Abstract
Bee-collected pollen is stored in the colony and mixed with bee saliva and microorganisms, resulting in pollen’s fermentation and acidification. Pathogens infecting the colony, including the deformed wing virus (DWV), may be transferred into the bee bread (BB). In this study, we used an in vivo infection assay to determine whether DWV remains infective in BB. We also used Lactobacillus kunkeei to ferment UV-treated fresh pollen. This fermented pollen in vitro (FPV) was used as a virus-free reference for the initial viral loads in BB and to evaluate the effect of controlled bacterial fermentation on the infectivity of DWV. Finally, we investigated the thermal inactivation of DWV in BB and FPV. To accomplish these goals, we added DWV inoculum to BB and FPV and heated them at 70 °C or 60 °C for 1 h. Suspensions of the virus recovered from the treated pollen samples were injected into pupae to evaluate the degree of viral viability and the efficacy of thermal inactivation. We found that the virus recovered from non-thermally treated BB and FPV samples remained highly infectious in the pupal injection assay. Overall, BB and FPV treated at 60 °C had an average reduction of 98.69% of viable DWV copies, while treatments at 70 °C inactivated DWV almost entirely (99.99%). This study demonstrates that DWV remains infective in BB and that thermal inactivation is an effective strategy for significantly reducing levels of viable DWV in contaminated pollen.
Introduction
In recent decades, honey bee colony losses have increased in the northern hemisphere. Winter colony losses have ranged between 22.2% and 40.5% in the USA from 2015 to 2020 (Bruckner et al., Citation2020; Kulhanek et al., Citation2017). One of the factors associated with this problem is the high prevalence of viruses (Highfield et al., Citation2009). Viral replication can occur through horizontal or vertical pathways based on developmental, physiological, ecological and pathological conditions (Chen et al., Citation2006). When colonies thrive under non-competitive conditions, bee viruses are primarily transmitted vertically and remain in a latent state without causing overt disease (Tentcheva et al., Citation2004; Yue et al., Citation2007). Alternatively, when colonies experience adverse conditions, including pest infestations and nutritional stress, horizontal routes of transmission become activated, resulting in the outbreak of viral infections and observable disease (Chen & Siede, Citation2007).
Among the approximately 90 viruses identified in the western honey bee (Apis mellifera) to date, deformed wing virus (DWV) is one of the most prevalent worldwide (Beaurepaire et al., Citation2020; Martin et al., Citation2012) and is closely correlated with winter losses (Dainat & Neumann, Citation2013; Highfield et al., Citation2009; Steinmann et al., Citation2015). Symptoms of DWV infection include missing or malformed wings, shortened abdomens and premature death (de Miranda & Genersch, Citation2010). DWV can be transmitted vertically and horizontally (Chen et al., Citation2006; Yue et al., Citation2007). Vertical transmission is important for DWV maintenance in the population, although the efficiency of this mode of transmission is low (Amiri et al., Citation2018). Varroa infestation in pupae and adults is the most efficient horizontal transmission route of DWV and is associated with acute symptomatic infection (Ball & Allen, Citation1988; Ball, Citation1989; Chen & Siede, Citation2007). However, several lines of evidence indicate that DWV is also transmitted horizontally via food ingestion during larval feeding: First, DWV has been detected in larval food (Yue & Genersch, Citation2005). Second, in vitro experiments show that DWV infects bee larvae by ingesting virus-contaminated sugar syrup (Ryabov et al., Citation2016; Citation2019) and pollen (Schittny et al., Citation2020). Third, high levels of DWV can be detected in all the body parts of honey bees showing disease symptoms, but not in the heads of asymptomatic bees (Yue & Genersch, Citation2005; Mazzei et al., Citation2014). These studies suggest horizontal transmission may occur as DWV replicates in the hypopharyngeal gland and is transferred into the larval food. DWV transmission via food ingestion is not exclusive to larval stages, as it has also been shown in adult stages through cannibalism and trophallaxis (Posada-Florez et al., Citation2021).
Several studies suggest that horizontal transmission can also occur through ingestion of DWV-contaminated pollen and honey stored within the colony. Queens from DWV-free colonies exposed to virus-contaminated food have been shown to produce DWV-positive eggs, indicating that the queen became infected (Singh et al., Citation2010). DWV has been detected in pollen sampled directly from visited flowers and in pollen pellets from uninfected foragers, suggesting that this pollen contains live viruses previously transferred by visiting infected bees (Mazzei et al., Citation2014; Singh et al., Citation2010). DWV has been detected in food (honey and pollen) after six months of storage at ambient and variable outdoor temperatures (Singh et al., Citation2010). These results suggest that DWV can resist the acidic environment of bee-fermented pollen and remain infectious for an extended period. Thus, DWV in stored pollen could cause colony re-infection several months after the natural decline of Varroa during the winter or after acaricide treatment. Notably, it has been shown that although colony DWV titers decline after acaricide treatment, subclinical DWV persists, and viral levels may increase gradually in the absence of Varroa (Locke et al., Citation2017).
