Abstract
The effect of sublethal doses of imidacloprid on protein content and activity of proteases on honey bees was analyzed. The study was conducted in three experimental groups: colonies from groups BE-5 and BE-200 were contaminated with 5 and 200 ppb of imidacloprid, respectively, via their food supply (syrup and pollen), while group BE was used as control (untreated). Bee samples were collected 3 and 10 weeks after feeding started. Protein concentration in bee tissue extracts was analyzed with reference: (a) to the dose of imidacloprid; and (b) duration of exposure to the chemical. The average quantity of protein content was significantly higher at the 3-week interval than in the 10-week interval and the bees from control colonies (BE) had significantly higher protein contents than contaminated bees (BE-5 and BE-200), even 3 weeks after feeding with imidacloprid started. Similarly, the activity of proteolytic enzymes (proteases) was found to be dependent on the dose of imidacloprid used, compared to bees from control colonies showing significantly higher activity.
Se ha analizado el efecto de las dosis subletales de imidacloprid en el contenido de proteínas y la actividad de las proteasas en las abejas melíferas. El estudio se realizó en tres grupos experimentales: las colonias de los grupos de BE-5 y BE-200 se contaminaron con 5 y 200 ppb de imidacloprid, respectivamente, a través del alimento suministrado (jarabe y polen), mientras que el grupo SER se utilizó como control (sin tratar). Se recogieron muestras de abejas en las semanas 3 y 10 después del comienzo de la alimentación. La concentración de proteína en extractos de tejido de abejas se analizó en relación con: a) la dosis de imidacloprid; y b) la duración de la exposición a la sustancia química. La cantidad promedio del contenido de proteína fue significativamente mayor en el intervalo de 3 semanas que en el intervalo de 10 semanas y las abejas de las colonias control (BE) tuvieron un contenido de proteína significativamente más alto que las abejas contaminadas (BE-5 y BE-200), incluso 3 semanas después de que comenzara la alimentación con imidacloprid. Del mismo modo se encontró que la actividad de las enzimas proteolíticas (proteasas) depende de la dosis de imidacloprid usada, en comparación con las abejas de colonias de control que muestran una actividad significativamente más alta.
Introduction
The honey bee (Apis mellifera L.) is regarded as one of the most important social insects in the world. A. mellifera has earned this prestigious position through the role it plays in natural ecosystems and in human life and activity, including its economic aspects (Garibaldi et al., Citation2011; Leonhardt, Gallai, Garibaldi, Kuhlmann, & Klein, Citation2013). Dramatic losses of honey bee colonies observed over the last decade have become an issue of almost global importance (Dainat, vanEngelsdorp, & Neumann, Citation2012; Neumann & Carreck, Citation2010). Many abiotic and biotic factors can have an adverse effect on the immunological ability of A. mellifera to ward off infection by viruses, bacteria, and molds (Nosema spp.); it can also result in interactions between pathogens (Di Prisco et al., Citation2013; Pettis, vanEngelsdorp, Johnson, & Dively, Citation2012). Factors such as the poor nutritive quality of bee food, concomitant parasitic Varroa destructor mites and viruses, the exposure of bees to lethal and sublethal effects of pesticides can also weaken the immune system of A. mellifera (Blacquière, Smagghe, van Gestel, & Mommaerts, Citation2012; Pettis et al., Citation2012).
Among many factors affecting honey bees, pesticides are a focus of growing attention, with neonicotinoid insecticides constituting the main subject of scientific studies (Blacquière et al., Citation2012; van Lexmond, Bonmatin, Goulson, & Noome, Citation2015). One of the substances most commonly used in crop protection and registered in over 120 countries is imidacloprid (IMD) (Jeschke, Nauen, Schindler, & Elbert, Citation2011). It is known that IMD, is an agonist of the nicotinic acetylcholine receptor (nAChRs) on the postsynaptic membrane, and like other compounds from this group of pesticides, it affects the central nervous system (CNS) of insects (Brown, Ihara, Buckingham, Matsuda, & Sattelle, Citation2006; Matsuda, Shimomura, Ihara, Akamatsu, & Sattelle, Citation2005) and subsequently the insect’s physiology and behavior (Desneux, Decourtye, & Delpuech, Citation2007; Piiroinen & Goulson, Citation2016; Teeters, Johnson, Ellis, & Mario, Citation2012; Van Dijk, Van Staalduinen, & Van der Sluijs, Citation2013). For example, it has been shown that sublethal doses of IMD have an adverse effect on the bee’s sense of smell and memory (Decourtye, Devillers et al., Citation2004, Decourtye, Armengaud et al., Citation2004; Kirchner, Citation1999; Williamson, Baker, & Wright, Citation2013), as well as on the insects’ intensity of feeding (Schneider, Tautz, Gruenewald, & Fuchs, Citation2012; Yang, Chuang, Chen, & Chang, Citation2008), territorial disorientation (Hatjina, Papachristoforou, Charistos, Bouga, & Arnold, Citation2012; Henry et al., Citation2012), and neurophysiologic changes (Goulson, Citation2013; Guez, Suchail, Gauthier, Maleszka, & Belzunces, Citation2001a, 2001b; Lambin, Armengaud, Raymond, & Gauthier, Citation2001). In recent in vivo studies, it has also been shown that the sublethal doses of imidacloprid not only cause decrease in the size of HPGs but also in the respiratory rhythm of A. mellifera (Hatjina et al., Citation2013). Besides imidacloprid itself, its degradation products released as a result of its metabolism have a further effect on bees (Suchail et al., Citation2004; Simon-Delso et al., Citation2014) and the proteins showing enzymatic qualities constitute an interesting group of compounds involved in different biochemical processes in insects (Frączek, Żółtowska, Lipiński, & Dmitryjuk, Citation2013; Shi, Dick, Ford, & Casida, Citation2009). Furthermore, all above-mentioned sublethal effects can also lead to losses of pollination services provided by honey bees and solitary bees (van der Sluijs et al., Citation2013).
