Abstract
The small hive beetle (SHB, Aethina tumida) is an invasive honey bee pest. It has been introduced into many countries worldwide and it will continue to spread. The lifecycle of the SHB is divided between a feeding and reproduction phase inside honey bee colonies and a pupation phase in the soil, surrounding colonies. Once larvae have achieved their ideal weight, they leave the hive in search of suitable soil in which to pupate. Trapping larvae when they leave the hive could reduce the reproductive success of SHBs, as this would break their lifecycle. Therefore, we investigated the larvae containment rate of different trap designs. Dry and wet larvae were released into traps and left to wander for 12 h, after which we counted the larvae remaining in the trap. Similarly, we tested the permeability of different mesh sizes for dry and wet larvae. Finally, we investigated the speed dry larvae are capable of crawling, by recording the time it took them to crawl a known distance. Dry larvae were contained by all traps. While most designs were unable to contain wet larvae, a trap with walls of sandpaper was able to contain all larvae successfully. Larvae could not pass through a mesh size of 1 mm in dry or wet conditions. The mean wandering larvae speed observed was 0.42 cm/sc. We recommend the use of traps for wandering SHB larvae as a mitigative measure for new introductions and a control method for established populations.
Introduction
The small hive beetle (SHB), Aethina tumida (Coleoptera: Nitidulidae), is a pest and scavenger of honey bee colonies and is native to sub-Saharan Africa (Lundie, Citation1940; Neumann, Pettis, & Schäfer, Citation2016). During the last 25 years, SHBs were introduced into many countries worldwide, leading to economic, societal, and ecological consequences for apiculture (Schäfer et al., Citation2019). The beetle is likely to continue spreading in the future (Cornelissen, Neumann, & Schweiger, Citation2019). SHBs typically spread through the exchange and imports of bees and beekeeping equipment and through transhumance (Mutinelli, Citation2011; Ouessou Idrissou, Huang, Yañez, & Neumann, Citation2019). The lifecycle of the SHB can be divided into two phases: (1) a feeding and reproductive phase inside the honey bee hive and (2) a pupation phase that occurs in the soil around hives. If SHBs are able to mate successfully, fertilized female beetles will lay irregular masses of eggs in small crevices around the hive and in brood cells (Ellis, Richards, Hepburn, & Elzen, Citation2003; Lundie, Citation1940). In some instances, honey bees can detect eggs oviposited in brood cells and abort the impacted brood (Ellis, Delaplane, et al., Citation2004). Nevertheless, some larvae do emerge from their eggs. When they do, they feed on honey, pollen, and brood and progress through three larval instars that differ in size, but otherwise little in appearance (de Guzman & Frake, Citation2007).
The larval SHBs cause most of the damage to host colonies. However, severe destruction usually only occurs if larvae develop en masse, possibly after the adult bees already absconded (completely abandoned the nest), leaving behind the resources necessary for SHB reproduction (Ellis, Citation2012; Ellis & Hepburn, Citation2006). Furthermore, larval faeces promote the fermentation of hive products, making them unsuitable for bee and human consumption (Lundie, Citation1940; Schäfer & Ritter, Citation2014; Schmolke, Citation1974). SHB reproduction is not clearly visible in all infested colonies; but cryptic, low-level reproduction seems to occur in most colonies in which adult SHBs are present (Spiewok & Neumann, Citation2006).
