Abstract
Phoridae, which belong to the Diptera order, were identified as the cause of serious infestations of Apis mellifera colonies in the American continent such as Phoridae from the genus “Melaloncha” in Central America and Apocephalus borealis in the USA. Some authors identified a novel phorid, Megaselia scalaris, as a possible parasitoid of honey bee in Brazil. Recently, in Europe, Diptera from the genus Megaselia was found able to parasitize adults of A. mellifera. So far, in the old continent, such infestation was reported only in bees dead or affected by other diseases, but never in healthy bees. From July through August 2014, the prevalence of myiases affecting A. mellifera was verified in Abruzzo and Molise regions. Most of the selected apiaries showed a great infestation of bees due to M. scalaris, even if they were apparently healthy, during trapping back from their foraging trip. Here, we report the results of our work to stress the impact that this parasitoid might have on health condition of beehive.
Los foridos pertenecientes al orden Diptera son reconocidos como responsables de graves infestaciones de colonias de Apis mellifera, en el continente americano. En particular fueron hallados foridos pertenecientes al género Melaloncha en América Central y Apocephalus borealis en los EE.UU. Otro forido, Megaselia scalaris ha sido indicado por algunos autores como posible parásito de las abejas en Brasil. En Europa recientemente se registraron foridos pertenecientes al género Megaselia capaces de parasitar adultos de A. mellifera.Sin embargo, hasta ahora, en Europa esta infestación solo fue citada sobre abejas muertas o enfermas por otras patologías, pero nunca sobre abejas sanas. Las investigaciones fueron realizadas durante los meses de julio y agosto de 2014 y tuvo como objetivo verificar la prevalencia de la miasis en Apis mellifera, en las Regiones de Abruzzo y Molise. En la mayor parte de los colmenares seleccionados, encontramos una fuerte infestación por M. scalaris sobre abejas aparentemente sanas capturadas al regreso de la actividad de forrajeo. Aquí presentamos los resultados de nuestro trabajo para poner en evidencia el impacto que este parásito podría tener sobre el estado sanitario de las colmenas.
Introduction
The “scuttle flies” or Phoridae are one of the largest families of Diptera. Normally Diptera were considered as the main cause of beehive infestation outside Europe. In particular, eight flies species of the genus Melaloncha Brues in Costa Rica (Ramirez, Citation1984) and the zombi fly Apocephalus borealis Brues 1924 in the USA were identified (Core et al., Citation2012). The specific way by which that parasite affects honey bees was described by Ramirez (Citation1984). The female fly attacks honey bees back from foraging flights or standing on a flower. By means of the ovipositor, it lays one or more eggs within the bee abdomen through the intersegmental membranes. Subsequently, larva from the hatched egg develops within the host, feeding of its tissues and hemolymph. Affected bees apparently continued their foraging activity and then they lost the capacity to fly and died (Ramirez, Citation1984). Conversely, honey bees affected by A. borealis were subject to disorientation and abandoned the beehive during the night, attracted by light sources (Core et al., Citation2012). After pupation, larvae become adult insects according to the humidity and temperature conditions. In Europe, Senotainia tricuspis (Macieira, et al., Citation1983) (Diptera: Sarcophagidae) is the most lethal attack to honey bees (Giordani, Citation1956; Orantes Bermejo, González Megías, & García Fernández, Citation1996). Similarly, to Melaloncha and Apocephalus, several species of the genus Megaselia Rondani were found to affect Apis mellifera in Brazil (Macieira, Chaud-Neto, & Zanon, Citation1983) and in Europe (Dutto & Ferrazzi, Citation2014; García Fernández, Santiago Álvarez, & Quesada Moraga, Citation2010). In Europe, this infestation was found only in honey bees which were dead or affected by other diseases. The most common species of the genus Megaselia is Megaselia scalaris, cosmopolitan species characterized by the capacity to colonize different ecological niches. Known as “the scuttle fly”, it is a polyphagous organism that generally acts as saprophagous, sarcophagous, and necrophagous (Costa et al., Citation2007). In addition, it could be a facultative parasitoid affecting different organisms such as humans (Campobasso, Disney, & Introna, Citation2004; Ghavami & Djalivand, Citation2015; James, Citation1947), plants (Disney, Citation1994; Karunaweera, Ihalamulla, & Kumarasinghe, Citation2002; Walter & Wene, Citation1951) and animals, including insects (Andreotti et al., Citation2003; De Gregorio & Leonide, Citation1980; Harrison & Gardner, Citation1991; Macieira et al., Citation1983; Ulloa & Hernandez, Citation1981). The aim of our investigation was to verify the prevalence of myiases affecting A. mellifera in central-southern Italy by two experiments in Abruzzo and Molise regions. The results of our observations highlight the impact that this parasitoid might have on health conditions of Italian beehives.
