Abstract
The honey bee parasite, Varroa destructor, is susceptible to removal by dusting agents that physically interfere with its ability to cling to its phoretic hosts. Here we describe modifications to established powdered sugar dusting techniques that modestly increased mite separation from hosts and allowed for greater reductions in whole colony mite infestation rates. These modifications increased body-to-body contact through crowding and mechanical agitation to supplement dusting effects on mite removal. Adult workers were isolated in a screened sugar shake box outside the colony, dusted with powdered sugar, then carefully shaken and bounced in crowded piles for one minute to separate mites from their hosts. Whole colony powdered sugar shake treatments resulted on average in a 92% reduction in post-treatment mite infestation rates over three successive mite treatments. Our modifications provided effective mite control comparable to the miticide Apiguard (thymol) without discernable negative effects on colonies. Four months after treatment, powdered sugar shake treated colonies had relatively similar-sized adult worker and brood populations as Apiguard treated colonies and larger adult populations than controls. Colonies also maintained queens and reared relatively few queen cells across treatments. Further comparisons of method components revealed that mechanical agitation of crowded workers enhanced mite separation from hosts due to powdered sugar dusting. Mechanical agitation alone only modestly increased mite separation compared to controls; however, high levels of mite separation associated with sugar dusting increased with agitation. These modified methods provide a rapid if laborious technique for reducing mite populations without chemical residues from restricted miticides.
Introduction
The Varroa mite (Varroa destructor) is an ectoparasite that has acquired the Western honey bee Apis mellifera as a host throughout much of the world (Nazzi & Le Conte, Citation2016; Traynor et al., Citation2020). The mite is widely considered the worst parasitic threat to honey bees worldwide both through direct parasitism of developing capped brood and adults and as a vector of honey bee pathogens (Boecking & Genersch, Citation2008; McMenamin & Genersch, Citation2015; Nazzi & Le Conte, Citation2016; Neumann & Carreck, Citation2010; Traynor et al., Citation2020). As a new host, A. mellifera is less adept in many coevolved behavioral resistance mechanisms present in the original host Apis cerana (Boecking & Spivak, Citation1999; Büchler et al., Citation1992; Guichard et al., Citation2023; Peng et al., Citation1987; Rosenkranz et al., Citation2010; Pritchard, Citation2016 for review). A. mellifera workers vary considerably in their resistance against Varroa infestations, with certain subspecies and selected genotypes showing substantial behavioral resistance against Varroa mites including grooming and removal of infested brood (Boecking & Spivak, Citation1999; Harbo & Harris, Citation1999; Pritchard, Citation2016 for reviews). However, most untreated A. mellifera colonies would be expected to perish within a few years without intervention (Carreck et al., Citation2010; Fries et al., Citation2006; Le Conte et al., Citation2010; Rosenkranz et al., Citation2010). Varroa mite infestations are primarily controlled through chemical treatments and targeted management practices (Rosenkranz et al., Citation2010; Traynor et al., Citation2020). Crucially, mite control methods do not have to eliminate all of the mites to be effective. Treatments that sharply reduce rather than completely eradicate female mites can outpace the mites’ slow rate of reproduction (Calis et al., Citation1999; Rosenkranz et al., Citation2010). However, the appearance of mite resistance to chemical treatments has put a premium on the development of novel effective treatments, especially non-toxic treatments that can be applied during honey flows (Pettis & Jadczak, Citation2005; Rinkevich, Citation2020).
One approach to mite control is the use of dusting agents that disrupt phoretic mites’ abilities to remain attached to their adult hosts (Fakhimzadeh et al., Citation2011; Macedo et al., Citation2002; Ramirez, Citation1987, Citation1989; Ramirez & Malavasi, Citation1991). Powdered sugar dusting was utilized by Fakhimzadeh (Citation2000) as a means to increase mite fall (i.e. permanent separation of a mite from a phoretic worker host) from treated nest workers. Powdered sugar interferes with the attempt of mites to attach themselves to the bee host (Fakhimzadeh et al., Citation2011, Macedo et al., Citation2002) and stimulates host grooming that dislodges mites (Stevanovic et al., Citation2012). Reported mite drop rates with efficacious application methods have varied from 77% to 93% in jars and colonies (Aliano & Ellis, Citation2005a; Fakhimzadeh, Citation2001a, Citation2001c; Macedo et al., Citation2002; Stanimirovic et al., Citation2011; but see Fakhimzadeh et al., Citation2011). Dusted mites are largely unable to reacquire new hosts as long as they remain dusted and may expire due to separation from hosts within several hours to a few days (Aliano & Ellis, Citation2005a; Macedo et al., Citation2002; Ramirez & Malavasi, Citation1991). Powdered sugar dusting is also advantageous in that it can be applied during nectar flows without leaving toxic chemical residues. The efficiency of the method to dislodge mites is dependent both upon host and mite dust coverage and fine dry granularity of the powdered sugar, and is improved by moderate mechanical agitation that separates powdered mites from dusted hosts (Aliano & Ellis, Citation2005a; Fakhimzadeh et al., Citation2011). Powdered sugar dusting (“sugar rolls” dusting hundreds of workers in a jar) has been widely adopted as an effective and accurate method for mite detection and quantification of phoretic mite infestation rates in colony worker subsamples (Bava et al., Citation2022; Dietemann et al., Citation2013; Fakhimzadeh, Citation2001c; Gregorc et al., Citation2017; Macedo et al., Citation2002).