The remarkable nutritional properties of pollen have been exploited in industries other than beekeeping. For example, fresh pollen collected by honey bees provides the primary source of nutrients for rearing bumble bees in captivity for fruit and vegetable pollination. However, utilizing untreated raw pollen has resulted in the transmission of pathogens, including DWV and Nosema ceranae, into the bumble bee industry (Graystock et al., Citation2013, Citation2016). In response to this threat, gamma irradiation has been successfully used to inactivate honey bee viruses. In these experiments, the effectiveness of treatment was evaluated by measuring the ability of IAPV to cause mortality after injection of suspensions obtained from treated pollen or by detecting DWV, SBV, BQCV, KBV and IAPV genomes in treated pollen by RT-PCR (Graystock et al., Citation2016; Meeus et al., Citation2014). Bee pollen also has nutritional benefits for humans (Kostić et al., Citation2020). The nutritional supplement industry has developed strategies for inactivating pollen microorganisms that might harm humans or cause pollen spoilage (De-Melo et al., Citation2015). One common practice involves maintaining a temperature over 55 °C throughout the dehydration processing. These thermal methods used in the commercial processing of pollen have been shown to have only a minor effect on the main components of pollen, such as amino acids and lipids, but they do have a significant effect on the abundance of bioactive compounds including, carotenoid pigments, flavonoids, polyphenols and some vitamins (Castagna et al., Citation2020; Domínguez-Valhondo et al., Citation2011). Therefore, a tradeoff must be balanced between the loss of some nutritional components and the benefit of eliminating pathogens during thermal treatment. An optimal temperature of 60 °C was proposed for commercially drying bee pollen based on the examination of microbiological, structural, and physical-chemical characteristics (Zuluaga-Domínguez et al., Citation2018).
In this study, we investigated the infectiousness of DWV in bee bread (BB) and tested the effectiveness of thermal treatments to inactivate DWV. We also used Lactobacillus kunkeei to ferment UV-treated fresh pollen. This virus-free fermented pollen in vitro (FPV) was used for two purposes: first, as a reference to determine the initial DWV loads of BB collected from field colonies, and second, to evaluate the effect of controlled bacterial fermentation on the infectivity of DWV. We assessed DWV infectivity before and after thermal treatment through an in vivo assay that measured replication following injection into pupae. This approach enabled us to directly assess viral infectivity in live bees because, in some cases, the genetic material of thermally inactivated viruses could still be detected using RT-PCR.
Materials and methods
Honey bees
Experiments were performed using honey bees from five colonies maintained during the summer of 2019 at Gordon College in Wenham, Massachusetts. The detailed genetic background of these colonies was not determined. However, they are presumably a mixture of European subspecies of Apis mellifera, predominantly Apis mellifera ligustica. Varroa infestation levels in these colonies ranged between 1.3% and 8.7%.
Preparation of DWV suspension
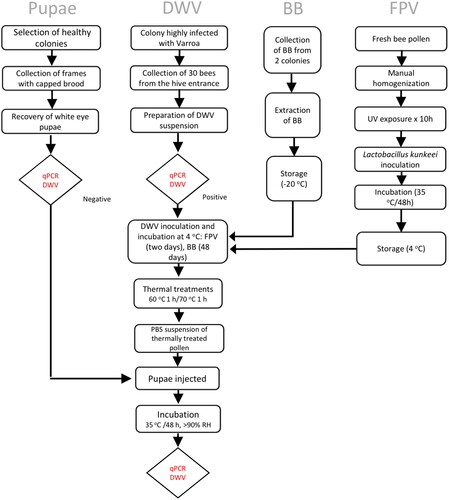
Pools of thirty adult bees were collected from the hive entrance of the colony with the highest Varroa infestation level (8.7%) (). Collected bees were brought to the laboratory and euthanized by exposure to CO2 for five minutes. Bees were stored at −80 °C until processing. Individual bees were manually crushed in 1.7 ml Eppendorf tubes with a plastic pestle in 700 µl of ice-cold PBS. Each bee homogenate was spun at 5000 rpm for 1 min at 4 °C in a benchtop microcentrifuge (Eppendorf). The resulting crude suspension (∼23 ml total) was filtered through a 0.22 µm syringe filter membrane (Millex-GV, MilliporeSigma). Finally, 10 µl of the filtered suspension was diluted 100-fold in water, and 5 µl of this was used as the starting template for RT-PCR. The remaining suspension was stored at −80 °C.
Collection of bee bread
Bee bread (BB) was collected from two field colonies during the winter of 2018 at Gordon College’s bee yard. The frames were transferred to the laboratory and BB was collected into 50 ml polyethylene tubes. BB samples were manually homogenized for 10 min using a spatula and stored frozen at −20 °C until further processing.
Fermented pollen in vitro (FPV)
Fresh bee pollen was obtained from pollen traps and stored at 4 °C until processing. One kilogram of fresh pollen was homogenized manually with a mortar and pestle. To minimize the influence of contaminating microbes in the pollen before fermentation, 900 g of the homogenized pollen was placed on a tray and exposed to UV-C (254 nm wavelength) illumination for 10 h in a biosafety cabinet. The pollen was stirred every hour during this process (Moreno Galarza, Citation2012). Meanwhile, 225 ml of sugar syrup solution was inoculated with Lactobacillus kunkeei, a fructose-fermenting bacteria present in the honey bee gut (Asama et al., Citation2015; Neveling et al., Citation2012). The syrup solution was composed of 40% fructose-yeast extract-polypeptone (FYP) broth (Filannino et al., Citation2016), and 60% sugar solution with a concentration of 15% fructose + glucose (1:1). The solution was incubated with shaking at 200 RPM for 24 h at 34 °C until reaching 108 CFU/ml, verified as an OD600 value of 0.4. Before fermentation, the pH of a sample diluted 1:1 in deionized water was measured as 4.0; after fermentation, the pH of a 1:1 diluted sample was 3.5. Measurements were repeated three times per sample/trial. Subsequently, 225 ml of this bacterial culture was mixed with 900 grams of pollen and poured into small sterile glass canning jars, filling them ¾ full. Jars were warmed in a water bath at 30 °C for 25 min to remove air bubbles. Jars were sealed and incubated for 48 h at 35 °C. After incubation, FPV was stored at 4 °C for ten days before inoculation with DWV.