The aim of this study was to determine possible differences in the protein profile and in the activity of proteases in the honey bee under chronic exposure of IMD, due to their important role in the different biological processes in A. mellifera. Our findings can contribute to a better knowledge of the physiological changes induced on honey bees by neonicotinoid insecticides.
Materials and methods
Honey bee colonies, treatments, and sampling
The study was carried out on A. m. carnica colonies in northeast Poland (53°53′49″ N longitude, 20°13′27″ E latitude) during summer 2013. Twenty-four colonies were randomly assigned to three experimental groups, after they were equalized in terms of brood combs and population. All colonies were kept at the same place, they had sister queens and they had no visible symptoms of diseases. Colonies from group BE (control) were given food free from imidacloprid, while the food administered to colonies from group BE-5 and BE-200 was contaminated with 5 and 200 ppb of imidacloprid, respectively. The concentrations of 5 and 200 ppb were chosen, firstly because they are both sublethal, and secondly because they represent an average of field realistic concentrations: the 5 ppb for pollen and nectar (Bonmatin et al., Citation2003, 2005) and the 200 ppb for guttation drops (Tapparo et al., Citation2011). The bees were fed with both bee syrup (Apifortuna) and pollen patties made from fresh pollen loads and inverted syrup Apifortuna in the ratio of 2.5:1.4. The colonies were given 5.5 kg of the liquid food and 0.3 kg of the patty in two portions for a total period of 2.5 months. The survival status of the colonies was assessed several times during the experiment and their overwintering ability was finally assessed in March 2014.
Bee samples were collected from the brood nest twice during the study period: the first sampling took place in late August, 3 weeks after feeding started, in order to ensure that the sampled bees had consumed the contaminated pollen and sugar solution as adults for several days; the second took place at the beginning of October 2013 (at the end of the experiment, 10 weeks after feeding started) in order to ensure the sampled bees had come in contact with the contaminated food not only as adults but also as larvae. Each sample group contained 20–30 adult honey bees. The bees were first weighed, then placed in Eppendorf tubes and were immediately frozen (anaesthetized) in liquid nitrogen. The bee material was stored at −70 °C until use.
Crude extract preparations from bees
Extracts from each sample of bees were obtained by homogenizing whole bees on ice for 2 min with 0.9% NaCl at 1:10 (w/v) ratio in an Omni TH-02 (5000–35,000 RPM, OMNI International, USA) homogenizer. The homogenates were then centrifuged at 2500 g for 15 min at 4 °C for debris removal and at 20,000 g for 40 min at the same temperature for supernatant clarification (Felicioli et al., Citation2004). The insect body extracts from all groups were coded accordingly to the group (as BE, BE-5, and BE-200). All collected materials were stored at −70 °C until use.
Determination of protein content and proteolytic ability
As proteases and inhibitors occur in many tissues and fulfill many different functions in insect biology, this investigation was performed on whole body extracts in order to capture any biochemical change under the influence of IMD (as in Farjan, Dmitryjuk, Lipiński, Biernat-Łopieńska, & Żółtowska, Citation2012; Farjan, Łopieńska-Biernat, Lipiński, Dmitryjuk, & Żółtowska, Citation2014). Extracts of each sample of bees were obtained according to Felicioli et al. (Citation2004). Protein concentrations in each sample were determined with the use of the modified BioRad Protein Assay System and the Bradford dye-binding procedure (Bradford, Citation1976). The results obtained were expressed in mg of protein per 100 mg of bee body weight. As the aim of the study was to determine possible differences in the protein profile and in the activity of proteases in the honey bee, no detailed identification of the proteins was performed at this stage.
The molecular weights of active proteins were estimated following procedures described by Laemmli (Citation1970) and Neuhoff, Stamm, and Eibl (Citation1985). The mix of proteins (Sigma, 6.5–200 kDa) was used as molecular weight markers. The gels were scanned with image scanner (GE Healthcare Life Science, with Lab Scan software, USA), and finally analyzed by densitometry using KTE Gel Scan software (Kucharczyk, Poland).
The proteolytic activity of the bee extracts was determined according to Mendiola, Alonso, Marquetti, and Finaly (Citation1996) using natural substrate as 1% (w/v) gelatin solution in 0.1 M Theorell and Steinhagen buffer (Küster & Thiel, Citation1993) at pH 7.5. The incubation mixtures contained: 25 μl of each one extracts (BE), (BE-5), and (BE-200) and 125 μl gelatin solution and were allowed to incubate for 30 min at 37 °C. The reaction was terminated by the addition of 100 μl of 10% trichloroacetic acid (TCA). After careful mixing and a 10-min incubation at 4 °C, the samples were centrifuged at 14,000 g for 15 min at 4 °C. The concentration of peptides released from gelatin by proteases was determined in the supernatant (Frączek, Żółtowska, Lipiński, & Dmitryjuk, Citation2012) and was expressed in units (U) expressing the mg peptides released per mg protein in bee extract.
Zymography
Zymography was performed with SDS-PAGE, using 12.5% polyacrylamide gels containing 0.1% gelatin in the presence of sodium dodecyl sulfate (SDS), according to Felicioli et al. (Citation2004). Proteins in the clarified extracts from each bee sample containing 15 μg proteins were used in the applied probe. Electrophoresis was carried out at 80 V for about 4 h at 4 °C. After electrophoresis, the gels were washed with gentle shaking at room temperature with 2% (v/v) aqueous solution of Triton X-100 for 30 min to remove SDS and restore the full activity of the peptidases and then rinsed with 0.1 M Theorell and Steinhagen buffer pH 7.5. The gels were transferred to Petri dishes filled with the above-mentioned buffer and incubated for 9 h at 37 °C, and then stained in 0.1% (w/v) colloidal Coomassie brilliant blue G-250. The active fractions appeared as unstained bands on the blue background of gels. Then, the gels were scanned.
Statistical analysis
A two-way ANOVA was carried out using ‘dose’ (0, 5, and 200 ppb) and ‘exposure period’ (3 and 10 weeks) as fixed effect factors using Statistica software package (Statsoft, V. 12.0). Significance of differences between the means was determined by the Duncan’s multiple range test.