By contrast, powdered sugar dusting has been considered less effective at removing mites from whole colonies and appears strongly dependent on application techniques and environmental conditions. Attempts to scale the method to whole colony mite reductions have met with mixed results and generally have been considered either ineffective or marginally effective at the colony level (Aliano & Ellis, Citation2005a; Berry et al., Citation2012; Ellis et al., Citation2009; Fakhimzadeh, Citation2000; Rosenkranz et al., Citation2010). Researchers initially explored powdered sugar techniques that relied on dusting effects alone and were practical for commercial applications (i.e. rapid and inexpensive with minimal colony manipulation (labor input) and colony disturbance (Berry et al., Citation2012; Ellis et al., Citation2009)). However, in-hive applications suffered from limited efficacy against mites as well as negative impacts on the bees themselves. Powdered sugar applications to brood nest top bars resulted in poor dust coverage, reacquisition of worker hosts by dropped mites, and significant, but inadequate for control, mite fall rates (Berry et al., Citation2012; Ellis et al., Citation2009). More even distribution of powdered sugar dust by blowers into nest frames may lead to insufficient mite fall rates and brood deaths from caked sugar, but only at excessive levels (Aliano & Ellis, Citation2005b). Other studies have indicated that seasonality, environmental conditions, and powdered sugar quality and consistency may significantly affect mite drop efficiencies (Fakhimzadeh et al., Citation2011; Gregorc et al., Citation2017).
A more effective and less destructive approach developed by Aliano and Ellis (Citation2005a) was to isolate workers from the hive and dust them in a screen box, thereby allowing dislodged mites to become physically separated from the colony. Bees were driven by bee repellent into the screen box, sealed with a top screen, dusted with powdered sugar, and lightly agitated, with affected mites dropping through the box’s bottom screen (Aliano & Ellis, Citation2005a). This method, combined with gentle agitation, improved dust coverage and sharply reduced brood exposure to excess powdered sugar (Aliano & Ellis, Citation2005b; Ellis et al., Citation2009; Fakhimzadeh, Citation2001b; Pettis et al., Citation2004 for effects (or lack thereof) on brood, workers, and queens). Notably, mite drop rates were substantially improved by using a solid floor instead of the original screen mesh floor, a substitution that likely increased dust coverage and the dislodging effects provided by mechanical agitation. This method reduced mite infestation rates (77%) to levels just below the threshold (90%) expected to outpace mite reproduction rates (Aliano & Ellis, Citation2005a). Powdered sugar shakes also must be applied repeatedly as only phoretic mites, and not mites in brood cells, are impacted by short term dusting. As a result, current powdered sugar application methods have generally been considered too laborious, time-consuming, and ineffective to be a large scale practical substitution for conventional chemical treatments (Aliano & Ellis, Citation2005a; Ellis et al., Citation2009; Haber et al., Citation2019; Rosenkranz et al., Citation2010). We initially used their sugar shake method to transfer mites for experiments; however, we noticed that we could achieve higher mite fall rates with certain protocol changes.
We describe modifications to Aliano’s and Ellis’s original powdered sugar shake method that modestly reduced mite infestation rates and decreased processing time in whole colonies. We adopted their use of an external screened sugar shake box and topped it with a funnel box to dust workers outside the colony. Our approach focused on deliberate worker crowding and more intensive mechanical agitation to increase mite separations from their hosts. These modifications employed rolling, crowding, and bouncing actions that are similar to those used for powdered sugar mite infestation rate assessments in Mason jars but applied at a larger scale and greater intensity than previously reported (Aliano & Ellis, Citation2005a; Fakhimzadeh et al., Citation2011). We also contrasted long term effects of powdered sugar treatments on mite infestation rates and colony performance with miticide-treated (Apiguard (thymol)) and untreated colonies. We also compared the relative contributions of the sugar shake method components (powdered sugar dusting and mechanical agitation) to the separation of mites from their worker hosts. The result is an improved mite treatment that is faster and more efficacious for reducing colony mite populations.
Materials and methods
Powdered sugar shake box for removal of phoretic mites
Powdered sugar shake treatments were administered using a sugar shake box with elements modeled directly after Aliano and Ellis (Citation2005a; ). All parts were constructed with the perimeter dimensions of a Langstroth deep (cross section 40.6 cm W × 42.2 cm L; interior box perimeter 37.5 cm W × 46.7 cm L) to allow telescopic stacking of shake box components top to bottom. Detachable shake box components were secured together with latches. The sugar shake box consisted of a Langstroth deep outfitted with a detachable mesh top slide screen (to open or close access to the box) and a mesh screen bottom (8 mesh hardware cloth, 0.28 cm openings). The shake box was topped by a second detachable Langstroth deep with a sheet metal funnel inside. The funnel was constructed to guide brushed bees into the box below and largely prevented their escape. A detachable sheet metal bottom catch board was attached underneath the shake box to catch excess powdered sugar and mites that fell through the shake box bottom screen.
Figure 1. (a) Assembled and (b) disassembled powdered sugar shake box used to isolate and treat nest workers against Varroa mite. Box components (top to bottom) include a detachable funnel box to guide bees in, a screened shake box to hold bees, a removable top screen to selectively allow bees in or enclose them, a bottom screen to allow dusted mites to fall out of the shake box, and a detachable bottom catch tray to catch falling mites and excess sugar. Handles allow controlled shaking and rolling of enclosed bees.