BB and FPV inoculation with DWV
Two trials were performed, as shown in . BB and FPV samples were placed into separate 15 ml plastic tubes before inoculating with DWV. For both trials, a suspension containing 6.69 x108 DWV copies/ml was used to infect both pollen sample groups to reach a final concentration of approximately 8 x107 DWV copies/g of pollen in the first trial and 1.5 x107 copies/g of pollen in the second trial. Samples containing 1.2 g of infected pollen were transferred into 2 ml tubes and stored at 4 °C for 48 days (FPV) or two days (BB) before thermal treatment.
Table 1. Trials, treatments (T1-T5), and replicates per treatment. Trials 1 and 2, describe independent experiments performed at 70 °C and 60 °C. Treatments include non-thermally treated (23 °C) and thermally treated BB and FPV samples in each trial. Five pollen samples were used per treatment, and three pupae were injected per sample (n = 15 per treatment).
Heat treatments of infected pollen samples
Four thermal treatments (T1-T4) were performed per trial, with five pollen replicates per treatment. Samples for treatments T1 and T3 were heated in a dry bath incubator (Fisher Scientific) at 70 °C or 60 °C for 1 h. The lower temperature was chosen based on a previous report of the optimal temperature for drying bee pollen for commercial purposes (Zuluaga-Domínguez et al., Citation2018). Control samples (T2, T4) were kept at room temperature (23 °C) for the same period. PBS-injected pupae were included as a control (T5) ().
Viral suspension preparation from pollen
For each replicate, 100 mg of pollen was diluted with 750 µl of sterile PBS in a screw-cap 2 ml tube. Each sample was homogenized in a FastPrep-24 homogenizer (MP Biomedicals) at 4.0 m/s for 20 s, then centrifuged at 6000 x g for 1 min. The resulting supernatant was then filtered through a 0.22 µm syringe filter (Millex-GV, MilliporeSigma) and stored at 4 °C for 24 h until further analysis.
Table 2. Primers for DWV and control gene (RPS5) used for qPCR.
In vitro infection assay
Pupae were collected from healthy colonies during the summer of 2019 at Gordon College, Wenham, MA. Colonies were previously treated for 15 days with commercial formic acid pads for controlling Varroa destructor. After the treatment, Varroa counts were conducted to validate treatment efficacy and identify colonies for sampling. Two frames with capped brood were collected from one colony. The frames were kept in an environmental chamber at 32 °C and 70% relative humidity. The process for selecting the pupae was completed by gently removing them from their cells using fine-tipped forceps within a controlled, sterile environment of a laminar flow biosafety cabinet. Initially, six pupae with white eyes (10 days old) were selected randomly from each frame and tested for DWV presence by RT-PCR before proceeding with the experiment. After this analysis to determine that pupae were not infected with DWV, 130 pupae with white eyes were collected and placed on a damp paper towel pad inside a 60 mm petri dish and kept at 35 °C, > 90% relative humidity. After 48 h, pupae were checked for viability and health status prior to injection. Dead or damaged pupae were excluded from the experiment. Healthy pupae were injected manually with 5 µl of the pollen supernatant suspension. The number of DWV copies in the suspensions of the non-thermally treated pollen samples was estimated at 5.5 x104 DWV copies per pupa in the first trial and 1 x104 copies per pupa in the second trial. Viral concentrations were estimated based on the initial viral loads introduced into the pollen samples upon inoculation, assuming no losses during the subsequent filtration step. Injections were performed using 30-Ga needles with a manually operated syringe control device. Injections were placed between the third and fourth tergite, and needles were replaced between injections. The injections were administered to groups consisting of three pupae, which were immediately returned to the environmental chamber in the same petri dish before proceeding to the next group. One group was injected with a sterile PBS solution as a negative control. Injected pupae were incubated for 48 h and then transferred into a 2 ml tube containing 750 µl of RLT buffer (QIAGEN, contains guanidine isothiocyanate) with 1% 2-mercaptoethanol and homogenized using a FastPrep-24 bead-based cell disruptor (MP Biomedicals) at 4.0 m/sec for 10 s. The homogenized samples were stored at −80 °C (Dietemann et al., Citation2013).