Results
Colony observations
During the first three weeks of the experiment, some colonies superseded their queens (1 from group BE, 1 from group BE-5, and 3 from group BE-200) and they continued with their new queens). However, by October, 1 colony from group BE-5 and 3 colonies from group BE-200 (the same colonies that had superseded) had lost half of their population and were removed from the apiary site. The remaining colonies proceeded successfully.
Protein content
The level of protein in tissue extracts was significantly reduced from the 1st to the 2nd sampling in all groups, including control (effect of ‘exposure period’: F2.479 = 388.73, p = 0.0000) (Table ). However, group BE-5 had reduced protein levels by 7% compared to the group BE (control) even during the 1st sampling, that is 3 weeks after exposure to imidacloprid and group BE-200 had a further reduction in protein levels by 22% (effect of ‘dose’: F2.479 = 118.55, p = 0.0000) (Table ). Whereas the difference in protein levels between 3 weeks and 10 weeks exposure for the group BE was 26%, in group BE-5 it was 35.7% and in group BE-200 the reduction was 30.1%, both significantly different from group BE (effect of ‘dose’ X ‘exposure period’: F2.279 = 1.64, p = 0.0027) (Table ). The differences between the groups are influenced by the time of sampling, which reflects the exposure to imidacloprid; the longer the exposure period, the greater the decrease in the protein levels compared to the control group.
Table 1. Protein concentration in bee body extracts (in mg/100 mg of body weight).
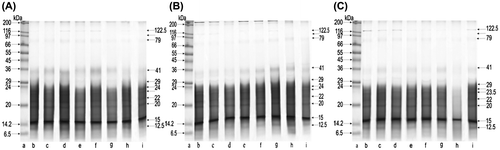
SDS-PAGE fractionations from BE, BE-5, and BE-200 (Figure ; A, B, and C, respectively) revealed the presence of proteins with molecular weights ranging from 8 to 205.5 kDa in the samples of tissues extracted from bees collected in August. The electropherogram BE showed 32 protein fractions, whereas 27 protein fractions were noted in BE-5. A further reduction to 23 fractions was observed on the electropherogram BE-200. The electrophoresis of BE-5, as compared to BE, showed the absence of fractions 39, 37, 23.5, 21, and 20.5 kDa. The lack of those proteins was also observed in BE-200, where an almost complete reduction of fractions 17 and 19 kDa was also noted. In the whole pool of proteins obtained through the electrophoresis of BE, BE-5, and BE-200, there were 7 dominant fractions with molecular weights 12.5; 20; 21.5; 22; 24; 29; and 41 kDa, constituting ca. 64% of all proteins.
Figure 1. SDS-PAGE of body extract proteins collected in August from groups: A, BE; B, BE-5; C, BE-200; lane a, molecular weight markers (the Wide Range Sigma Marker TM 6.5–200 kDa); lanes b–i, different colonies.
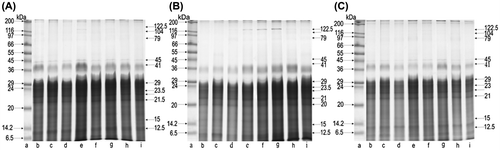
The range of molecular weights of protein fractions obtained from the biological material collected in October for BE, BE-, and BE-200 can be seen in electropherogram images of Figure (A)–(C), respectively. The protein profiles showed an identical range of molecular weights as compared with those obtained from bees examined in August. SDS-PAGE protein fractionation revealed the presence of 27 fractions. However, in the electrophoresis of BE-5, we noted a reduction to 23 fractions and the electropherogram obtained from BE-200 showed a further reduction in the number of fractions to 21. October protein fractionation from BE-5, as compared to BE, revealed the absence of fractions 16, 17, 19, and 22.5 kDa, that were not found in BE-200, either. Moreover, fractions 21 and 23 kDa in BE-200 were almost entirely reduced. Protein fractionation from BE, BE-5, and BE-200 showed that 6 fractions of molecular weights 12.5; 15; 20; 22; 24; and 41 kDa contained 53% of all proteins. The dominant fraction among these was fraction 15 kDa, constituting 18% of the whole protein content.
Proteolytic activity
The highest mean activity of proteolytic enzymes was in control samples, with 0.27 U/mg obtained from BE in August, and 0.26 U/mg obtained from BE in October (Table ). The statistical analysis revealed that the mean activity of enzymes in all tested bee extracts depended on the administered dose of imidacloprid (effect of ‘dose’: F2.479 = 63.842, p = 0.0000). It was also confirmed that the exposure period with the pesticide had no effect on the activity of proteases (effect of ‘exposure period’: F1.479 = 0.718, p = 0.397315). The analysis also showed that there is no significant interaction between these factors (effect of ‘dose’ X ‘exposure period’: F2.279 = 1.64, p = 0.195171); therefore, there was no further reduction due to the exposure duration and the difference existing between the groups at the 1st sampling, 3 weeks after the exposure, was exactly the same even after 10 weeks of exposure.
Table 2. Proteolytic activity of bee body extracts (in U/100 mg of body weight).
