested S-HMWK. A typical result of three independent assays is shown. H, S-HMWK; dH, digested S-HMWK; lanes 1, 5 and 9, saline; lanes 2, 6 and 10, DPPG-HbV; Lanes 3, 7 and 11, PEG-DPPG-HbV; lanes 4, 8 and 12, PEG-DPEA-HbV.
Complement Consumption
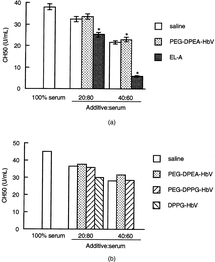
The residual complement titer in serum mixed with PEG-DPEA-HbV or saline was decreased in accordance with the dilution ratio of the additives (). No difference in the complement titer was observed between PEG-DPEA-HbV and saline, indicating that PEG-DPEA-HbV did not consume the complement component. The negatively charged liposome, EL-A, further reduced the complement titer, suggesting that the complement was activated by EL-A (Fig. . No difference was observed between PEG-DPEA- and PEG-DPPG-HbV in the complement consumption (). However, DPPG-HbV significantly consumed complement as no residual complement titer was detected with a mixing ratio of 40%.
Figure 2 Consumption of complement by HbVs. HbVs, saline or EL-A were mixed with serum as indicated ratio (v/v) at 37°C for 24 h. A: Data are represented as the mean ± SEM using serum from five individuals. *Significantly different from saline (p < 0.05). B: Representative data are shown using one serum.
DISCUSSION
We have evaluated the biocompatibility of HbVs using human plasma in vitro. We first investigated the effects of HbVs on the plasma coagulation activity. It is important for HbVs to have no effect on the coagulation activity of the plasma. As shown in , significant prolongations of both PT and APTT were observed as the increase of HbVs or saline ratio to plasma. However, even with the mixing ratio of HbVs or saline at 40%, PT and APTT were maintained in the normal range, 10–14 sec and 26–38 sec, respectively. Interestingly, the shortening of APTT was observed with the mixing ratio at 20% and its degree was significant for DPPG-HbV and PEG-DPPG-HbV compared to saline. The important observation was that only PEG-DPEA-HbV had no effect on APTT compared to saline at any plasma ratio. In addition, HMWK degradation was observed in the plasma incubated with PEG-DPPG-HbV and DPPG-HbV. Cleavage of HMWK reflects the activation of the kallikrein-kinin cascade and the release of bradykinin. HMWK cleavage by PEG-DPPG-HbV and DPPG-HbV may become of great concern because bradykinin is considered to be one of the causes of adverse hypotensive reactions in transfusion [Citation[23]]. On the other hand, PEG-DPEA-HbV did not induce HMWK digestion. Both the intrinsic coagulation pathway and the kallikrein-kinin cascade are initiated by physical contact with FXII or FXII/HMWK with negatively charged surfaces, such as liposomes. Although PEG-DPPG-HbV has a negative charge (−3.4 mV) similar to PEG-DPEA-HbV (−2.6 mV), only PEG-DPPG-HbV affected the intrinsic coagulation system and kallikrein-kinin cascade. It is unclear as to the reason why PEG-DPPG-HbV shortened APPT and activated the kallikrein-kinin cascade. It should be noted that the lipid composition is different between PEG-DPPG-HbV and PEG-DPEA-HbV; PEG-DPPG-HbV and DPPG-HbV have a phosphate group and PEG-DPEA-HbV has a carbonyl group. It is unclear whether the difference in lipid composition affects the intrinsic coagulation system and kallikrein-kinin cascade.
Several kinds of adverse reactions have been reported in the administration of PEGylated liposomes, such as hypotension, flush, respiratory distress, decrease of mean arterola pressure and chest pain [Citation[18], Citation[19]]. Similarly, the decrease of C3, B and C4 was observed in patients, suggesting complement activation by the liposome [Citation[24]]. These reports suggest that PEGylation may be insufficient to avoid complement activation. In this study, however, PEGylation of DPPG-HbV dramatically decreased its reactivity to the complement (). It is well known that negatively charged liposomes reduced the complement titer [Citation[4-6]], and we successfully reproduced this phenomenon in this study (Fig. , indicating that our complement assay system adequately estimated the complement titer. Therefore, the discrepancy of the effect of PEGylation between our study and others was unclear and may depend on the differences regarding lipid composition, liposome size or surface charge.
In summary, PEG-DPEA-HbV has an excellent biocompatibility to human plasma regarding coagulation and kallikrein-kinin systems. Most importantly, the property of PEG-DPEA-HbV of its lacking the ability of complement activation is an essential advantage not only as an oxygen carrier, but also in the pharmaceutical field, such as in liposomal therapeutic drugs and diagnostic liposomes.
This study was supported, in part, by a Health Science Research Grant (Artificial Blood Project) from the Ministry of Health and Welfare, Japan, and by Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture.