After larvae of the SHB have achieved their ideal weight, they cease feeding and begin a wandering stage. In this phase, they seek a place in the ground in which to pupate. Larvae in the wandering stage primarily wait until early evening to emerge from the hive, possibly to avoid predators (Schmolke, Citation1974). Once out of the hive, the wandering larvae can travel long distances in search of a suitable pupation site in the soil (Bernier, Fournier, & Giovenazzo, Citation2014; Ellis, Hepburn, Luckman, & Elzen, Citation2004; Meikle & Patt, Citation2011). The wandering stage can extend longer than a month if the larvae are unable to find a suitable pupation site (Schmolke, Citation1974). Trapping larvae as they leave the hive to pupate might be a good way to control SHBs, as this method could break the SHB lifecycle. Arbogast et al. (Citation2012) tested a larval trap that they mounted below the hive entrance. This trap was primarily developed as a research tool that could be used to investigate the population dynamics of SHBs (Arbogast et al., Citation2012). They were not designed to capture all larvae leaving a hive. Moreover, these traps could only be used on hives with solid bottom boards, though many beekeepers use screened bottom boards. Furthermore, wandering larvae can exit hives from small cracks and openings around the nest easily. Therefore, we recognized a need to develop a larval trap that encompasses the entire hive footprint. Such a trap possibly could serve as a monitoring tool in areas SHBs are not known to be established (Neumann et al., Citation2016; Schäfer et al., Citation2019) or as a SHB control device. Here, we investigated various trap designs en route to developing a trap for wandering SHB larvae. Furthermore, we determined the speed a wandering larva is able to travel under laboratory conditions.
Materials and methods
Experiments were conducted at the Honey Bee Research and Extension Laboratory at the University of Florida in Gainesville, Florida, USA, in May 2017 and October 2018. Wandering larvae were reared from field caught adult SHBs following standard protocols (Neumann et al., Citation2013). The fermented honey that was produced during the rearing process was collected and used in the trap tests to simulate the conditions of natural mass reproduction of SHBs in honey bee colonies in the field. All tests were conducted at room temperature (22 °C).
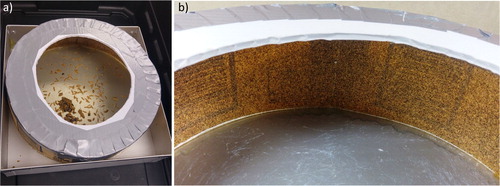
Four trap designs (Traps A–D) were tested in this study. Trap A consisted of a square-shaped container (29 × 29 cm) with a vertical wall of 7.3 cm in height (). Trap B was similar in design and size to trap A, with an additional 1 cm edge at the top of the vertical wall curved at a right-angle (90°) toward the center of the trap (). Trap C was similar to trap B, with an additional downward curve at a 90° angle from the 90° edge at the top of the trap ( and ). Trap D was circular in shape, with an inside diameter of 29 cm. Its vertical walls (9.4 cm) were made of sandpaper (60 Grit 336 U Aluminium Oxid 3 MTM) secured to the wall using epoxy resin. Around the top edge, there was a 5 cm wide ledge of corrugated plastic, covered with duct tape, at an angle of 90° to the trap wall and facing inward ().
Figure 1. Traps A (center), B (right), and C (left) during the “wet test”. Wandering small hive beetle larvae can be seen in the top left corner of trap C (left). Fermented honey is visible in all the traps (photo: K. Stief).

Figure 2. Trap C with dry (left) and wet (right) small hive beetle larvae. Note the larvae scaling the wall surface in wet conditions (Photo’s: K. Stief).

Figure 3. Trap D (a), with wet small hive beetle larvae. Trap D consisted of a vertical wall covered with sandpaper (b) (photos K. Stief).
All trap designs were first tested with dry wandering larvae. For this purpose, 50 dry larvae were introduced into each of the traps for 1 h. This was repeated three times for traps A and D and four times for traps B and C. Additionally, 1000 dry larvae were introduced into trap A and 100 larvae into traps B, C, and D. All traps were observed for 12 h. During observations, we noted if the SHB larvae were able to crawl up the vertical surface and escape the trap.
For the “wet larvae” test, 100–500 wandering larvae were allowed to crawl in 50 g fermented honey and were then placed in the traps and left to wander overnight. After 15 h, we determined the number of larvae that had exited the traps or remained trapped. To recover all larvae that escaped traps during the experiments, the traps were placed in large, lid-covered plastic boxes (60 × 42 × 17.5 cm). The experiment was conducted once with trap A and five times for traps B, C, and D. The efficacy of the test traps can be impacted by SHB larva exposure to honey, as honey can make it easier for larvae to crawl up vertical surface. Thus, we determined if “wet” SHB larvae are able to crawl up a cardboard strip coated with StoLotusan ColorTM (Sto Corp., Atlanta, USA). To do this, five SHB larvae were placed in the middle of a horizontally oriented, 10 cm wide strip until they latched onto the surface. Then, the strip was turned to the vertical position to determine if the larvae were able to cling to and climb up the surface. This was repeated five times with dry larvae, larvae wet with water, and larvae wet with processed honey ().