Zymography
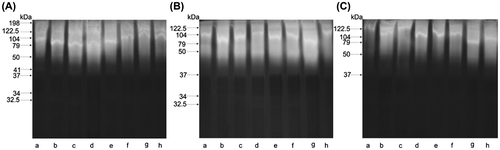
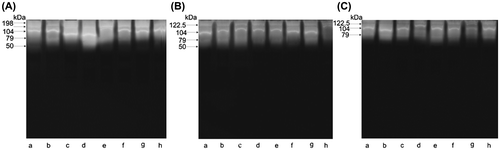
Zymogram scans also provide evidence confirming an effect of the administered dose of imidacloprid on proteolytic activity. The fractionation of samples of BE collected in August after the incubation of gels at pH 7.5 revealed a wide hydrolysis zone in a form of unstained bands against the blue background of gels (Figure (A)). The presence of 9 active fractions with molecular weights ranging from 32.5 to 198 kDa was confirmed (Figure (A)). A clear decrease in the activity of proteases was observed in BE-5 (reduction of two proteins 41 and 198 kDa), as compared to BE (Figure (B)). A loss of further 2 fractions of active proteases, with molecular weights 32.5 and 34 kDa, was observed in BE-200, as compared to BE (Figure (C)). Zymograms obtained from October bee extracts BE, BE-5, and BE-200 can be seen in images of (Figure (A)–(C) respectively). The images in Figure also show a reduction of active fractions of proteases in relation to the administered dose of imidacloprid. Control samples (BE) contained 5 fractions of active enzymes with molecular weights ranging from 50 to 198 kDa. A decrease by 1 fraction of proteases with the molecular weight of 198 kDa was revealed in BE-5, as compared to BE. A further decrease in proteolytic activity, as compared to BE, was confirmed in BE-200 where the absence of active fraction 50 kDa was noted.
Discussion
Neonicotinoid insecticides, including imidacloprid (IMD), belong to a group of stress factor insecticides affecting among others, the performance and the susceptibility of honey bees to biological agents (Alburaki et al., Citation2015; Wu, Smart, Anelli, & Sheppard, Citation2012) due to the effect on the nervous system (Brown et al., Citation2006; Matsuda et al., Citation2005). It has recently been observed that sublethal doses of imidacloprid have a decreasing effect on hypopharyngeal glands (HPGs) of the nursing bees (Hatjina et al., Citation2013). The changes in the size of HPGs (which refers to the diameter of acini) constitute an indicator of the activity of HPGs. The synthesis of royal jelly (RJ) takes place in HPGs; and ca. 82% of RJ proteins belong to the major royal jelly proteins (MRJP) (Knecht & Kaatz, Citation1990; Schmitzová et al., Citation1998). The above results confirm the presence of four major proteins of RJ (i.e., 50, 56, 57, and 64 kDa) (Hanes & Šimuth, Citation1992; Kubo et al., Citation1996). Ohashi, Sawata, Takeuchi, Natori, and Kubo (Citation1996) showed that the gene of the 64 kDa protein/RJP57 is expressed specifically in the nurse bee glands, while the gene encoding the 56 kDa protein is expressed both in nurse bees and foragers. Information concerning the existence of different forms of major proteins MRJP-1 to MRJP-5 can be found in the proteomic studies of Sato et al. (Citation2004). Also Li, Feng, Zhang, and Pan (Citation2008), who based their research on analysis of the proteome from the HPGs of honey bees, showed the presence of six different forms of MRJP-1 with molecular weights from 49 to 60 kDa. The results of these studies are in contrast to the findings of Hanes and Šimuth (Citation1992), and correspond with the results of Santos et al. (Citation2005).
Our work is the first study presenting a profile of proteolytic activity along with protein profile. In our study, we used extracts from whole bees, so it is important to remember that proteases and inhibitors occur in many tissues and fulfill different functions in the biology of insects, from food secretion and digestion (Chapman, Citation1998; Deseyn & Billen, Citation2005; Li et al., Citation2008; Liu et al., Citation2013) to metamorphosis (Lima, Brochetto-Braga, & Chaud-Netto, Citation2000; Malone, Todd, Burgess, & Christeller, Citation2004; Strachecka, Gryzińska, & Krauze, Citation2010; Strachecka, Paleolog, & Grzywnowicz, Citation2008) and immune mechanisms (Evans et al., Citation2006; Frączek et al., Citation2013). It is also well known that some enzymes fulfill detoxification functions, because they take part in the metabolism of pesticides (Shi et al., Citation2009). Another group is represented by a proteolytic system that catalyzes the hydrolytic degradation of peptide bonds and takes part in many biological processes (Costa & Cruz-Landim, Citation2005; Frączek et al., Citation2013; Lima et al., Citation2000; Strachecka et al., Citation2008, 2010; Walter & Clélia, Citation1994). Many such proteins are involved in the immunological response and not in the functioning of digestive enzymes (Ji, Wang, Guo, Hartson, & Jiang, Citation2004; Jiang et al., Citation2005).
In our study, the above-presented protein profiles of tissue extracts from the worker bees of A. mellifera showed statistically significant relationships between the tested parameters, i.e., the content of protein and the dose of administered IMD, as well as the time of exposure to the pesticide. The bees which were fed pollen contaminated with 5 ppb IMD showed a decreased level of proteins by 7% just after 3 weeks and by 10% after 10 weeks of exposure compared to the control. In the case of 200 ppb imidacloprid dose, a further decrease in the concentration of proteins was noted, i.e., by 15% and a further 8%, respectively, 3 and 10 weeks after feeding. This is a clear indication that chronic exposure to imidacloprid even for 3 weeks can substantially reduce the protein content on honey bees. Furthermore, the SDS-PAGE images of the October samples showed the lack of 4 bonds and a 21% decrease in protein content in BE-5, and the absence of 6 bonds and a ca. a 54% decrease in protein content was observed in BE-200, as compared to BE (Figure (A)–(C)). Based on our results, we can conclude that pollen containing both doses of IMD decreases the level of the honey bee proteins and it suggests an intense negative effect of the pesticide on the activity of the honey bee’s HPGs. The causes of this phenomenon presumably lie in a decrease in the size of HPG lobules (Hatjina et al., Citation2013). This leads to a disturbed protein synthesis in the glands, and in particular to a decrease in the production of royal jelly by nurse bees (Ohashi, Natori, & Kubo, Citation1997). Soybean trypsin inhibitor (SBTI) can also have a negative effect on the development of HPGs. Malone et al. (Citation2004), Sagili, Pankiw, and Zhu-Salzman (Citation2005), and Babendreier et al. (Citation2005) also demonstrated a decrease in HPGs protein content in adult honey bees whose diet contained transgenic products as toxin Bt, biotin-binding protein (avidin), and a protease inhibitor (aprotinin).