DWV quantitation
Quantitative RT-PCR was conducted according to the manufacturer’s recommendations using the Luna® Universal One-Step RT-qPCR Kit (New England Biolabs, Ipswich, MA). The analysis was carried out on a Bio-Rad CFX96 Real-Time System running CFX Manager 3.1 Software (Bio-Rad, Hercules, CA). The RLT pupae homogenate was diluted 1:100 with PCR-grade water before direct addition to the RT-qPCR reaction to minimize inhibition and streamline processing. Individual reactions were conducted in 20 μl volumes. The reactions included 5.0 μl of the diluted RLT pupae homogenate as the template, 10 μl of Luna Universal One-Step Reaction Mix, 1 μl of RT enzyme, 1.6 μl forward and reverse primers (0.4 μM final concentration/primer) and 2.4 μl of molecular grade water.
The cycling protocol was as recommended: 55 °C for 10 min for reverse transcription, 95 °C for 1 min for initial denaturation, followed by 40 amplification cycles of 95 °C for 10 sec and 60 °C for 30 sec, followed by a melting curve analysis from 65-95 °C. A plasmid template containing the 130 bp DWV amplicon (Supplementary Table 1) was synthesized and cloned into the pUCIDT-AMP plasmid (Integrated DNA Technologies, Coralville, IA). A standard curve was conducted using five serial 10-fold dilutions of this plasmid. Molar calculations for copy numbers were completed and verified using online tools: http://cels.uri.edu/gsc/cndna.html and http://scienceprimer.com/copy-number-calculator-for-realtime-pcr. All samples were analyzed in triplicate. Relative values were calculated from the differences (ΔCt) between the Ct values of the DWV and the control gene, ribosomal protein S5 (RPS5), which were transformed into relative values. Both sets of primers used for DWV and RPS5 have been used extensively for studies of DWV, as previously reported (Gregorc et al., Citation2012) ().
Statistical analysis
Initial analyses of normality of the qPCR values were conducted using the Kolmogorov-Smirnov/Lilliefor test (Fox, 2011). The distribution of the qPCR values in both trials was nonparametric, and the data were analyzed using the Mann-Whitney U test (de Mendiburu, Citation2016). Statistical analyses were conducted using Statplus (AnalystSoft).
Results
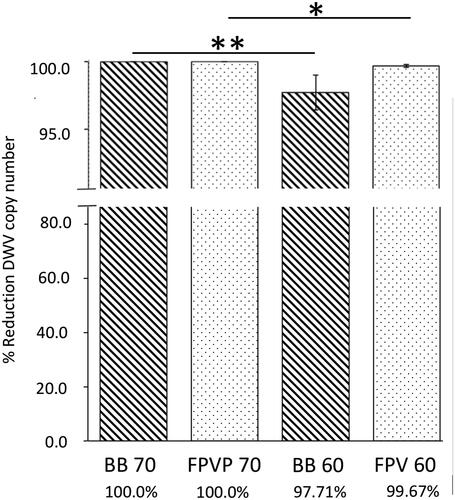
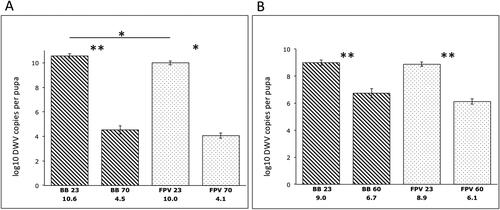
Our results indicate that DWV remained infective after 48 days of incubation at 4 °C in fermented pollen (BB or FPV). Significant amplification in the non-thermally treated samples occurred after injecting 5.5 x104 and 1 x 104 DWV copies per pupa for the first and second trials, respectively. The average viral loads 48 h post-infection were 3.04 x1010 and 1.26 x109 per pupa for the first and second trials, respectively. We observed a significant reduction in viable DWV copies in both BB (p = 0.009, U = 25) and FPV (p = 0.014, U = 0) samples after 1 h at 70 °C (first trial). Similar results were obtained after 1 h at 60 °C (second trial) (BB: p = 0.009, U = 25, FPV: p = 0.009. U = 25). In comparison with the thermally untreated controls, pollen samples treated at 70 °C for 1 h exhibited a 6-log reduction in DWV copies, whereas a 2.5-log reduction was recorded when treated at 60 °C for 1 h (). There were no significant differences when comparing thermally treated BB and FPV samples at 70 °C (p = 1) or 60 °C (p = 0.25, U = 18). Non-thermally treated samples showed significantly higher DWV copies in BB compared with FPV in the first trial (p = 0.028, U = 2). In contrast, there was no significant difference between DWV copies in non-thermally treated BB and FPV samples in the second trial (p = 0.6) (). PBS-injected control pupae groups showed undetectable DWV levels and were not included in the analysis or graphs.
Figure 2. Infectivity of DWV recovered from thermally treated and untreated pollen 48 h post-infection. Reduction in viable DWV following thermal treatment of stored pollen (BB, striped bars) and in vitro fermented pollen (FPV, dotted bars). Reduction of DWV copies at 70 °C was 6 logs (A), whereas DWV copies were reduced 2.5 logs after the treatment at 60 °C (B). Error bars represent SE. Non-parametric data were analyzed with the Mann-Whitney U test. *p < 0.05; **p < 0.01.
Our analysis of the first trial revealed a DWV copy reduction of 99.998% in BB and 99.999% in FPV after one hour at 70 °C. In the second trial (60 °C for 1 h), BB samples had a DWV copy reduction of 97.71%, compared with a DWV copy reduction of 99.67% in FPV. No significant differences in the reduction of DWV copies were found between BB and FPV within each trial. Comparisons across trials of both BB and FPV samples treated at 70 °C for 1 h demonstrated a higher reduction of DWV copies compared with samples treated at 60 °C for 1 h. However, this reduction was more significant between BB samples compared with FPV samples: BB 70/60 °C (p = 0.009, U = 0), FPV 70/60 °C (p = 0.014, U = 20) ().