Figure 4. A small hive beetle larvae crawling up the surface of a piece of cardboard coated with StoLotusan ColorTM (photo: K. Stief).

Given that the traps are constructed to go underneath hives, thus exposing them to the weather, the traps must be fitted with a drain to allow rainwater to leave the trap. Such a drain should prohibit SHB larvae from escaping the trap. Therefore, stainless steel meshes with different mesh widths (2.5, 1.5, 1 mm) were tested to determine larvae ability to pass through the mesh. Dry larvae (n = 30 for mesh widths 2.5 and 1.5 mm; n = 80 for 1 mm) were introduced into 10 cm long containers made of 4 cm PVC-tube (Ø = 4 cm). The tubes were sealed with one of the test meshes () on the bottom end. The top was covered with a solid surface to prevent larvae from exiting the tube from that end. The tubes were placed in a container for >16 h, providing the larvae with an opportunity to escape through the mesh screen. The test was repeated with 120 wet larvae for mesh width 1 mm. The number of larvae remaining in the tubes was determined.
Figure 5. The ability of small hive beetle larvae to pass through three sizes of test meshes (1, 1.5, and 2.5 mm, from left to right) was determined (photo: K. Stief).

Finally, an experiment was performed to calculate at the approximate speed wandering larvae travels under laboratory conditions. Two concentric circles were drawn on a smooth concrete surface, the inner circle with a radius of 4.5 cm and the outer circle with a radius of 24.5 cm. Dry wandering larvae (n = 150) were placed in the inner circle. Thereafter, the time was recorded when the first larvae crossed the inner circle and when the first larvae reached the outer circle. The difference between the two points was the time it took a wandering larva to crawl 20 cm. This experiment was repeated 10 times with the same larvae.
Statistical analysis
We calculated mean containment rates for different trap prototypes and treatments (dry/wet). As all larvae in a dry state were contained in the traps, we only compared trap containment statistically for wet larvae. We used binomial data where each larva was used as a function of containment (0 or 1). A Generalized Linear Mixed Model was used, with containment as a target and trap prototype as a fixed effect. A number of variables were added as a random effect block with diagonal as the best covariance type fit (). The variables included were replicate, duration of the experiment (minutes), total number of SHBs used and the two-factor interaction between time and number of SHBs. No statistical analysis was performed for the other tests, as the data were limited and we were only interested in the question if all larvae were contained. The mean speed was calculated for the wandering larvae.
Table 1. Overview of the generalized linear mixed model (GLMM) parameters for analyzing the containment rate of wet wandering small hive beetle larvae by different trap types.
Results
Dry larvae were contained by all traps, with no larvae escaping. Trap performance varied significantly when wet larvae were used (p < .001, F 23.460; ). The containment rate of trap A was significantly lower than that of all other traps (p < .001), while the performance of traps B and C was comparable (p = .339). Trap D contained all introduced larvae and this resulted in a significantly higher containment rate (p < .001) for this trap than for the others. Trap A and trap C showed different levels of containment (p < .05, F 1.964), with trap C trapping more SHB larvae. All other pairwise comparisons were not significant.
Table 2. Average containment rate (%) of wet, wandering small hive beetle larvae for different trap types.
Neither dry larvae nor larvae wet with water were able to crawl up the vertical strip that was coated with the self-cleaning paint (StoLotusan ColorTM). However, in five tests, an average of three wandering larvae that were wet with fermented honey were able to crawl to the top of the strip.