The shake box was initially set up with the top funnel box attached, the top screen slide open, and the bottom catch board attached (Supplemental Video 1). First, the resident queen was located and removed into a temporary queen cage. Workers were then gently knocked off or swept off frames and other hive equipment through the funnel into the screened shake box below. Separated workers tended to congregate in the shake box or the dark underside of the funnel rather than fly out of the funnel. Once all workers were swept into the shake box, the apparatus was carefully knocked to drop the bees from the funnel underside to the shake box below. The top screen slide was quickly closed and the funnel box removed. The workers were tapped down again and tilted to the side to create a pile of unsteady workers.
The bees were treated with approximately 250 g of dried powdered sugar spread over the bees using a household 475 mL flour sifter (Supplemental Video 1). Care was taken to ensure that the powdered sugar was dried and not clumped before use. The bees were rolled around by a bouncing, rolling motion of the shake box (5–10 cm bounce, ∼20–40 bounces/minute, at least 30° rolling motion side-to-side) for one minute. With this motion, bees were unable to stand steady and continuously collided with one another, thereby repeatedly rubbing body against body. The bees were then left undisturbed in the shake box for 3 min to allow workers to recover and groom themselves. Dislodged mites and excess powdered sugar fell through the bottom screen to the bottom catch plate below. The bees were tapped down again and bounced for 5–10 s more to remove more mites. Then the bottom catch plate was removed, the bees carefully knocked off balance in the shake box, the top screen slide opened, and the bees dumped into the colony by inverting the shake box over the brood nest center. At this point, the sugar-coated workers were largely disoriented and grooming themselves rather than flying about. However, most sugar-coated workers moved down to cover the brood frames within a few minutes. The queen was carefully returned to the brood nest center and the colony closed once the pile of sugar-coated bees retreated into the nest core.
The box design also allowed for recovery of both excess powdered sugar and dislodged mites (Dietemann et al., Citation2013; Noble et al., Citation2021). Mites, excess sugar, and other debris caught by the bottom catch plate could be sifted with a soft fine mesh screen that allowed only fine particles to pass through (Supplemental Video 1). Powdered sugar that passed through this fine mesh was relatively free of debris and clumped bits of moisture. Dislodged mites could be recovered en masse for other experimental uses as well. Filtered mites and debris on the fine mesh screen were knocked onto slightly dampened paper towels and cleared of powdered sugar by light water rinsing (Dietemann et al., Citation2013). Mites could then be reintroduced to new hosts by placing mite-infested paper towels among workers.
Experiment 1: Effects of powdered sugar shakes on mite infestation rates and colony performance
We compared powdered sugar shake-treated colonies with miticide-treated (Apiguard (thymol), Vita Europe Limited, Augusta, Georgia, USA) colonies and untreated control colonies to evaluate treatment effects on phoretic mite infestation rates, colony performance, and queen retention. Experimental colonies were constructed from overwintering colonies and new colonies were established from packages in April 2013 at USDA-ARS Carl Hayden Bee Research Center (CHBRC, Tucson, Arizona, USA). Twenty-four colonies containing on average 14.6K workers, 2.7 frames of brood (63% capped), and an ovipositing queen were assembled in single Langstroth deeps in late May 2013. All bees used directly in the experiments were of the same commercial stock. The queen stock used here was unselected with regard to mite resistance characteristics including grooming and infested brood removal (Harbo & Harris, Citation1999). As such, these bees were meant to represent standard mite-susceptible Italian stock that is widely used in commercial colonies in the United States. The prior exposure of bees used in these experiments to miticides was minimal if at all. Bees from overwintering colonies were last treated with Apiguard (Vita Europe Limited, Augusta, Georgia, USA) in October 2012, package bees were treated with unknown miticides before package establishment in April 2013, and queens were exposed to unknown miticides in commercial queen breeder colonies before transfer to experimental colonies. Critically, none of the bees present in the experiments except for queens had been physically exposed to miticides and were removed at least three brood cycle generations (the time since package establishment) from earlier mite treatments. In addition, all of the experimental colonies were provisioned with newly drawn combs that had not been previously exposed to miticides. To limit drift, these experimental colonies were separated at least 40 m behind vegetation from other colonies.
Our mite treatments were scheduled in early June immediately after the spring nectar flow, the major forage period for local colonies. In the lower Sonoran desert, mite treatments are commonly applied outside of two peak forage periods to avoid honey contamination. Colonies at this site experience major forage availability from early spring through early summer (late March to early June) and minor forage availability from late summer through mid-fall (late July to October), with pronounced mid-summer (mid-June to late July) and early winter (November to mid-January) forage dearths (O’Neal & Waller, Citation1984). Locally, mite treatments are often minimally applied twice a year either in early spring or early summer (March/April or June/July, outside of almond pollination and the spring nectar flow) and again in fall (September–November) before overwintering. A significant fraction of beekeepers in the United States use thymol as a miticide treatment in warm weather conditions when the product is effective, although usually at lower temperatures than occurred in our experiment (De Carolis et al., Citation2023; Giacomelli et al., Citation2016; Haber et al., Citation2019).