Discussion
Virological studies are complicated by the necessity of measuring active viruses in a system involving living cells. Enumerating viable honey bee viruses such as DWV is no exception. Injection of viral suspensions into developing pupae is the recommended method for quantifying viable viruses in a sample, as robust honey bee cell lines are unavailable (McMenamin et al., Citation2021). In this method, pupae are individually inoculated with an ultrafiltered viral suspension. After incubation, which allows for replication of the injected virus, the viral copy number can be measured by standard quantitative PCR. Assessing the viability of viral particles using pupa is presumably more relevant for evaluating the efficiency of thermal treatments compared with direct detection of viral genomes in the treated pollen. Direct detection by PCR may amplify a significant percentage of thermally damaged, and therefore inactivated, viral particles.
In this study, we evaluated the effect of temperature on DWV viability using naturally fermented pollen (BB) and artificially fermented pollen (FPV). Our results indicated a 97.71% reduction in average levels of viable DWV after injecting the virus recovered from BB that was treated for one hour at 60 °C, compared with the non-thermally treated control. A more intensive treatment at 70 °C resulted in nearly complete inactivation of DWV. We did not find significant differences between BB and FPV samples regarding the copy number of viable viral particles amplified in pupae post-thermal treatments. However, when assessing the efficiency of DWV inactivation, a higher statistical significance was observed between the different thermal treatments (70 °C/60 °C) in BB samples compared with FPV samples. This difference suggests that thermal treatment at 60 °C could be more effective in eliminating viable DWV in FPV compared with BB samples. A possible explanation for this result is the differences in acidity between naturally fermented pollen (BB) and artificially fermented pollen (FPV). Indeed, the resulting pH after in vitro fermentation of fresh pollen was 3.5, which is slightly lower than the typically reported pH value of bee bread (pH 3.9-4.0) (Degrandi-Hoffman et al., Citation2012; Dranca et al., Citation2020). However, additional measurements with a larger sample size would be required to support this hypothesis.
The number of viral particles required to produce overt infection depends on the method of viral introduction in the host organism, with a higher number of DWV copies required to produce infection via the oral route compared to direct injection. The DWV OID50 (overt infection dosage) has been reported to be 2.5 x103 copies in injected pupae (Möckel et al., Citation2011). High infection percentages are typically obtained with 1 x 105 copies per bee, which produces 1 x1010−1011 copies after infection (Evans et al., Citation2022). Thus, in this study, both the range of viral concentration injected (1-5 x104) and copies produced post-infection (3.7 x109 − 9.1 x1011), are consistent with previous reports. For lab experiments involving adult workers, the DWV concentration required to produce high percentages of overt infection by spiked pollen ingestion is approximately 1.0 x109 copies per gram of pollen. However, 1 x 107 and 1 x 106 DWV copies per gram of pollen resulted in 47% and 20% of bees infected, respectively (Schittny et al., Citation2020). Therefore, the DWV concentrations in the pollen used in this study (1.5 − 8 x107 per g/pollen) are in the range reported to cause overt infection.
After the thermal treatment, a filtered suspension derived from DWV-contaminated pollen samples was used to infect pupae. We did not determine whether the treated pollen was able to produce overt infection in adult bees. However, our results, showing considerable reductions in viable DWV copies after heating at 60 °C and 70 °C, suggest that these thermal treatments could effectively reduce varying levels of DWV contamination in BB. Based on Schittny et al. (Citation2020) estimations of the number of DWV copies per gram of pollen required to produce different percentages of overt infection by pollen ingestion, we estimate that BB harboring low (≤1.0 x106) and medium (∼1.0 x107) DWV copy numbers could be treated at 60 °C. Meanwhile, BB harboring a high DWV copy number (≥1.0 x109), could require heating at 70 °C. Additional studies are required to measure naturally occurring DWV concentration in pollen during different seasons and under different colony health conditions (e.g., Varroa infestation and DWV infection levels). These studies could determine the conditions in which the concentrations of DWV in BB are in the range expected to cause overt disease, and if heat treatments can effectively reduce pollen viral loads to prevent such disease.
Our results showing the viability of DWV inoculated into fermented pollen when stored for up to 6 weeks at 4 °C is consistent with previous studies in which DWV has been detected in bee bread stored for six months at ambient outdoor temperatures fluctuating from −6 °C to 32 °C (Singh et al., Citation2010). Although our study is the first to demonstrate that DWV is viable and able to produce overt infection in pupae, additional studies are required to determine whether DWV remains infective at typical hive temperatures, in which the effect of low pH in the fermented pollen could be more significant. Overall, these results support the idea that DWV can remain infectious in pollen for long periods, especially during winter. Infective DWV present in bee bread could be ingested when temperatures increase at the beginning of spring, causing the colony to collapse in a relatively short period of time. This early spring colony mortality could be wrongly attributed to winter mortality, which is more likely caused by other non-viral agents, such as low temperatures and starvation. This hypothesis aligns with the common observation of significant colony mortality in early spring, even when there are more favorable temperature conditions and the dead colonies had sufficient remaining honey and pollen reserves. Reduction of contaminating viruses in stored pollen has practical implications for colony management, as current efforts to reduce colony viral infections focus chiefly on reducing Varroa levels (Locke et al., Citation2017; Roth et al., Citation2020).