Only the 1 mm stainless steel mesh was able to contain the SHB larvae () completely. All larvae passed through the 2.5 mm mesh within 16 h. Most (22 of 30) of the SHB larvae passed through the 1.5 mm mesh within 16 h. SHBs never passed through the 1 mm mesh during the study (> 20 h), even when the mesh was turned horizontally and fermented honey was added ().
Table 3. Containment of dry wandering small hive beetle (SHB) larvae by different mesh sizes.
Wandering larvae (n = 10) took a minimum of 48.09 s (SD ± 2.93) to cover a distance of 20 cm (). This calculates to an average speed of 0.42 (max: 0.48, min: 0.38) cm/s.
Table 4. The speed of wandering small hive beetle (SHB) larvae.
Discussion
Our results demonstrate that it is possible to trap wandering SHB larvae effectively. Depending on the design of its vertical wall, a trap can prevent larvae that exit a hive from pupating in the soil around the hive, thus disrupting the SHB lifecycle (). Dry larvae were not able to climb the vertical walls of the trap, except at the joints where they could establish a grip. While it was relatively easy to prevent dry larvae from escaping a tray with vertical walls, wet larvae were more difficult to stop. They were able to climb nearly all surfaces of the trap, probably due to adhesive forces between the smooth surface and the fermented honey covering the larvae. During routine SHB rearing, we observed > 1000 wet wandering larvae that had escaped the rearing container that had a 40 cm high plastic wall each of four consecutive nights. The larvae aggregated approximately 15 m away from the container at a light source. Coating walls with self-cleaning paint (StoLotusan ColorTM) did not prevent wet larvae from climbing the walls (). Furthermore, the wet larvae could navigate the modified top-edges of the walls (Traps B and C; ).
Figure 6. A prototype trap (design A) placed underneath a hive. This picture shows the principle concept of catching wandering small hive beetle larvae while they exit the hive (photo K. Stief).

Only the sandpaper-lined walls in trap D () prevented the wet larvae from escaping. We assume that the granular structure of the sandpaper lowered the force of adhesion on the wall, which made it impossible for the larvae to overcome this barrier. Our results also showed that wandering SHB larvae cannot pass through a 1 mm mesh (, ). Therefore, wandering larvae traps that are created to stop SHBs before they reach the soil have to be constructed with walls lined with a material such as sandpaper and include a water-permeable bottom (≤ 1 mm mesh) to allow rainwater to escape the trap.
Wandering larvae that are not trapped can crawl from the hive until they find suitable soil in which to pupate. Sanford (Citation1998) observed wandering larvae crawling a distance over 200 m from the point of origin. We observed larvae wandering for up to three days, which at the average speed we calculated could theoretically amount to > 1 km distance. This, however, has not been shown under field conditions thus far.
Survival and reproduction of an introduced species are critical to its development as an invasive species (Blackburn et al., Citation2011). These phases determine if a species becomes established or not. The SHB has become established widely beyond its natural area of distribution, from the tropics to temperate climatic zones, and it seems there are few environmental factors preventing its establishment in areas with these climates (Schäfer et al., Citation2019). However, breaking the lifecycle by trapping wandering larvae could aid the eradication of incipient populations of SHBs after introduction into SHB-free areas. The outcome, however, depends on a number of factors to be considered and there are no general rules that can be applied for the successful eradication of invasive alien species (Pluess et al., Citation2012). The honey bee is the preferred host for the SHB. Therefore, installing larvae traps on managed colonies could prevent an incipient population from becoming established as part of an early detection or monitoring system. However, feral colonies, which nest in tree cavities and some artificial cavities, such as those in buildings for example (Seeley & Morse, Citation1976), cannot be fitted with such traps. Thus, the presence of unmanaged colonies should be considered when designing a plan of action to prevent the introduction of or to eradicate SHBs.