We equalized colony mite infestation rates toward targeted values (5 to 10 mites/100 bees) across colonies by exchanging mite-infested workers and brood from heavily infested colonies to less infested colonies on three occasions (weekly before the experiment). Phoretic mite infestation rates were estimated by 70% ethanol wash of 300 bees in source colonies and allocated proportionally to obtain more even mite distributions in the newly constructed colonies (Lee et al., Citation2010). Colonies were heavily infested (6.9 ± 0.9 mites/100 bees) in late May two weeks before the beginning of Experiment 1. Colonies were briefly assessed for worker and brood populations and equalized once more before the experimental treatments. Colonies initially subsisted on honey and stored pollen from the late spring-early summer forage period but were also given 1 L dilute (1:1) sucrose syrup weekly to help them survive the summer dearth. Critically, all colonies were outfitted with an empty top feeder, shaded from the afternoon sun, and given ready access to nearby water to limit the known deleterious effects of thymol on bees under very hot climatic conditions (see label instructions Vita Europe Limited, Augusta, Georgia, USA; Giacomelli et al., Citation2016 for discussion of temperature sensitivity).
Mite treatments were applied in early June 2013 during the transition to the mid-summer dearth (locally late June to early August) in hot, dry summer conditions before the onset of the summer monsoon. Each of the 24 colonies was randomly assigned to either the powdered sugar shake, Apiguard (thymol) miticide, or untreated control treatment groups (n = 8). Powdered sugar shake-treated colonies were dusted and shaken as previously described on three separate occasions at 7-day intervals (21 days total) to provide treatment over the full reproductive cycle of female mites (i.e. treat all mites emerging from brood cells). Miticide-treated colonies were treated three times at 7-day intervals with Apiguard (Vita Europe Limited, Augusta, Georgia, USA) applied as recommended by label instructions for warm summer conditions. One 25 g Apiguard tray was placed on the top bars of the brood frames each week. Untreated colonies were not treated for mites during the treatment phase. Colonies were examined again four months after initial treatment in October 2013.
Phoretic mite counts were made from nest worker subsamples immediately before and one day after each treatment round (pre-treatment and post-treatment checks), as well as 4 months after initial treatment (Aliano & Ellis, Citation2005a). To assess the efficacy of the treatments, approximately 300 workers were collected and checked for mites with 70% ethanol washes (Dietemann et al., Citation2013). To obtain a highly accurate sample count near 300 bees, nest workers were initially estimated by mass (against the average worker mass). Samples were gently agitated for 20 min by an orbital shaker to ensure full separation of mites from their hosts.
Colony strength (adult worker and brood populations) was assessed immediately before and four months after the initial mite treatments. The adult worker population was estimated by weighing colonies with and without the full adult worker complement (Carroll et al., Citation2018). Colonies were weighed during the early morning of the experiment to obtain a mass with all workers present inside the colony. To obtain mass without the workers, brood, frames, and hive equipment were weighed while the adult workers were isolated by brushing and shaking into the shake box. Brood populations were assessed visually by a single observer who estimated brood cell frame coverage to the nearest 1/10× frame equivalent (Delaplane et al., Citation2013). The presence of the queen and queen cell formation were noted during routine biweekly colony checks to detect queen supersedure attempts and queen replacement attempts by invasion swarms.
For Experiment 1, we wanted to compare changes in each colony’s phoretic mite infestation rates before and after mite treatments (pre-treatment and post-treatment) for each treatment round. For experimental purposes, we assumed that the post-treatment mite infestation rates would have been similar to the pre-treatment mite infestation rates had the treatments not been applied. This approach presumed that there would be minimal changes in the mite infestation rates that would have naturally occurred over the one-day period between pre-treatment and post-treatment assessments (i.e. differences in the number of mites emerging from cells, mites entering cells, mites dying from natural causes).
Pre-treatment to post-treatment changes in the phoretic mite infestation rate were calculated as: (post-treatment infestation rate/pre-treatment infestation rate)*100.
Experiment 2: Effects of powdered sugar dusting and mechanical agitation on mite drop rates
A second experiment was conducted to assess the relative contributions of powdered sugar dusting and mechanical agitation to mite separation from their hosts (Aliano & Ellis, Citation2005a). Approximately 300 workers were collected from highly mite-infested source colonies (14.6 ± 0.8 SE mites/100 bees) and placed in 473 mL (pint) glass Mason jars capped with the same screen mesh as the shake box (Fakhimzadeh, Citation2001a, Citation2001c; Fakhimzadeh et al., Citation2011; Macedo et al., Citation2002 for similar jar experiments). Both mites and bees originated from colonies prepared by the same methods as colonies used for Experiment 1. Bees were subjected to one of four treatments: (1) control (no sugar dusting or shaking), (2) mechanical agitation/shaking only, (3) sugar dusting only, or (4) a combination of both sugar dusting and mechanical agitation/shaking. Treatments were administered in a manner similar to the whole colony sugar shake experiment. Jars were kept upright for sugar application but otherwise kept inverted (screened top down) during the experiment. For dusted bees, approximately 20 g of powdered sugar was added by flour sifter to lightly coat the workers with powdered sugar. Bees were mechanically shaken or left unshaken for one minute, then left alone for three minutes, then (for shaken bees) briefly bounced again. Shaken bees were agitated with a bouncing motion as previously described, while unshaken bees were left undisturbed in their inverted jars. Mites that had separated from their hosts and excess powdered sugar were allowed to fall through the inverted mesh lid to a white painted top board below. Care was taken to also count mites that had fallen off bees but become stuck on the jar or mesh. Jar bees were then subjected to a 70% ethanol wash to count the enclosed bees and any remaining mites (Dietemann et al., Citation2013). Samples were gently agitated by an orbital shaker for 20 min as before to ensure the separation of mites from their hosts.