In the EU, it is estimated that the bumble bee industry demands at least 50% of all the imported bee pollen for rearing bumble bees for pollination services (Haefeker, Citation2021). As a strategy, this industry has adopted gamma irradiation as the primary technology to prevent the spread of honey bee diseases to bumble bees (Álvarez Hidalgo et al., Citation2020). The use of this technology has been supported by its use in removing the Israeli acute paralysis virus (IAPV). For comparison with thermal treatments, 16.9 kGy gamma radiation reduced IAPV’s infectivity by 100-1000 fold (Meeus et al., Citation2014), which is similar to what we observed in our study after heating at 60 °C.
Beeswax has a melting temperature of 61-63 °C (Tulloch, Citation1980). Thus, although the temperature range tested in our study could not be used for heating frames containing contaminated bee bread, it can be used to inactivate contaminating viruses in fresh pollen. Alternative strategies for avoiding viral infections from contaminating bee bread could include removing virus-contaminated bee bread at the end of the winter and replacing it with virus-free pollen substitutes. Finally, it is possible that a more extended heat treatment just below the melting point, such as 50 or 55 °C, could help reduce levels of viable virus from frames.
In conclusion, we showed that DWV remained infective in bee bread and established an effective thermal protocol for its inactivation. Our study also provides initial information on the effect of a specific bacterial strain on bee bread’s pH and its impact on DWV infectivity. However, further experiments with other bacteria and temperatures are required to explore whether different temperatures combined with the lower pH of bee bread may help to reduce DWV infectivity. Our results have potential implications for understanding how the viability of DWV in bee bread may contribute to winter colony losses.
Supplemental Material
Download MS Word (15.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Álvarez Hidalgo, E., Hernandez-Flores, J. L., Andrade Moreno, V. D., Ramos López, M., Romero Gómez, S., Vázquez Cruz, M. A., Torres Ruíz, A., Alvarado Osuna, C., Jones, G. H., Arvizu Hernández, I., Estrada Martínez, A., & Campos-Guillén, J. (2020). Gamma irradiation effects on the microbial content in commercial bee pollen used for bumblebee mass rearing. Radiation Physics and Chemistry, 168, 108511. https://doi.org/10.1016/j.radphyschem.2019.108511
- Amiri, E., Kryger, P., Meixner, M. D., Strand, M. K., Tarpy, D. R., & Rueppell, O. (2018). Quantitative patterns of vertical transmission of deformed wing virus in honey bees. PLoS One, 13(3), e0195283. https://doi.org/10.1371/journal.pone.0195283
- Asama, T., Arima, T. H., Gomi, T., Keishi, T., Tani, H., Kimura, Y., Tatefuji, T., & Hashimoto, K. (2015). Lactobacillus kunkeei YB38 from honeybee products enhances IgA production in healthy adults. Journal of Applied Microbiology, 119(3), 818–826. https://doi.org/10.1111/jam.12889
- Ball, B. (1989). Varroa jacobsoni as a virus vector. In R. Cavalloro (Ed.), Present status of varroatosis in Europe and progress in the varroa mite control (pp. 241–244).
- Ball, B., & Allen, M. F. (1988). The prevalence of pathogens in honey bee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Annals of Applied Biology, 113(2), 237–244. https://doi.org/10.1111/j.1744-7348.1988.tb03300.x
- Beaurepaire, A., Piot, N., Doublet, V., Antunez, K., Campbell, E., Chantawannakul, P., Chejanovsky, N., Gajda, A., Heerman, M., Panziera, D., Smagghe, G., Yañez, O., de Miranda, J. R., & Dalmon, A. (2020). Diversity and global distribution of viruses of the western honey bee, Apis mellifera. Insects, 11(4), 239. https://doi.org/10.3390/insects11040239
- Bruckner, S., Steinhauer, N., Engelsma, J., Fauvel, A. M., Kulhanek, K., et al. (2020). 2019–2020 Honey bee colony losses in the United States preliminary results, Bee Informed Partnership.
- Castagna, A., Benelli, G., Conte, G., Sgherri, C., Signorini, F., Nicolella, C., Ranieri, A., & Canale, A. (2020). Drying techniques and storage: Do they affect the nutritional value of bee-collected pollen? Molecules (Basel, Switzerland), 25(21), 4925. https://doi.org/10.3390/molecules25214925
- Chen, Y., Evans, J., & Feldlaufer, M. (2006). Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. Journal of Invertebrate Pathology, 92(3), 152–159. https://doi.org/10.1016/j.jip.2006.03.010
- Chen, Y., & Siede, R. (2007). Honey bee viruses. Advances in Virus Research, 70, 33–80. https://doi.org/10.1016/S0065-3527(07)70002-7
- Dainat, B., & Neumann, P. (2013). Clinical signs of deformed wing virus infection are predictive markers for honey bee colony losses. Journal of Invertebrate Pathology, 112(3), 278–280. https://doi.org/10.1016/j.jip.2012.12.009
- de Mendiburu, F. (2016). Agricolae: Statistical procedures for agricultural research. R Package Version 1.2-4.