We also compared changes in the expression of protein profiles in samples BE-5 and BE-200 with those of BE. A high level of the mean proteolytic activity was observed for BE collected both in August and October. Both levels of the activity of enzymes in the samples were very similar to each other. Our analysis showed that the mean activity of enzymes in BE-5 and BE-200 depended on the administered dose of IMD in the bee diet, and that the duration of administering the pesticide had no effect on the activity of proteases. The PAGE zymogram of the protein profile changes provided further confirmation of these results. On the basis of the analysis of our results, we can conclude that the IMD doses administered with pollen in the diet had a negative effect on the level of proteolytic activity in honey bees. A reduction in some of the proteolytic activity in the midgut of the honey bee, after administering a 1% dose of SBTI in the diet, was also noted by Sagili et al. (Citation2005). A confirmation of these results can be found in the earlier studies by Burgess, Malone, and Christeller (Citation1996). These researchers observed that a 1% dose of SBTI in the diet reduced protein biosynthesis in the HPGs of bees and considerably decreased the level of three endopeptidases, such as chymotrypsin, elastase, and trypsin.
In conclusion, our studies show that a reduction in protein biosynthesis and a decrease in the level of proteolytic activity in A. mellifera could be a result of the bees’ exposure to sublethal doses of IMD even for the duration of 3 weeks. Longer duration of exposure increases the impact of IMD in relation to protein levels but not in relation to proteolytic activity. Furthermore, an intensified adverse effect of IMD was observed for the 200 ppb dose (still sublethal, although very high). The reduced protein content in our study signifies also the reduced quality of royal jelly, therefore, a possible change in the feeding behavior towards the queen, thus a stress factor resulting in reduced egg laying and depopulation of the colonies. Along with the above, the question to be answered is: what is the impact of this reduced protein synthesis and activity on the honey bee function or colony development? Taking as an example the indirect effect which was observed on the colony development, especially under the increased levels of the pesticide, this could possibly be the result of the imidacloprid feeding; however, as this was not the scope of the study it has not been thoroughly monitored and it is a hypothesis that needs further investigation. It is however important to remember that population growth is the best predictor of a colony’s ability to survive over the winter and has been demonstrated recently by several studies (Büchler et al., Citation2014; Hatjina et al., Citation2014). Furthermore, the population growth of a colony is influenced by its health status (Di Prisco et al., Citation2013; Pettis et al., Citation2012) and the environment (Khoury, Myerscough, & Barron, Citation2011). It has been shown that an environment contaminated with a neuro-toxic substance can also have delayed and time-cumulative toxicity (Rondeau et al., Citation2014).
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the UWM –Olsztyn, Poland [grant number 528.0113.0811].
Acknowledgments
We wish to thank the anonymous referees for their valuable comments with improve the manuscript.
References
- Alburaki, M., Boutin, S., Mercier, P.-L., Loublier, Y., Chagnon, M., & Derome, N. (2015). Neonicotinoid-coated Zea mays seeds indirectly affect honey bee performance and pathogen susceptibility in field trials. PLOS ONE, 10, e0125790. doi:10.1371/journal.pone.0125790
- Babendreier, D., Kalberer, N. M., Romeis, J., Fluri, P., Mulligan, E., & Bigler, F. (2005). Influence of Bt-transgenic pollen, Bt-toxin and protease inhibitor (SBTI) ingestion on development of the hypopharyngeal glands in honey bees. Apidologie, 36, 585–594. doi:10.1051/apido:2005049
- Blacquière, T., Smagghe, G., van Gestel, C. A. M., & Mommaerts, V. (2012). Neonicotinoids in bees: A review on concentrations, side-effects and risk assessment. Ecotoxicology, 21, 1581–1581. doi:10.1007/s10646-012-0863-x
- Bonmatin, J. M., Marchand, P. A., Charvet, R., Moineau, I., Bengsch, E. R., & Colin, M. E. (2005). Quantification of imidacloprid uptake in maize crops. Journal of Agricultural Food and Chemistry, 53, 5336–5341.10.1021/jf0479362
- Bonmatin, J. M., Moineau, I., Charvet, R., Fleche, C., Colin, M. E., & Bengsch, E. R. (2003). A LC/APCI-MS/MS method for analysis of imidacloprid in soils, in plants, and in pollens. Analytical Chemistry, 75, 2027–2033.10.1021/ac020600b
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. doi:10.1016/0003-2697(76)90527-3
- Brown, L. A., Ihara, M., Buckingham, S. D., Matsuda, K., & Sattelle, D. B. (2006). Neonicotinoid insecticides display partial and super agonist actions on native insect nicotinic acetylcholine receptors. Journal of Neurochemistry, 99, 608–615. doi:10.1111/j.1471-4159.2006.04084.x
- Büchler, R., Costa, C., Hatjina, F., Andonov, S., Meixner, M., Conte, Y., … Wilde, J. (2014). The influence of genetic origin and its interaction with environmental effects on the survival of Apis mellifera L. colonies in Europe. Journal of Apicultural Research, 53, 205-214. doi:10.3896/IBRA.1.53.2.03
- Burgess, E. P. J., Malone, L. A., & Christeller, J. T. (1996). Effects of two proteinase inhibitors on the digestive enzymes and survival of honey bees (Apis mellifera). Journal of Insect Physiology, 42, 823–828. doi:10.1016/0022-1910(96)00045-5
- Chapman, R. F. (1998). The insects. Cambridge, UK: Cambridge University Press. doi:10.1017/CBO9780511818202
- Costa, R. A. C., & Cruz-Landim, C. (2005). Hydrolases in the hypopharyngeal glands of workers of Scaptotrigona postica and Apis mellifera (Hymenoptera, Apinae). Genetics and Molecular Research, 4, 616–623.