Beyond the scope of eradication, an effective larval trap could benefit beekeepers in areas where the SHB has already become established. Thus far, control has focused mainly on trapping adult beetles, with only a limited number of traps and methods designed for the purpose of capturing larvae or killing pupae (Neumann et al., Citation2016). It has been shown that SHB populations increase in an apiary over time and it is advised to alternate apiary locations in the US to disrupt the population growth of SHBs (Hood, Citation2011). Applying an effective larvae trap could lower SHB populations in an apiary. This would be an advantage over all known methods of control targeting SHB larvae or pupae (Benuszak et al., Citation2019), as most of these include the use of active substances that might have unwanted side-effects. The actual efficacy of such a trap should be established using field trials.
There is an urgent need to slow the ongoing global spread of SHBs (Cornelissen et al., Citation2019; Neumann et al., Citation2016). However, to eradicate an invasive species or contain an incipient population from spreading, an action plan is needed and this plan must be based on the biology of the species concerned, the prevailing environmental conditions in the affected region and the appropriate involvement of all stakeholders (Schäfer et al., Citation2019). Our study provides baseline knowledge for the design and implementation of an effective SHB larvae trap that could limit the impact of established SHB populations on managed honey bees’ colonies and slow the spread of new introductions.
Acknowledgements
We thank Peter Neumann for promoting the idea of a trap for wandering SHB larvae and introducing the author team members. We further thank Branden Stanford, Marc Hendriks, Logan Cutts and Brandi Simmons for their assistance with the experiments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arbogast, R. T., Torto, B., Willms, S., Fombong, A. T., Duehl, A., & Teal, P. E. A. (2012). Estimating reproductive success of Aethina tumida (Coleoptera: Nitidulidae) in honey bee colonies by trapping emigrating larvae. Environmental Entomology, 41(1), 152–158. doi:10.1603/EN11186
- Benuszak, J., Brown, M., Chauzat, M.-P., Duquesne, V., Franco, S., Goulrlay-France, C., Kryger, P., … Watkins, R. (2019). Guidance document for the management of the small hive beetle (Aethina tumida) infestation in soil. European Union Reference Laboratory for Bee health (EURL), First Version, April 2019. Retrieved from https://sites.anses.fr/en/system/files/Guidance_document_management_SHB_in_soil_vf.pdf
- Bernier, M., Fournier, V., & Giovenazzo, P. (2014). Pupal development of Aethina tumida (Coleoptera: Nitidulidae) in thermo-hygrometric soil conditions encountered in temperate climates. Journal of Economic Entomology, 107(2), 531–537. doi:10.1603/EC13288
- Blackburn, T. M., Pyšek, P., Bacher, S., Carlton, J. T., Duncan, R. P., Jarošík, V., … Richardson, D. M. (2011). A proposed unified framework for biological invasions. Trends in Ecology & Evolution, 26(7), 333–339. doi:10.1016/j.tree.2011.03.023
- Cornelissen, B., Neumann, P., & Schweiger, O. (2019). Global warming promotes biological invasion of a honey bee pest. Global Change Biology, 25(11), 3642–3655. doi:10.1111/gcb.14791
- de Guzman, L. I., & Frake, A. M. (2007). Temperature affects Aethina tumida (Coleoptera: Nitidulidae) development. Journal of Apicultural Research, 46(2), 88–93. doi:10.1080/00218839.2007.11101373
- Ellis, J. D. (2012). Small hive beetle (Aethina tumida) contributions to colony losses. In D. Sammataro & J. A. Yoder (Eds.), Honey bee colony health: Challenges and sustainable solutions (pp. 135–144). Boca Raton, FL: CRC Press, Taylor & Francis Group, LLC.