Materials and methods
Sample collection
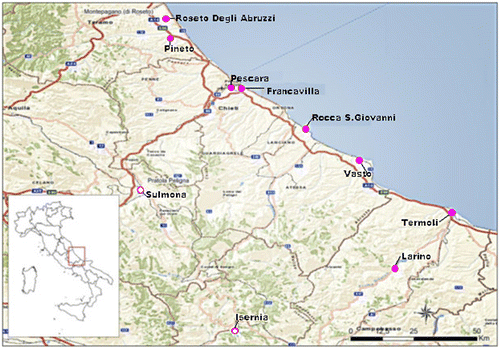
From August to September 2014, the sampling was carried out in different apiaries in Abruzzo and Molise regions. Firstly, we performed a clinical inspection in order to evaluate health condition of honey bees and their population. Then, by closing the entrance of the beehive, we caught the bees back from foraging trip. Samples were sealed in plastic jars (120 ml) with perforated lid (1.5 mm diameter holes) protected by sterile gauze allowing only the air flow in, but preventing also the escape of bees collected. Subsequently, they were introduced in a special box containing a double layer of wet absorbent papers to increase the level of environmental humidity. Samples were incubated at 24 °C. The apiaries selected for our experiments are illustrated in Figure . During the first experiment (23 and 24 August), we selected eight apiaries, six in Abruzzo and two in Molise, respectively. The same apiaries were used also for the second experiment (12 and 13 September), plus the apiary of Sulmona and Larino.
Figure 1. Collection sites in Abruzzo and Molise regions in central-southern Italy where honey bees were collected.
Sample examination
Once dead, bees were subject to specific analysis. For each jar, we counted the number of bees and we performed the visual inspection of each bee. Sometimes, we used also the stereomicroscope to detect some possible physical alterations of bees. Taking note of all observations, we followed this procedure every day for three weeks.
The construction of light trap
A light trap was made according to the method of the Natural History Museum of Los Angeles County for night capture of honey bees parasitized by A. borealis (Natural History Museum Los Angeles County [NHM], Citation2015). On 20 October, we installed the light trap in the apiary 1 (Roseto, Abruzzo) and we left it for three consecutive nights. On the fourth day, we collected bees from the trap and delivered them to our laboratory to detect parasites.
Results
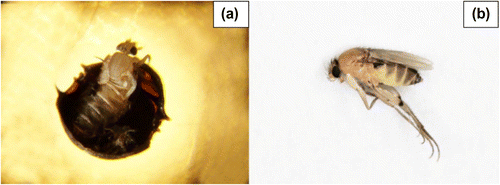
The results of both experiments are reported in the Tables and . Since the beginning of the analysis, we found the first signs of the affection that allowed us to discriminate bees which were M. scalaris positive. They were characterized by blackish color and moist and grubby appearance (Figure a), combined to smell alcohol unlike healthy ones. Once developed, larvae started to perforate head and thorax of each bee which appeared completely broken down (Figure b).
Table 1. Table summary of the results. Number of M. scalaris emerged from honey bees in eight apiaries of Abruzzo and Molise on 23–24 August 2014.
Table 2. Table summary of the results. Number of M. scalaris emerged from honey bees in nine apiaries of Abruzzo and Molise regions on 12–13 September 2014.
The first collection
We performed the first collection on 23 and 24 August in six and two apiaries of Abruzzo and Molise regions, respectively (Table ). Sample analysis was conducted from 28 August to 20 September 2014. The apiaries 1, 2, 6, and 7 resulted positive five days after sampling in contrast with the samples 3 and 5, which became positive after eight days. At the same time, the samples 1, 2, and 5 had already shown the first signs of the transformation from larva to pupa. At first, pupa was characterized by light color and then became darker (Figure c), until the rupture of its outer envelope from which the adult insect emerged. Although sample 4 (Francavilla) was initially negative, it resulted positive after twelve days, in contrast with the sample 8, the only negative one. In the samples 1, 3, and 6 (Roseto, Pescara, Vasto), we observed a relevant increase in larvae for about 23 days (Table ).