- Dainat, B., vanEngelsdorp, D., & Neumann, P. (2012). Colony collapse disorder in Europe. Environmental Microbiology Reports, 4, 123–125. doi:10.1111/j.1758-2229.2011.00312.x
- Decourtye, A., Armengaud, C., Renou, M., Devillers, J., Cluzeau, S., Gauthier, M., & Pham-Delègue, M. H. (2004b). Imidacloprid impairs memory and brain metabolism in the honey bee (Apis mellifera L.). Pesticide Biochemistry and Physiology, 78, 83–92. doi:10.1016/j.pestbp.2003.10.001
- Decourtye, A., Devillers, J., Cluzeau, S., Charreton, M., & Pham-Delègue, M. H. (2004a). Effects of imidacloprid and deltamethrin on associative learning in honey bees under semi-field and laboratory conditions. Ecotoxicology and Environmental Safety, 57, 410–419. doi:10.1016/j.ecoenv.2003.08.001
- Deseyn, J., & Billen, J. (2005). Age-dependent morphology and ultrastructure of the hypopharyngeal gland of Apis mellifera workers (Hymenoptera, Apidae). Apidologie, 36, 49–57. doi:10.1051/apido:2004068
- Desneux, N., Decourtye, A., & Delpuech, J. M. (2007). The sublethal effects of pesticides on beneficial arthropods. Annual Review of Entomology, 52, 81–106. doi:10.1146/annurev.ento.52.110405.091440
- Di Prisco, G., Cavaliere, V., Annoscia, D., Varricchio, P., Caprio, E., Nazzi, F., … Pennacchio, F. (2013). Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proceedings of the National Academy of Sciences, 110, 18466–18471. doi:10.1073/pnas.1314923110
- Evans, J. D., Aronstein, K., Chen, Y. P., Hetru, C., Imler, J. L., Jiang, H., … Hultmark, D. (2006). Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Molecular Biology, 15, 645–656. doi:10.1111/j.1365-2583.2006.00682.x
- Felicioli, A., Donadio, E., Balestreri, E., Montagnoli, G., Felicioli, R., & Podesta, A. (2004). Expression profile of water-soluble proteinases during ontogenesis of Megachile rotundata: An electrophoretic investigation. Apidologie, 35, 595–604. doi:10.1051/apido:2004064
- Farjan, M., Dmitryjuk, M., Lipiński, Z., Biernat-Łopieńska, E., & Żółtowska, K. (2012). Supplementation of the honey bee diet with vitamin C: The effect on the antioxidative system of Apis mellifera carnica brood at different stages. Journal of Apicultural Research, 51, 263–270. doi:10.3896/IBRA.1.51.3.07
- Farjan, M., Łopieńska-Biernat, E., Lipiński, Z., Dmitryjuk, M., & Żółtowska, K. (2014). Supplementing with vitamin C the diet of honey bees (Apis mellifera carnica) parasitized with Varroa destructor: effects on antioxidative status. Parasitology, 141, 770–776. doi:10.1017/S0031182013002126
- Frączek, R. J., Żółtowska, K., Lipiński, Z., & Dmitryjuk, M. (2012). Proteolytic activity in the extracts and in the excretory/secretory products from Varroa destructor parasitic mite of honey bee. International Journal of Acarology, 38, 101–109. doi:10.1080/01647954.2011.610357
- Frączek, R. J., Żółtowska, K., Lipiński, Z., & Dmitryjuk, M. (2013). The mutual influence of proteins from Varroa destructor extracts and from honey bee haemolymph on their proteolytic activity – in vitro study. Acta Parasitologica, 58, 317–323. doi:10.2478/s11686-013-0144-8
- Garibaldi, L. A., Aizen, M. A., Marcelo, A., Klein, A. M., Cunningham, S. A., & Harder, L. D. (2011). Global growth and stability of agricultural yield decrease with pollinator dependence. Proceedings of the National Academy of Sciences U.S.A., 108, 5909–5914. doi:10.1073/pnas.1012431108
- Goulson, D. (2013). Review: An overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology, 50, 977–987.10.1111/jpe.2013.50.issue-4
- Guez, D., Suchail, S., Gauthier, M., Maleszka R., & Belzunces L. P. (2001a). Sublethal effects of imidacloprid on learning and memory in honey bees. In Proceedings of the 7th International Symposium “Hazards of pesticides to bees”, September 7–9, 1999, Avignon (Belzunces L. P., Pelissier C., & Lewis G.B., Eds). Les Colloques de I’INRA, 98, 279.
- Guez, D., Suchail, S., Gauthier, M., Maleszka, R., & Belzunces, L. P. (2001b). Contrasting effects of imidacloprid on habituation in 7- and 8-day-old honey bees (Apis mellifera). Neurobiology of Learning and Memory, 76, 183–191. doi:10.1006/nlme.2000.3995
- Hanes, J., & Šimuth, J. (1992). Identification and partial characterization of the major royal jelly protein of the honey bee (Apis mellifera L.). Journal of Apicultural Research, 31, 22–26. doi:10.1080/00218839.1992.11101256
- Hatjina, F., Papachristoforou, A., Charistos, L., Bouga, M., & Arnold G. (2012). Monitoring the effect of imidacloprid under semi-field conditions using electronic bee counters. Poster in Proceedings of the 5th European Conference of Apidology, September 4–6, 2012, Halle an der Saale, Germany, 208.