- Ellis, J. D., & Hepburn, H. R. (2006). An ecological digest of the small hive beetle (Aethina tumida), a symbiont in honey bee colonies (Apis mellifera). Insectes Sociaux, 53(1), 8–19. doi:10.1007/s00040-005-0851-8
- Ellis, J. D., Delaplane, K. S., Richards, C. S., Hepburn, H. R., Berry, J. A., & Elzen, P. J. (2004). Hygienic behavior of Cape and European Apis mellifera (Hymenoptera: Apidae) toward Aethina tumida (Coleoptera: Nitidulidae) eggs oviposited in sealed bee brood. Annals of the Entomological Society of America, 97(4), 860–864. doi:10.1603/0013-8746(2004)097[0860:HBOCAE]2.0.CO;2
- Ellis, J. D., Hepburn, R., Luckman, B., & Elzen, P. J. (2004). Effects of soil type, moisture, and density on pupation success of Aethina tumida (Coleoptera: Nitidulidae). Environmental Entomology, 33(4), 794–798. doi:10.1603/0046-225X-33.4.794
- Ellis, J. D., Richards, C. S., Hepburn, H. R., & Elzen, P. J. (2003). Oviposition by small hive beetles elicits hygienic responses from Cape honeybees. Naturwissenschaften, 90(11), 532–535. doi:10.1007/s00114-003-0476-6
- Hood, W. M. (2011). Handbook of Small Hive Beetle IPM. Clemson: Clemson University, Cooperative Extension Service.
- Lundie, A. E. (1940). The small hive beetle Aethina tumida. Science Bulletin 220. Pretoria, South Africa: Department of Agriculture and Forestry, Government Printer.
- Meikle, W. G., & Patt, J. M. (2011). The effects of temperature, diet, and other factors on development, survivorship, and oviposition of Aethina tumida (Coleoptera: Nitidulidae). Journal of Economic Entomology, 104 (3), 753–763. doi:10.1603/EC10364
- Mutinelli, F. (2011). The spread of pathogens through trade in honey bees and their products (including queen bees and semen): Overview and recent developments. Revue Scientifique et Technique-Office International des Épizooties. (Ed. S. MacDiarmid). Revue Scientifique et Technique de L'oie, 30(1), 257–271. doi:10.20506/rst.30.1.2033
- Neumann, P., Evans, J. D., Pettis, J. S., Pirk, C. W. W., Schäfer, M. O., Tanner, G., & Ellis, J. D. (2013). Standard methods for small hive beetle research. Journal of Apicultural Research, 52(4), 1–32. doi:10.3896/IBRA.1.52.4.19
- Neumann, P., Pettis, J. S., & Schäfer, M. O. (2016). Quo vadis Aethina tumida? Biology and control of small hive beetles. Apidologie, 47(3), 427–466. doi:10.1007/s13592-016-0426-x
- Ouessou Idrissou, F., Huang, Q., Yañez, O., & Neumann, P. (2019). International beeswax trade facilitates small hive beetle invasions. Scientific Reports, 9(1), 10665. doi:10.1038/s41598-019-47107-6
- Pluess, T., Cannon, R., Jarošík, V., Pergl, J., Pyšek, P., & Bacher, S. (2012). When are eradication campaigns successful? A test of common assumptions. Biological Invasions, 14(7), 1365–1378. doi:10.1007/s10530-011-0160-2
- Sanford, M. T. (1998). Aethina tumida: A new beehive pest in the Western Hemisphere, Apis, 16, 1–5.
- Schäfer, M., & Ritter, W. (2014). The small hive beetle (Aethina tumida). In W. Ritter (Ed.), Bee health and veterinarians (pp. 149–156). Paris: World Organization for Animal Health.
- Schäfer, M. O., Cardaio, I., Cilia, G., Cornelissen, B., Crailsheim, K., Formato, G., … Neumann, P. (2019). How to slow the global spread of small hive beetles, Aethina tumida. Biological Invasions, 21(5), 1451–1459. doi:10.1007/s10530-019-01917-x
- Seeley, T. D., & Morse, R. A. (1976). The nest of the honey bee (Apis mellifera L.). Insectes Sociaux, 23(4), 495–512. doi:10.1007/BF02223477
- Schmolke, M. D. (1974). A study of Aethina tumida: The small hive beetle (Project Report, p. 178). University of Rhodesia.
- Spiewok, S., & Neumann, P. (2006). Cryptic low-level reproduction of small hive beetles in honeybee colonies. Journal of Apicultural Research, 45(1), 47–48. doi:10.3896/IBRA.1.45.1.11