The second collection
We performed the second collection on 12 and 13 September in the same apiaries of the previous sampling plus two new ones in Sulmona (Abruzzo) and Larino (Molise) (Table ). Sample examination was performed from 16 September to 10 October. Five days after collection, we observed the presence of the first larvae in samples 1, 3, 4, 5, 6, 7, and 8. However, during sample collection, we were not able to see the conformational change of larva to pupal stage because the process occurred within the bodies of parasitized bees (dead for a long time). This was confirmed by immediate appearance of adult insect without the intermediate pupal stage after eight days from the collection. Of nine, samples 2 and 9 only resulted negative. In samples 1 (Roseto, Abruzzo) and 8 (Larino, Molise), we observed an increase in the number of larvae for approximately 29 days in contrast with the first collection that took 23 days (Table ). This was probably due to a decrease of environmental temperatures during that period.
Morphological analysis of M. scalaris
During both experiments, the samples were subject to an accurate analysis observing in detail the parasite, focusing on its different developmental stages. At the beginning, a gray-white larva, 3 mm long, (Figure a), increased in length until 7–11 mm during later development (Figure b). Then, about ten days after collection, larva began to pupate and presented an oval shape with 11 striations on the back. White pupa became brownish yellow and finally dark brown (Figure c). Two days later pupation, adults emerged from puparium and appeared as blackish-yellowish striped flies (2.5–4 mm) (Figure a), characterized by a large humpbacked thorax and little flattened head (Figure b).
Light trap
On 29 October 2014, we analyzed bees trapped by light trap in Roseto apiary. After three days, the trap collected 28 bees with blackish color and moist and grubby aspect. Two days after collection, the accurate observation of trapped bees, allowed us to find a little white larva emerging from the thorax of parasitized bee. Although it sounds like only one larva was found, data not shown reported that all of the remaining bees (27) that were apparently M. scalaris negative resulted equally parasitized by larvae of M. scalaris which were unable to hatch because of lack of optimal conditions.
The identification of the parasites
For the entomological identification of the species, some of adult flies frozen at −20 °C and stored in ethanol 70% were analyzed by Prof. Antonio Felicioli (Department of Veterinary Sciences of the University of Pisa) and by Dr Francesco De Filippo (Istituto Zooprofilattico Sperimentale of Lombardia and Emilia Romagna). In both cases, the adult parasites were identified as M. scalaris (Phoridae family).
Discussion
Normally honey bees have to counteract with numerous pathogens, parasites, and chemical substances such as insecticides, whose combination represents the cause of Colony Collapse Disorder. This syndrome is characterized by foraging honey bees abandoning their hive (Ellis, Evans, & Pettis, Citation2010; vanEngelsdorp et al., Citation2009). Relevant bee losses were recorded also outside the USA, in Europe, as well as in other countries (Neumann & Carreck, Citation2010). Since 2009 to 2014, a monitoring program was established in Italy to investigate the health status of bee colonies (http://www.reterurale.it). Furthermore, between 2012 and 2014, the European Commission established an epidemiological study that investigated the bee colonies losses, in 17 European countries (Chauzat et al., Citation2014). None of these programs found this phorid fly as possible threat for honey bees. However, in California, Core et al. (Citation2012) provided the first evidence that the phorid fly A. borealis might represent an emerging threat for North American apiculture. Nocturnally active bees were found to be parasitized leaving their hive and dying finally. Wandering like zombies (hence the term “Zoom-Bees”), they could be easily caught by light traps (Core et al., Citation2012) because they were attracted by light sources. Diptera from the genus Melaloncha was previously identified as parasitoid responsible for high mortality of beehives in Central America (Ramirez, Citation1984). Recently, a similar phenomenon was reported also in Europe where Diptera from the genus