- de Miranda, J., & Genersch, E. (2010). Deformed wing virus. Journal of Invertebrate Pathology, 103 Suppl 1, S48–S61. https://doi.org/10.1016/j.jip.2009.06.012
- De-Melo, A., Estevinho, M. L., & Almeida-Muradian, L. B. (2015). A diagnosis of the microbiological quality of dehydrated bee-pollen produced in Brazil. Letters in Applied Microbiology, 61(5), 477–483. https://doi.org/10.1111/lam.12480
- Degrandi-Hoffman, G., Eckholm, B., & Huang, M. (2012). A comparison of bee bread made by Africanized and European honey bees (Apis mellifera) and its effects on hemolymph protein titers. Apidologie, 44(1), 52–63. https://doi.org/10.1007/s13592-012-0154-9
- Dietemann, V., Ellis, J. D., & Neumann, P. (2013). The COLOSS BEEBOOK Volume II: Standard methods for Apis mellifera pest and pathogen research. International Bee Research Association IBRA.
- Domínguez-Valhondo, D., Bohoyo Gil, D., Hernández, M. T., & González-Gómez, D. (2011). Influence of the commercial processing and floral origin on bioactive and nutritional properties of honeybee-collected pollen. International Journal of Food Science & Technology, 46(10), 2204–2211. https://doi.org/10.1111/j.1365-2621.2011.02738.x
- Dranca, F., Ursachi, F., & Oroian, M. (2020). Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods (Basel, Switzerland), 9(10), 1358. https://doi.org/10.3390/foods9101358
- Evans, J., Banmeke, O., Palmer-Young, E. C., Chen, Y., & Ryabov, E. V. (2022). Beeporter: Tools for high-throughput analyses of pollinator-virus infections. Molecular Ecology Resources, 22(3), 978–987. https://doi.org/10.1111/1755-0998.13526
- Filannino, P., Di Cagno, R., Addante, R., Pontonio, E., & Gobbetti, M. (2016). Metabolism of fructophilic lactic acid bacteria isolated from the Apis mellifera L. bee gut: Phenolic acids as external electron acceptors. Applied and Environmental Microbiology, 82(23), 6899–6911. https://doi.org/10.1128/AEM.02194-16
- Graystock, P., Yates, K., Evison, S. E. F., Darvill, B., Goulson, D., & Hughes, W. H. O. (2013). The Trojan hives: Pollinator pathogens, imported and distributed in bumblebee colonies. Journal of Applied Ecology, 50(5), 1207–1215. https://doi.org/10.1111/1365-2664.12134
- Graystock, P., Jones, J. C., Pamminger, T., Parkinson, J. F., Norman, V., Blane, E. J., Rothstein, L., Wäckers, F., Goulson, D., & Hughes, W. O. H. (2016). Hygienic food to reduce pathogen risk to bumblebees. Journal of Invertebrate Pathology, 136, 68–73. https://doi.org/10.1016/j.jip.2016.03.007
- Gregorc, A., Evans, J. D., Scharf, M., & Ellis, J. D. (2012). Gene expression in honey bee (Apis mellifera) larvae exposed to pesticides and Varroa mites (Varroa destructor). Journal of Insect Physiology, 58(8), 1042–1049. https://doi.org/10.1016/j.jinsphys.2012.03.015
- Haefeker, W. (2021). Pollen supplements and substitutes in the EU feed market: A product/market survey for bees and other animal species. EFSA Supporting Publications, 18(2), 1–43.
- Highfield, A. C., El Nagar, A., Mackinder, L. C. M., Noël, L. M.-L J., Hall, M. J., Martin, S. J., & Schroeder, D. C. (2009). Deformed wing virus implicated in overwintering honeybee colony losses. Applied and Environmental Microbiology, 75(22), 7212–7220. https://doi.org/10.1128/AEM.02227-09
- Kostić, A., Milinčić, D. D., Barać, M. B., Ali Shariati, M., Tešić, ŽL., & Pešić, M. B. (2020). The Application of Pollen as a Functional Food and Feed Ingredient-The Present and Perspectives. Biomolecules. Biomolecules, 10(1), 84. https://doi.org/10.3390/biom10010084
- Kulhanek, K., Steinhauer, N., Rennich, K., Caron, D. M., Sagili, R. R., Pettis, J. S., Ellis, J. D., Wilson, M. E., Wilkes, J. T., Tarpy, D. R., Rose, R., Lee, K., Rangel, J., & vanEngelsdorp, D. (2017). A national survey of managed honey bee 2015–2016 annual colony losses in the USA. Journal of Apicultural Research, 56(4), 328–340. https://doi.org/10.1080/00218839.2017.1344496
- Locke, B., Semberg, E., Forsgren, E., & de Miranda, J. R. (2017). Persistence of subclinical deformed wing virus infections in honeybees following Varroa mite removal and a bee population turnover. PLoS One, 12(7), e0180910. https://doi.org/10.1371/journal.pone.0180910
- Martin, S. J., Highfield, A. C., Brettell, L., Villalobos, E. M., Budge, G. E., Powell, M., Nikaido, S., & Schroeder, D. C. (2012). Global honey bee viral landscape altered by a parasitic mite. Science (New York, N.Y.), 336(6086), 1304–1306. https://doi.org/10.1126/science.1220941
- Mazzei, M., Carrozza, M. L., Luisi, E., Forzan, M., Giusti, M., Sagona, S., Tolari, F., & Felicioli, A. (2014). Infectivity of DWV associated to flower pollen: Experimental evidence of a horizontal transmission route. PLoS One, 9(11), e113448. https://doi.org/10.1371/journal.pone.0113448
- McMenamin, A., Parekh, F., Lawrence, V., & Flenniken, M. L. (2021). Investigating virus-host interactions in cultured primary honey bee cells. Insects, 12(7), 653. https://doi.org/10.3390/insects12070653
- Meeus, I., Mosallanejad, H., Niu, J., de Graaf, D. C., Wäckers, F., & Smagghe, G. (2014). Gamma irradiation of pollen and eradication of Israeli acute paralysis virus. Journal of Invertebrate Pathology, 121, 74–77. https://doi.org/10.1016/j.jip.2014.06.012
- Möckel, N., Gisder, S., & Genersch, E. (2011). Horizontal transmission of deformed wing virus: Pathological consequences in adult bees (Apis mellifera) depend on the transmission route. The Journal of General Virology, 92(Pt 2), 370–377. https://doi.org/10.1099/vir.0.025940-0
- Moreno Galarza, L. (2012). Aislamiento y Selección de Lactobacillus sp con potencial probiótico a partir de pan de abejas (isolation and selection of Lactobacillus sp with probiotic potential from bee bread). Facultad de Ciencias Universidad Nacional de Colombia. Master in Microbiology Sciences (pp. 1–93).