REFERENCES
- Wakamoto, S., Fujihara, M., Abe, H., Sakai, H., Takeoka, S., Tsuchida, E., Ikeda, H., Ikebuchi, K. (2001). Artif. Cells Blood Substit. Immobil. Biotechnol. 29: 191–201. [PUBMED], [INFOTRIEVE], [CSA]
- Wakamoto, S., Fujihara, M., Abe, H., Yamaguchi, M., Takeoka, S., Tsuchida, E., Azuma, H., Ikeda, H. Artif. Cells Blood Substit. Immobil. Biotechnol. (in press). [CSA]
- Ito, T., Fujihara, M., Abe, H., Yamaguchi, M., Wakamoto, S., Takeoka, S., Sakai, H., Tsuchida, E., Ikeda, H., Ikebuchi, K. (2001). Artif. Cells Blood Substit. Immobil. Biotechnol. 29: 427–437. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Chonn, A., Cullis, P.R., Devine, D.V. (1991). J. Immunol. 146: 4234–4241. [PUBMED], [INFOTRIEVE], [CSA]
- Cunningham, C.M., Kingzette, M., Richards, R.L., Alving, C.R., Lint, T.F., Gewurz, H. (1979). J. Immunol. 122: 1237–1242. [PUBMED], [INFOTRIEVE], [CSA]
- Devine, D.V., Wong, K., Serrano, K., Chonn, A., Cullis, P.R. (1994). Biochim. Biophys. Acta 1191: 43–51. [PUBMED], [INFOTRIEVE], [CSA]
- Griep, M.A., Fujikawa, K., Nelsestuen, G.L. (1985). Biochemistry 24: 4124–4130. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Mitropoulos, K.A., Martin, J.C., Reeves, B.E.A., Esnouf, M.P. (1989). Blood 73: 1525–1533. [PUBMED], [INFOTRIEVE], [CSA]
- Alving, C.R., Richards, R.L., Guirguis, A.A. (1977). J. Immunol. 118: 342–347. [PUBMED], [INFOTRIEVE], [CSA]
- Alving, C.R. (1984). Biochemical Society Transactions 12: 342–344. [PUBMED], [INFOTRIEVE], [CSA]
- Bradley, A.J., Devine, D.V., Ansell, S.M., Janzen, J., Brooks, D.E. (1998). Arch. Biochem. Biophys. 357: 185–194. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Klibanov, A.L., Maruyama, K., Torchilin, V.P., Huang, L. (1990). FEBS Lett. 268: 235–237. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Woodle, M.C., Matthay, K.K., Newman, M.S., Hidayat, J.E., Collins, L.R., Redemann, C., Martin, F.J., Papahadjopoulos, D. (1992). Biochim. Biophys. Acta 1105: 193–200. [PUBMED], [INFOTRIEVE], [CSA]
- Szebeni, J., Wassef, N.M., Rudolph, A.S., Alving, C.R. (1996). Biochim. Biophys. Acta 1285: 127–130. [PUBMED], [INFOTRIEVE], [CSA]
- Laverman, P., Boerman, O.C., Oyen, W.J.G., Corstens, F.H.M., Storm, G. (2001). Crit. Rev. Ther. Drug Carrier Syst. 18: 551–566. [PUBMED], [INFOTRIEVE], [CSA]
- Szebeni, J., Baranyi, L., Savay, S., Bodo, M., Morse, D.S., Basta, M., Stahl, G.L., Bunger, R., Alving, C.R. (2000). Am. J. Physiol. Heart Circ. Physiol. 279: H1319–H1328. [PUBMED], [INFOTRIEVE], [CSA]
- Wassef, N.M., Johnson, S.H., Graeber, G.M., Swartz, Jr., G.M., Schultz, C.L., Hailey, J.R., Johnson, A.J., Taylor, D.G., Ridgway, R.L., Alving, C.R. (1989). J. Immunol. 143: 2990–2995. [PUBMED], [INFOTRIEVE], [CSA]
- Laing, R.B., Milne, L.J., Leen, C.L., Malcolm, G.P., Steers, A.J. (1994). Lancet 344: 682. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Ringden, O., Andstrom, E., Remberger, M., Svahn, B.M., Tollemar, J. (1994). Lancet 344: 1156–1157. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sakai, H., Takeoka, S., Park, S.I., Kose, T., Nishide, H., Izumi, Y., Yoshizu, A., Kobayashi, K., Tsuchida, E. (1997). Bioconjug. Chem. 8: 23–30. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Sou, K., Naito, Y., Endo, T., Takeoka, S., Tsuchida, E. (2003). Biotechnol. Prog. 19: 1547–1552. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Abe, H., Ikebuchi, K., Hirayama, J., Fujihara, M., Takeoka, S., Sakai, H., Tsuchida, E., Ikeda, H. (2001). Artif. Cells Blood Substit. Immobil. Biotechnol. 29: 381–388. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Abe, H., Ikebuchi, K., Shimbo, M., Sekiguchi, S. (1998). Transfusion 38: 411–412. [PUBMED], [INFOTRIEVE], [CSA], [CROSSREF]
- Brouwers, A.H., De Jong, D.J., Dams, E.T., Oyen, W.J., Boerman, O.C., Laverman, P., Naber, T.H., Storm, G., Corstens, F.H. (2000). J. Drug. Target 8: 225–233. [PUBMED], [INFOTRIEVE], [CSA]