Megaselia was able to parasitize adults of A. mellifera (Dutto & Ferrazzi, Citation2014; García Fernández et al., Citation2010). Indeed, this infestation was found in sick or dead honey bees only, but never in healthy ones (Dutto & Ferrazzi, Citation2014; García Fernández et al., Citation2010). Here, we describe the occurrence of myiases in two regions of central-southern Italy: Abruzzo and Molise through multiple collections of bees and investigations. Although we caught bees back from foraging trip (still alive), showing no clinical symptom, they were found to be M. scalaris positive. Therefore, our results suggested that the phorid fly M. scalaris was able to parasitize also healthy bees. Unfortunately, we did not establish the absolute infestation rate because of high variability of the number of eggs laid within each bee as reported for other kinds of myiases (Core et al., Citation2012; Ramirez, Citation1984). Moreover, it is noteworthy that the infestation caused by M. scalaris was detected only in the apiaries which were placed near the coast of Adriatic sea; instead, samples collected in apiaries away from the Adriatic sea (Isernia and Sulmona) were M. scalaris negative. This may suggest that around seacoast, parasites could find more favorable conditions for their development. The infestation caused by M. scalaris is serious because of its spread in the Abruzzo and Molise regions where most of the selected apiaries were M. scalaris positive. Moreover, especially during the second sampling (12–13 September), there was a decrease in the number of adult bees. This suggested a severe loss of foraging bees accompanied by negative effects on the development and health status of the beehive. No alterations of brood were observed. Despite the severity of the situation, the beehives affected by myiasis due to M. scalaris survived. As reported by Core et al., (Citation2012), for A. borealis, the confirmation of the nocturnal activity of parasitized bees abandoning their hive was provided by the construction of the light trap where trapped bees resulted M. scalaris positive. However, further studies are needed to better understand this phenomenon. Therefore, our research will continue during the productive season of beehives in order to monitor the persistence of M. scalaris in Abruzzo and Molise regions, and to test the possibility to find this parasite in other Italian regions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgements
We thank Prof. Antonio Felicioli, Department of Veterinary Sciences, University of Pisa, Italy, and Dr Francesco De Filippo, Istituto Zooprofilattico Sperimentale of Lombardia and Emilia Romagna, Italy, for the entomological identification of M. scalaris. We express our gratitude also to the partner designer www.mkdgrafica.com for its support with graphic work.
References
- Andreotti, R., Koller, W. W., Tadei, W.J., do Prado, A. P., Barros, J. C., Dos Santos, F., & Gomes, A. (2003). Occurrence of the Megaselia scalaris (Loew, 1866) (Diptera, Phoridae) as a parasitoid of Boophilus microplus in Campo Grande, MS, Brazil. Revista Brasileira de Parasitologia Veterinaria, 12, 46–47. Retrieved from http://www.cbpv.org.br/rbpv/documentos/1212003/c12146_47.pdf
- Campobasso, C. P., Disney, R. H. L., & Introna, F. (2004). A case of Megaselia scalaris (Loew) (Dipt., Phoridae) breeding in a human corpse. Aggrawal’s Internet Journal of Forensic Medicine and Toxicology, 5, 3–5. Retrieved from http://anilaggrawal.com/ij/vol_005_no_001/pdf/forensic_entomology_special_issue_low_resolution.pdf
- Chauzat, M. P., Laurent, M., Riviere, M. P., Saugeon, C., Hendrikx, P., & Ribiere-Chabert, M. (2014). A pan-European epidemiological study on honey bee colony losses 2012-2013. European Union Reference Laboratory for honey bee health (EURL). Anses, Honey bees pathology Unit, 32 pp. Retrieved from http://ec.europa.eu/food/animals/live_animals/bees/docs/bee-report_en.pdf
- Core, A., Runckel, C., Ivers, J., Quock, C., Siapno, T., DeNault, S., & Hafernik, J. (2012). A new threat to honey bees, the parasitic phorid fly Apocephalus borealis. PLoS ONE, 7, e29639. doi:10.1371/journal.pone.0029639
- Costa, J., Almeida, C. E., Esperança, G. M., Morales, N., Mallet, J. R. D. S., Gonçalves, T. C. M., & do Prado, A. P. (2007). First record of Megaselia scalaris (Loew) (Diptera: Phoridae) infesting laboratory colonies of Triatoma brasiliensis Neiva (Hemiptera: Redu- viidae). Neotropical Entomology, 36, 987–989. doi:10.1590/S1519-566X2007000600026