- Hatjina, F., Papaefthimiou, Ch., Charistos, L., Dogaroglu, T., Bouga, M., & Emmanouil, Ch (2013). Sublethal doses of imidacloprid decreased size of hypopharyngeal glands and respiratory rhythm of honey bees in vivo. Apidologie, 44, 467–480. doi:10.1007/s13592-013-0199-4
- Hatjina, F., Costa, C., Büchler, R., Uzunov, A., Drazic, M., Filipi, J., … Kezic, N. (2014). Population dynamics of European honey bee genotypes under different environmental conditions. Journal of Apicultural Research, 53, 233–247. doi:10.3896/IBRA.1.53.2.05
- Henry, M., Beguin, M., Requier, F., Rollin, O., Odoux, J.-F., Aupinel, P., … Decourtye, A. (2012). A common pesticide decreases foraging success and survival in honey bees. Science, 336, 348–350. doi:10.1126/science.1215039
- Jeschke, P., Nauen, R., Schindler, M., & Elbert, A. (2011). Overview of the status and global strategy for neonicotinoids. Journal of Agricultural and Food Chemistry, 59, 2897–2908. doi:10.1021/jf101303g
- Ji, C., Wang, Y., Guo, X., Hartson, S., & Jiang, H. (2004). A pattern recognition serine proteinase triggers the prophenoloxidase activation cascade in the tobacco hornworm, Manduca sexta. Journal of Biological Chemistry, 279, 34101–34106. doi:10.1074/jbc.M404584200
- Jiang, H., Wang, Y., Gu, Y., Guo, X., Zou, Z., Scholz, F., … Kanost, M. R. (2005). Molecular identification of a bevy of serine proteinases in Manduca sexta hemolymph. Insect Biochemistry and Molecular Biology, 35, 931–943. doi:10.1016/j.ibmb.2005.03.009
- Khoury, D. S., Myerscough, M. R., & Barron, A. B. (2011). A quantitative model of honey bee colony population dynamics. PLoS ONE, 6, e18491. doi:10.1371/journal.pone.0018491
- Kirchner, W. H. (1999). Mad-bee-disease? Sublethal effects of Imidacloprid (Gaucho®) on the behavior of honey-bees. Apidologie, 30, 422.
- Knecht, D., & Kaatz, H. H. (1990). Patterns of larval food production by hypopharyngeal glands in adult worker honey bees. Apidologie, 21, 457–468. doi:10.1051/apido:19900507
- Kubo, T., Sasaki, M., Nakamura, J., Sasagawa, H., Ohashi, K., Takeuchi, H., & Natori, S. (1996). Change in the expression of hypopharyngeal-gland proteins of the worker honey bees (Apis mellifera L.) with age and/or role. Journal of Biochemistry, 119, 291–295.10.1093/oxfordjournals.jbchem.a021237
- Küster, F. W., & Thiel, A. (1993). Rechentafeln für die chemische Analytik. Berlin (NY): Walter de Gruyter.
- Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685. doi:10.1038/227680a0
- Lambin, M., Armengaud, C., Raymond, S., & Gauthier, M. (2001). Imidacloprid-induced facilitation of the proboscis extension reflex habituation in the honey bee. Archives of Insect Biochemistry and Physiology, 48, 129–134. doi:10.1002/arch.1065.abs
- Leonhardt, S. D., Gallai, N., Garibaldi, L. A., Kuhlmann, M., & Klein, A. M. (2013). Economic gain, stability of pollination and bee diversity decrease from southern to northern Europe. Basic and Applied Ecology, 14, 461–471. doi:10.1016/j.baae.2013.06.003
- Li, J., Feng, M., Zhang, Z., & Pan, Y. (2008). Identification of the proteome complement of hypopharyngeal glands from two strains of honey bees (Apis mellifera). Apidologie, 39, 199–214. doi:10.1051/apido:2007059
- Lima, P. R. M., Brochetto-Braga, M. R., & Chaud-Netto, J. (2000). Proteolytic activity of Africanized honey bee (Apis mellifera: Hymenoptera, Apidae) venom. Journal of Venomous Animals and Toxins, 6, 104–113. doi:10.1590/S0104-79302000000100004
- Liu, Z., Ji, T., Yin, L., Shen, J., Shen, F., & Chen, G. (2013). Transcriptome sequencing analysis reveals the regulation of the hypopharyngeal glands in the honey bee, Apis mellifera carnica Pollmann. PLoS ONE, 8, e81001. doi:10.1371/journal.pone.0081001
- Malone, L. A., Todd, J. H., Burgess, E., & Christeller, J. T. (2004). Development of hypopharyngeal glands in adult honey bees fed with a Bt toxin, a biotin-binding protein and a protease inhibitor. Apidologie, 35, 655–664. doi:10.1051/apido:2004063
- Matsuda, K., Shimomura, M., Ihara, M., Akamatsu, M., & Sattelle, D. B. (2005). Neonicotinoids show selective and diverse actions on their nicotinic receptor targets: Electrophysiology, molecular biology, and receptor modeling studies. Bioscience, Biotechnology, and Biochemistry, 69, 1442–1452. doi:10.1271/bbb.69.1442
- Mendiola, J., Alonso, M., Marquetti, M. C., & Finaly, C. (1996). Boophilus microplus, multiple proteolytic activities in the midgut. Experimental Parasitology, 82, 655–664. doi:10.1006/expr.1996.0004
- Neuhoff, V., Stamm, R., & Eibl, H. (1985). Clear background and highly sensitive protein staining with Coomassie Blue dyes in polyacrylamide gels: A systematic analysis. Electrophoresis, 6, 427–448. doi:10.1002/elps.1150060905
- Neumann, P., & Carreck, N. L. (2010). Honey bee colony losses. Journal of Apicultural Research, 49(1), 1–6. doi:10.3896/IBRA.1.49.1.01
- Ohashi, K., Natori, S., & Kubo, T. (1997). Change in the mode of gene expression of the hypopharyngeal gland cells with an age-dependent role change of the worker honey bee Apis mellifera L. European Journal of Biochemistry, 249, 797–802. doi:10.1111/j.1432-1033.1997.t01-1-00797.x
- Ohashi, K., Sawata, M., Takeuchi, H., Natori, S., & Kubo, T. (1996). Molecular cloning of cDNA and analysis of expression of the gene for α-glucosidase from the hypopharyngeal gland of the honey bee Apis mellifera L. Biochemical and Biophysical Research Communications, 221, 380–385.10.1006/bbrc.1996.0604
- Pettis, J. S., vanEngelsdorp, D., Johnson, J., & Dively, G. (2012). Pesticide exposure in honey bees results in increased levels of the gut pathogen Nosema. Naturwissenschaften, 99, 153–158. doi:10.1007/s00114-011-0881-1
- Piiroinen, S., & Goulson, D. (2016). Chronic neonicotinoid pesticide exposure and parasite stress differentially affects learning in honey bees and bumble bees. Proceeding of the Royal Society B, 20160246, doi:10.1098/rspb.2016.0246
- Rondeau, G., Sánchez-Bayo, F., Tennekes, A. H., Decourtye, A., Ramírez-Romero, R., & Desneux, N. (2014). Delayed and time-cumulative toxicity of imidacloprid in bees, ants and termites. Scientific Reports, 4, 5566. doi:10.1038/srep05566
- Sagili, R. R., Pankiw, T., & Zhu-Salzman, K. (2005). Effects of soybean trypsin inhibitor on hypopharyngeal gland protein content, total midgut protease activity and survival of the honey bee (Apis mellifera L.). Journal of Insect Physiology, 51, 953–957. doi:10.1016/j.jinsphys.2005.04.003
- Santos, K. S., Santos, L. D., Mendes, M. A., Souza, B. M., Malaspina, O., & Palma, M. S. (2005). Profiling the proteome complement of the secretion from hypopharyngeal gland of Africanized nurse-honey bees (Apis mellifera L.). Insect Biochemistry and Molecular Biology, 35, 85–91.10.1016/j.ibmb.2004.10.003
- Sato, O., Kunikata, T., Kohno, K., Iwaki, K., Kieda, M., & Kurimoto, M. (2004). Characterization of royal jelly proteins in both Africanized and European honey bees (Apis mellifera) by two dimensional gel electrophoresis. Journal of Agricultural and Food Chemistry, 52, 15–20.