- Neveling, D., Endo, A., & Dicks, L. M. (2012). Fructophilic Lactobacillus kunkeei and Lactobacillus brevis isolated from fresh flowers, bees and bee-hives. Current Microbiology, 65(5), 507–515. https://doi.org/10.1007/s00284-012-0186-4
- Posada-Florez, F., Lamas, Z. S., Hawthorne, D. J., Chen, Y., Evans, J. D., & Ryabov, E. V. (2021). Pupal cannibalism by worker honey bees contributes to the spread of deformed wing virus. Scientific Reports, 11(1), 8989. https://doi.org/10.1038/s41598-021-88649-y
- Roth, M., Wilson, J. M., Tignor, K. R., & Gross, A. D. (2020). Biology and management of Varroa destructor (Mesostigmata: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies. Journal of Integrated Pest Management, 11(1), 1. https://doi.org/10.1093/jipm/pmz036
- Ryabov, E., Childers, A. K., Lopez, D., Grubbs, K., Posada-Florez, F., Weaver, D., Girten, W., vanEngelsdorp, D., Chen, Y., & Evans, J. D. (2019). Dynamic evolution in the key honey bee pathogen deformed wing virus: Novel insights into virulence and competition using reverse genetics. PLoS Biology, 17(10), e3000502. https://doi.org/10.1371/journal.pbio.3000502
- Ryabov, E., Fannon, J. M., Moore, J. D., Wood, G. R., & Evans, D. J. (2016). The Iflaviruses Sacbrood virus and deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ, 4, e1591. https://doi.org/10.7717/peerj.1591
- Schittny, D., Yañez, O., & Neumann, P. (2020). Honey bee virus transmission via hive products. Veterinary Sciences, 7(3), 96. https://doi.org/10.3390/vetsci7030096
- Singh, R., Levitt, A. L., Rajotte, E. G., Holmes, E. C., Ostiguy, N., Vanengelsdorp, D., Lipkin, W. I., Depamphilis, C. W., Toth, A. L., & Cox-Foster, D. L. (2010). RNA viruses in hymenopteran pollinators: Evidence of inter-Taxa virus transmission via pollen and potential impact on non-Apis hymenopteran species. PLoS One, 5(12), e14357. https://doi.org/10.1371/journal.pone.0014357
- Steinmann, N., Corona, M., Neumann, P., & Dainat, B. (2015). Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PLoS One, 10(6), e0129956. https://doi.org/10.1371/journal.pone.0129956
- Tentcheva, D., Gauthier, L., Zappulla, N., Dainat, B., Cousserans, F., Colin, M. E., & Bergoin, M. (2004). Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Applied and Environmental Microbiology, 70(12), 7185–7191. https://doi.org/10.1128/AEM.70.12.7185-7191.2004
- Tulloch, A. (1980). Beeswax—Composition and analysis. Bee World, 61(2), 47–62. https://doi.org/10.1080/0005772X.1980.11097776
- Yue, C., & Genersch, E. (2005). RT-PCR analysis of deformed wing virus in honeybees (Apis mellifera) and mites (Varroa destructor). The Journal of General Virology, 86(Pt 12), 3419–3424. https://doi.org/10.1099/vir.0.81401-0
- Yue, C., Schröder, M., Gisder, S., & Genersch, E. (2007). Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). The Journal of General Virology, 88(Pt 8), 2329–2336. https://doi.org/10.1099/vir.0.83101-0
- Zuluaga-Domínguez, C., Serrato-Bermudez, J., & Quicazán, M. (2018). Influence of drying-related operations on microbiological, structural and physicochemical aspects for processing of bee-pollen. Engineering in Agriculture, Environment and Food, 11(2), 57–64. https://doi.org/10.1016/j.eaef.2018.01.003