- De Gregorio, R., & Leonide, J. C. (1980). Un nouveau cas de phoride parasite d’Orthopteres adultes (Dipt.). Bulletin de la Société entomologique de France, 85, 103–105. Retrieved from http://www.lasef.org/new/new_sommaires.html
- Disney, R. H. L. (1994). Scuttle Flies: The Phoridae (1 st ed., 464 pp). London: Chapman & Hall. doi:10.1007/978-94-011-1288-8
- Dutto, M., & Ferrazzi, P. (2014). Megaselia rufipes (Diptera: Phoridae): A new cause of facultative parasitoidism in Apis mellifera. Journal of Apicultural Research, 53, 141–145. doi:10.3896/IBRA.1.53.1.15
- Ellis, J. D., Evans, J. D., & Pettis, J. (2010). Colony losses, managed colony population decline, and Colony Collapse Disorder in the United States. Journal of Apicultural Research, 49, 134–136. doi:10.3896/IBRA.1.49.1.30
- García Fernández, P., Santiago Álvarez, C., & Quesada Moraga, E. (2010). Primera cita de Megaselia scalaris (Loew, 1866), (Diptera: Phoridae) en Apis mellifera iberiensis. Revista Ibero Latino americana de Parasitologia, 69, 72–76. Retrieved from http://www.rilparasitologia.org/journal/download/pdf/id/37
- Ghavami, M. B., & Djalivand, A. (2015). First record of urogenital myiasis induced by Megaselia scalaris (Diptera: Phoridae) from Iran. Journal of arthropod-borne diseases, 9, 274–280. Retrieved from http://jad.tums.ac.ir
- Giordani, G. (1956). Contributo alla conoscenza della “Senotainia tricuspis” Meig., dittero sarcofagide, endoparassita dell’ape domestica (Contribute to the knowledge of Senotainia triscusipis, diptera from sarcophagidae family, endo-parasite of domestic bee). Bollettino dell’Istituto di Entomologia “Guido Grandi” della Università degli Studi di Bologna (Bulletin of entomology “Guido Grandi” Institute of the University of Bologna), 21, 61–84.
- Harrison, R.D., & Gardner, W.A. (1991). Parasitism of the pecan weevil (Coleoptera: Curculionidae) by Megaselia scalaris (Diptera: Phoridae). Journal of Entomological Science, 26, 301–302. Retrieved from http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1479-8298/issues
- James, M. T. (1947). The flies that cause myiases in man (631 pp). Miscellaneous Publication US Department of Agriculture. Retrieved from https://archive.org/details/fliesthatcausemy631jame
- Karunaweera, N.D., Ihalamulla, R. L., & Kumarasinghe, S. P. (2002). Megaselia scalaris (Diptera: Phoridae) can live on ripe bananas-a potential health hazard? Ceylon Medical Journal, 47, 9–10. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12001616
- Macieira, O. J. D., Chaud-Neto, J., & Zanon, A. M. (1983). Oviposition rate and relative viability of descendants from couples of Megaselia scalaris (Diptera: Phoridae) reared in different experimental conditions. Revista Brasileira de Biologia, 43, 223–228.
- Natural History Museum Los Angeles County. (2015). Collecting ZomBees. Retrieved from http://www.nhm.org/site/activities-programs/citizen-science/zombee-watch/collecting-zombees
- Neumann, P., & Carreck N. L. (2010). Honey bee colony losses. Journal of Apicultural Research, 49, 1–6. doi:10.3896/IBRA.1.49.1.01
- Orantes Bermejo, F. J., González Megías, A., & García Fernández, P. (1996). Prevalence of parasitization by Diptera in Apis mellifera L. in southern Spain. Apidologie, 27, 467–471. doi:10.1051/apido:19960605
- Ramirez, W. (1984). Biología del género Melalancha (Phoridae), moscas parasitoides de la abeja doméstica (Apis mellifera L.) en Costa Rica. Revista de Patologia Tropical, 39, 25–28. Retrieved from http://biblat.unam.mx/es/revista/revista-de-biologia-tropical/articulo/biologia-delgeneromelaloncha-phoridae-moscas-parasitoides-de-la-abeja-domestica-apis-mellifera-l-en-costa-rica
- Ulloa, P., & Hernandez, M. (1981). Biología y control natural de Peridroma saucia, plaga de la flor de la curuba. Revista Colombiana de Entomología, 7, 47–53. Retrieved from http://ring.ciard.net/node/10972
- vanEngelsdorp, D., Evans, J. D., Saegerman, C., Mullin, C., Haubruge, E., Nguyen Bach, K., & Pettis, J. S. (2009). Colony collapse disorder: A descriptive study. PLoS ONE, 4, e6481. doi:10.1371/journal.pone.0006481
- Walter, E. V., & Wene, G. P. (1951). Tests of insecticides to control larvae of Euxesta stigmatias and Megaselia scalaris. Journal of Economic Entomology, 44, 998–999. doi:10.1093/jee/44.6.999