- Schmitzová, J., Klaudiny, J., Albert, Š., Schröder, W., Schreckengost, W., Hanes, J., … Šimúth, J. (1998). A family of major royal jelly proteins of the honey bee Apis mellifera L. Cellular and Molecular Life Sciences CMLS, 54, 1020–1030.10.1007/s000180050229
- Schneider, Ch. W., Tautz, J., Gruenewald, B., & Fuchs, S. (2012). RFID tracking of sublethal effects of two neonicotinoids insecticides on the foraging behavior of Apis mellifera. PLoS ONE, 7, 54819–54822. doi:10.1371/journal.pone.0030023
- Shi, X., Dick, R. A., Ford, K. A., & Casida, J. E. (2009). Enzymes and inhibitors in neonicotinoid insecticide metabolism. Journal of Agricultural and Food Chemistry, 57, 4861–4866. doi:10.1021/jf900250f
- Simon-Delso, N., Amaral-Rogers, V., Belzunces, L. P., Bonmatin, J.-M., Chagnon, M., Downs, C., … Wiemers, M. (2014). Systemic insecticides (neonicotinoids and fipronil): Trends, uses, mode of action and metabolites. Environmental Science and Pollution Research, 22, 5–34. doi:10.1007/s11356-014-3470-y
- Suchail, S., De Sousa, G., Rahmani, R., & Belzunces, L. P. (2004). In vivo distribution and metabolisation of 14C-imidacloprid in different compartments of Apis mellifera L. Pest Management Science, 60, 1056–1062. doi:10.1002/ps.895
- Strachecka, A., Gryzińska, M., & Krauze, M. (2010). The Influence of environment al pollution on the protective proteolytic barrier of the honey bee Apis mellifera׳s body surface. Polish Journal of Environmental Studies, 19, 855–859.
- Strachecka, A., Paleolog, J., & Grzywnowicz, K. (2008). The surface proteolytic activity in Apis mellifera. Journal of Apicultural Science, 52, 49–56.
- Tapparo, A., Giorio, C., Marzaro, M., Marton, D., Solda, L., & Girolami, V. (2011). Rapid analysis of neonicotinoid insecticides in guttation drops of corn seedlings obtained from coated seeds. Journal of Environmental Monitoring, 6, 1564–1568. doi:10.1039/c1em10085h
- Teeters, B. S., Johnson, R. M., Ellis, M. D., & Mario, D. (2012). Using video-tracking to assess sublethal effects of pesticides on honey bees (Apis mellifera L.). Environmental Toxicology and Chemistry, 31, 1349–1354. doi:10.1002/etc.1830
- Van Dijk, T. C., Van Staalduinen, M. A., & Van der Sluijs, J. P. (2013). Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS ONE, 8, e62374. doi:10.1371/journal.pone.0062374
- van Lexmond, M. B., Bonmatin, J.-M., Goulson, D., & Noome, D. A. (2015). Worldwide integrated assessment on systemic pesticides. Environmental Science and Pollution Research, 22, 1–4. doi:10.1007/s11356-014-3220-1
- van der Sluijs, J. P., Simon-Delso, N., Goulson, D., Maxim, L., Bonmatin, J.-M., & Belzunces, L. P. (2013). Neonicotinoids, bee disorders and the sustainability of pollinator services. Current Opinion in Environmental Sustainability, 5, 293–305. doi:10.1016/j.cosust.2013.05.007
- Walter, R. T., & Clélia, F. (1994). Insect digestive enzymes: Properties, compartmentalization and function. Comparative Biochemistry and Physiology, 109B, 1–62.
- Williamson, S. M., Baker, D. D., & Wright, G. A. (2013). Acute exposure to a sublethal dose of imidacloprid and coumaphos enhances olfactory learning and memory in the honey bee Apis mellifera. Invertebrate Neuroscience, 13, 63–70. doi:10.1007/s10158-012-0144-7
- Wu, J. Y., Smart, M. D., Anelli, C. M., & Sheppard, W. S. (2012). Honey bees (Apis mellifera) reared in brood combs containing high levels of pesticide residues exhibit increased susceptibility to Nosema (Microsporidia) infection. Journal of Invertebrate Pathology, 109, 326–329. doi:10.1016/j.jip.2012.01.005
- Yang, E. C., Chuang, Y. C., Chen, Y. L., & Chang, L. H. (2008). Abnormal foraging behavior induced by sublethal dosage of imidacloprid in the honey bee (Hymenoptera: Apidae). Journal of Economic Entomology, 101, 1743–1748. doi:10.1603/0022-0493-101.6.1743