The percentage of mites that became separated from their phoretic hosts was calculated as:
(number of mites separated from worker hosts/total number of mites recovered)*100.
Statistical analysis
Most statistical metrics were assessed on a relative scale to allow comparisons of different sized colonies and to correct for natural colony-to-colony variation. Comparisons were made across colonies by treatment groups and all statistical tests were performed with SAS 9.4, 9.4, Citation2016 (SAS, Inc., Cary, NC, USA). Data sets were checked for normality by Shapiro-Wilk tests and examination of residuals (PROC UNIVARIATE). Changes in mite infestation rates (Experiment 1) and mite separations from hosts (Experiment 2) were reported as percentages. All data sets lacked normality and were compared across treatments by non-parametric Kruskal-Wallis tests with DSCF multiple comparisons between treatment group ranks (NPAR1WAY with dscf option).
Results
Experiment 1: Effects of powdered sugar shake treatments on whole colony mite infestation rates
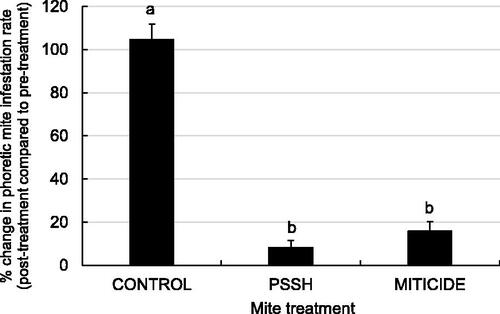
Powdered sugar shake treatments reduced infestation rates by an average of 91.8 ± 2.7% SE in treated colonies over three successive weekly treatment rounds (see Supplemental File 2 (data sets) for all data). Sugar shakes reduced infestation rates by an estimated 93.0 ± 3.4%, 89.9 ± 2.4%, and 92.5 ± 2.4 SE% of phoretic mites in the first, second, and third treatment applications. Averaged post-treatment changes in infestation rates for powdered sugar shake-treated colonies were significantly different than untreated controls and comparable to Apiguard-treated miticide colonies (, Kruskal-Wallis test, Χ2=12.551, df = 2, p < 0.0001; DSCF multiple comparisons (ranks), p < 0.05). Colonies treated by powdered sugar shake or Apiguard experienced relatively lower increases in infestation rates than untreated control colonies four months after initial treatment. Untreated control colonies had on average 3.7x ± 0.9x SE in their initial mite load four months after initial treatment compared to 1.4x ± 0.6x SE and 1.2x ± 0.4x SE for powdered sugar shake-treated and miticide treated colonies, respectively.
Figure 2. Average pre-treatment to post-treatment change in phoretic mite infestation rates (mean ± SE) across three successive mite treatment rounds. Colonies were treated by powdered sugar shake (PSSH), the miticide Apiguard (MITICIDE) or no mite treatment (CONTROL) (n = 6). Colonies were treated three times (every 7 days) to target mites emerging from brood cells over a full mite reproductive cycle. Treatment groups that do not share a superscript differ by DSCF multiple comparisons (ranks, p < 0.05).
Effects of powdered sugar shake treatments on whole colony performance and queen retention
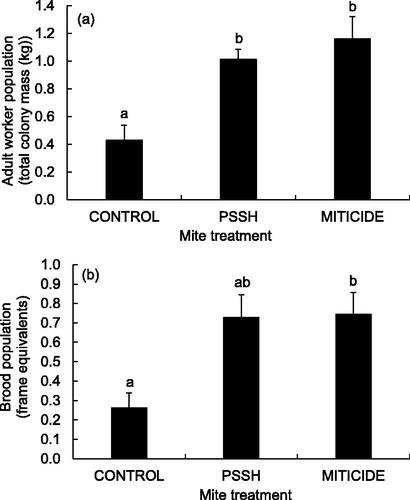
Powdered sugar shake treatments showed little discernable effect on adult worker populations or brood rearing four months after initial treatment. Colony populations in all treatments decreased slightly relative to summer populations by October due to extended drought and natural seasonal declines. Over the long term, adult worker populations decreased relatively more in untreated control colonies than powdered sugar shake-treated colonies or miticide-treated colonies (, Kruskal-Wallis test, Χ2=9.852, df = 2, p = 0.0073 (adult workers); DSCF multiple comparisons (ranks), p < 0.05). Miticide treated, but marginally not sugar shake treated colonies, had relatively larger brood populations than untreated control colonies (, Kruskal-Wallis test, Χ2=7.417, df = 2, p = 0.0245 (brood); DSCF multiple comparisons (ranks), p < 0.05).
Figure 3. Relative sizes of (a) adult worker and (b) brood populations (mean ± SE) in October colonies (four months after initial treatment) compared to pre-treatment values. Adult worker populations were estimated by total mass (colony mass with and without workers) while brood populations were estimated by frame coverage. The October adult and brood populations of each colony are scaled against the initial June populations to provide estimates of relative changes in population size. Colonies were treated by powdered sugar shake (PSSH), the thymol-based miticide Apiguard (MITICIDE) or no mite treatment (CONTROL) (n = 5 (CONTROL or MITICIDE) or 6 (PSSH)). Treatment groups that do not share a superscript differ by DSCF multiple comparisons (ranks, p < 0.05).
Reductions in mite infestation rates were achieved in sugar shake colonies with no noticeable effects on queen retention or queen supersedure attempts across treatments. All experimental colonies save one untreated control and one miticide-treated colony retained their resident queens for four months. Missing queens were replaced once detected but affected colonies were eliminated from further analysis. Colonies from all three treatments made relatively few queen supersedure attempts as evidenced by low numbers of untreated capped queen cells through the four-month experimental period (2, 3, and 2 queen cups produced, respectively, in the control, sugar-shake, and miticide colonies).
Experiment 2: Effects of powdered sugar dusting and mechanical agitation on mite drop rates
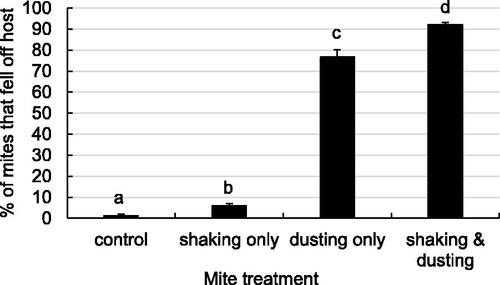
In the second experiment, all treatment components resulted in different levels of mite separation from their phoretic hosts (, Kruskal-Wallis test, Χ2=28.334, df = 3, p < 0.0001; DSCF multiple comparisons (ranks), p < 0.05). Mechanical agitation alone caused higher separation than untreated controls, but was significantly lower than jar preparations subjected to powdered sugar dusting or powdered sugar dusting plus mechanical agitation. Powdered sugar dusting without mechanical agitation resulted in moderately high separation from hosts, but was significantly lower than a combination of powdered sugar dusting and mechanical agitation
Figure 4. Percentage of mites that became separated from their phoretic hosts (mean ± SE) after treatment by different method components of powdered sugar shake treatments. Approximately 300 nest workers were treated in Mason jars by techniques similar to whole colony treatments. Enclosed bees were either shaken, dusted with powdered sugar, treated by both techniques, or left untreated as controls (n = 8). Treatment groups that do not share a superscript differ by DSCF multiple comparisons (ranks; p < 0.05).
Discussion
Effective control of Varroa mite relies on mite removal (commonly cited as above 90%) that outstrips the pace of mite reproduction. Mature Varroa mite females have a relatively low rate of reproduction (variously estimated at 1.4 to 2.3 mite offspring per brood cell cycle) such that methods that do not remove every mite can sufficiently suppress mites for months at a time (Nazzi & Le Conte, Citation2016; Pritchard, Citation2016). Our study applied minor modifications to established techniques that yielded reductions in phoretic mite infestation rates similar to or higher than previous whole colony powdered sugar applications (Aliano & Ellis, Citation2005a; Berry et al., Citation2012; Ellis et al., Citation2009; Fakhimzadeh, Citation2000; Macedo et al., Citation2002; Stanimirovic et al., Citation2011). Our modifications also differed from Aliano and Ellis (Citation2005a) methods in that we separated workers from the colony by mechanical shaking rather than with the bee repellent BeeGo (Better bee, Greenwich, NY, USA). Butyric acid, the main active ingredient used to repel bees in BeeGo, also acts as a Varroa mite excitant at low volatile exposures with unknown effects at recommended label applications (Teal et al., Citation2014; Mark Carroll, personal communication). Reductions in phoretic mite infestation rates by powdered sugar shake were similar to or greater than those reported for Apiguard treatments (Giacomelli et al., Citation2016; Mattila & Otis, Citation2000).
Powdered sugar dusting is thought to cause phoretic mites to lose their hosts not only by reducing mite attachment but also by interfering with mechanisms of host retention and reacquisition. Like other physical dusting methods, powdered sugar dusting causes fouling of the ambulacra that the mite uses to cling to its host to cause separation of the mite from its host (Fakhimzadeh, Citation2000). Dusting is also thought to prevent mites from successfully moving to new host structures or new phoretic hosts (Nazzi & Le Conte, Citation2016; Ramirez & Malavasi, Citation1991). Thorough dusting of workers with powdered sugar results in fewer host structures that repositioning mites can readily grip (Fakhimzadeh et al., Citation2011). Powdered sugar dusting also triggers host grooming responses to sugar residues present on the host body (Macedo et al., Citation2002; Stevanovic et al., Citation2012). Workers remove mites primarily by autogrooming with some allogrooming, although A. mellifera workers vary considerably in the degree of mite removal by their grooming behaviors (Büchler et al., Citation1992; Harbo & Harris, Citation1999; Peng et al., Citation1987; Stevanovic et al., Citation2012). However, mites are known to reposition themselves on the host in response to host grooming, a response that may lead to dislodging when conducted on dusted surfaces (Ramirez & Malavasi, Citation1991). Certain application methods may physically separate fallen mites from other potential hosts, eventually leading to starvation (Aliano & Ellis, Citation2005a; Ramirez, Citation1987). Aliano and Ellis (Citation2005a) use of an external screen box to treat infested workers allowed for more thorough dust coverage and prevented host reacquisition for mites that fell through the screen.
Our modifications further enhanced the effects of powdered sugar dusting through the deliberate crowding, piling and rolling (mechanical agitation) of massed worker hosts. In our experiments, mechanical agitation of massed workers alone only modestly increased mite separation from their hosts. However, mechanical agitation in combination with powdered sugar dusting improved mite separation rates compared to dusting alone. Our workers were concentrated into piles of thousands of bees and agitated for an extended period such that individual workers bounced and rubbed repeatedly against other workers. These actions likely sharply increased body-to-body contacts of phoretic mites with non-host bees in forceful and frequent collisions not observed in undisturbed frame bees. It is difficult to say how the mechanical agitation of massed workers affected worker grooming, a major behavioral mechanism for the removal of phoretic mites from the host body (Peng et al., Citation1987; Stevanovic et al., 2011). Bees did not visibly engage in autogrooming while being bounced around in large unsteady masses. However, we observed both mechanically agitated and unagitated dusted workers intermittently grooming off the thin coat of powdered sugar on their bodies for tens of minutes after treatment. The application of shaking, bouncing, and rolling motions to crowded dusted bees also served to evenly coat workers with powdered sugar and remove excess sugar. At the same time, we had to balance mite separation by mechanical agitation of dusted bees against worker stress. We carefully employed a gently bouncing, rolling action that did not smash or crush workers against the screen box sides. Our study agrees with previous ones noting that powdered sugar shakes can be repeatedly applied without long term deleterious effects on workers, queens, brood rearing, or colony performance except at the most extreme applications (Ellis et al., Citation2009; Fakhimzadeh, Citation2001b; Fakhimzadeh et al., Citation2011; Pettis et al., Citation2004). Because powdered sugar is applied outside the colony, our experimental brood were not exposed to lethal sugar caking levels sometimes encountered with direct applications of powdered sugar to brood frames (Aliano & Ellis, Citation2005b; Pettis et al., Citation2004).
A second critical aspect of our modifications was to sharply decrease the time brood were left unattended. Our use of active shaking reduces the exposure time required to remove mites from tens of minutes (observed in passive applications) to a few minutes. Direct pouring of workers on top of the brood box ensures a more rapid return of workers to the nest core than waiting for workers to return on their own. Other studies have used much longer exposure times to powdered sugar dusting to increase mite drop efficiencies that nonetheless may result in extended brood exposures (Aliano & Ellis, Citation2005a). Capped brood in particular are highly sensitive to temperature fluctuations and tolerant of a narrow temperature range of brood nest temperatures (Wang et al., Citation2016). More prolonged exposures may render brood vulnerable to temperature extremes in the absence of worker thermal control. Powdered sugar dusting applications also may be more efficacious under the hot, dry conditions (21 °C to 43 °C) present during our experiments. Varroa mites are more active at or near hive temperatures compared to even moderately cooler temperatures. Dry conditions may also better preserve powdered sugar in a fine granular consistency required for dusting than very humid or wet conditions (Currie & Tahmasbi, Citation2008; Fakhimzadeh et al., Citation2011; Gregorc et al., Citation2017). Locally, we have used powdered sugar shake treatments to remove or transfer phoretic mites under a variety of conditions and only found it ineffective in cold (below 10 °C) or extremely wet conditions (Mark Carroll, personal communication).
Practical application of powdered sugar dusting to colony level mite control remains a tradeoff between mite removal efficiency and the labor intensity of the methods themselves (Aliano & Ellis, Citation2005a). Most past explorations of powdered sugar dusting focused on low intensity in-colony applications without worker isolation or agitation (Ellis et al., Citation2009; Fakhimzadeh, Citation2000). These techniques pragmatically aimed at rapidly applied, inexpensive, and effective applications that would affect mite removal primarily by dusting and grooming alone. Direct application of sugar to top bars resulted in incomplete adult worker dust coverage and insufficient reductions in mite infestation rates. As our results indicate, neither dusting or agitation alone is sufficient to separate mites from their phoretic hosts at adequate control levels (Fakhimzadeh et al., Citation2011). An added benefit of the shorter processing time is the ability to treat colonies more rapidly. Our more intensive exposure techniques allow for a shorter worker isolation period to produce similar effects on mite infestation rates. Worker removal by brushing and frame knocking is relatively quick across all colony sizes. The main factor determining our colony treatment rates was locating and removing the queen. We estimate that an experienced two person team could likely treat 8-10 single deep colonies per hour if they have two sugar shake box rigs (Mark Carroll, personal communication).
Despite its efficacy, powdered sugar shakes remain an intensive and laborious control method compared to established miticide alternatives (Aliano & Ellis, Citation2005a). Like all control methods that target phoretic mites, powdered sugar shakes must be applied three times over successive weeks to remove mites emerging from brood cells before they complete reproductive maturation for the next rounds of cell invasion (Nazzi & Le Conte, Citation2016; Traynor et al., Citation2020). Our treatment did not inflict higher adult worker mortality or reduced brood rearing. However, like many phoretic mite treatments, our treatments resulted in repeated disturbance of colonies within a short period. As such, experimental colonies were more readily agitated by additional disturbance than untreated colonies by the third treatment round (Nicholas Brown, personal observation). For these reasons, we recommend that powdered sugar shakes be avoided during periods when colonies are sensitive to external disturbance, such as queen introductions. Aliano and Ellis (Citation2005b) noted that powdered sugar shake may be particularly effective during broodless or nearly broodless periods when colony mites are almost entirely phoretic and vulnerable to one application. As a physical technique, powdered sugar shake may also be useful for applications during nectar flows and honey production when most chemical treatments are restricted. Given the importance of low-toxicity Varroa mite control methods amidst increasing miticide resistance, novel techniques have their place in honey bee management (Gregorc & Sampson, Citation2019).
Supplemental Material
Download MP4 Video (422.8 MB)Supplemental File 2.xlsx
Download MS Excel (19.8 KB)Acknowledgments
The authors would like to thank Madeline Saunders and Craig Goodall for assisting with colony management and evaluations. This research was supported by internal research funds of the USDA-ARS. The USDA is an equal opportunity employer and provider.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
All relevant data are within the paper and its Supporting Information files (Supplemental File 2 (data sets)).
References
- Aliano, N. P., & Ellis, M. D. (2005a). A strategy for using powdered sugar to reduce Varroa populations in honey bee colonies. Journal of Apicultural Research, 44(2), 54–57. https://doi.org/10.1080/00218839.2005.11101148
- Aliano, N. P., & Ellis, M. D. (2005b). Only large amounts of powdered sugar applied directly to brood cells harms immature honey bees. Journal of Apicultural Research, 44(1), 33–35. https://doi.org/10.1080/00218839.2005.11101144
- Bava, R., Castagna, F., Carresi, C., Cardamone, A., Federico, G., Roncada, P., Palma, E., Musella, V., & Britti, D. (2022). Comparison of two diagnostic techniques for the Apis mellifera varroatosis: Strengths, weaknesses and impact on the honey bee health. Veterinary Sciences, 9(7), 354. https://doi.org/10.3390/vetsci9070354
- Berry, J. A., Afik, O., Nolan, M. P., & Delaplane, K. (2012). Revisiting powdered sugar for Varroa control on honey bees (Apis mellifera L.) Journal of Apicultural Research, 51(4), 367–368. https://doi.org/10.3896/IBRA.1.51.4.14
- Boecking, O., & Genersch, E. (2008). Varoosis: The ongoing crisis in bee keeping. Journal of Consumer Protection and Food Safety, 3, 221–228. https://doi.org/10.1007/s00003-008-0331-y
- Boecking, O., & Spivak, M. (1999). Behavioral defenses of honey bees against Varroa jacobsoni Oud. Apidologie, 30(2-3), 141–158. https://doi.org/10.1051/apido:19990205
- Büchler, R., Drescher, W., & Tornier, I. (1992). Grooming behavior of Apis cerana, Apis mellifera and Apis dorsata and its effect on the parasitic mites Varroa jacobsoni and Tropilaelaps claraeae. Experimental and Applied Acarology, 16(4), 313–319. https://doi.org/10.1007/BF01218573
- Calis, J. N. M., Fries, I., & Ryrie, S. C. (1999). Population modeling of Varroa jacobsoni Oud. Apidologie, 30(2-3), 111–124. https://doi.org/10.1051/apido:19990203
- Carreck, N. L., Ball, B. V., & Martin, S. J. (2010). Honey bee colony collapse and changes in viral prevalence associated with Varroa destructor. Journal of Apicultural Research, 49(1), 93–94. https://doi.org/10.3896/IMBRA.1.49.1.13
- Carroll, M. J., Meikle, W. G., McFrederick, Q. S., Rothman, J. A., Brown, N., Weiss, M., Ruetz, Z., & Chang, E. (2018). Pre-almond supplemental forage improves colony survival and alters queen pheromone signaling in overwintering honey bee colonies. Apidologie, 49(6), 827–837. https://doi.org/10.1007/s13592-018-0607-x
- Currie, R. W., & Tahmasbi, G. H. (2008). The ability of high- and low-grooming lines of honey bees to remove the parasitic mite Varroa destructor is affected by environmental conditions. Canadian Journal of Zoology, 86(9), 1059–1067. https://doi.org/10.1139/Z08-083
- De Carolis, A., Newmark, A. J., Kim, J., Cazier, J., Hassler, E., Pietropaoli, M., Robinette, C., Formato, G., & Song, J. (2023). Results of an international survey for risk assessment of honey bee health concerning Varroa management. Applied Sciences, 13(1), 62. https://doi.org/10.3390/app13010062
- Delaplane, K. S., van der Steen, J., & Guzman-Novoa, E. (2013). Standard methods for estimating strength parameters of Apis mellifera colonies. Journal of Apicultural Research, 52(1) https://doi.org/10.3896/IBRA/1.52.1.03
- Dietemann, V., Nazzi, F., Martin, S. J., Anderson, D., Locke, B., Delaplane, K. S., … Ellis, J. D. (2013). Standard methods for Varroa research. In V. Dietemann, J. D. Ellis & P. Neumann (Eds.), The COLOSS BEEBOOK, Volume II: Standard Methods for Apis mellifera pest and pathogen research. Journal of Apicultural Research, 52(1) https://doi.org/10.3896/IBRA.1.52.1.09
- Ellis, A. M., Hayes, G. W., & Ellis, J. D. (2009). The efficacy of dusting honey bee colonies with powdered sugar to reduce Varroa mite populations. Journal of Apicultural Research, 48(1), 72–76. https://doi.org/10.3896/IBRA.1.48.1.14
- Fakhimzadeh, K. (2000). Potential of super-fine ground, plain white sugar dusting as an ecological tool for the control of varroasis in the honey bee (Apis mellifera). American Bee Journal, 140, 487–491.
- Fakhimzadeh, K. (2001a). Effectiveness of confectioner sugar dusting to knock down Varroa destructor from adult honey bees in laboratory trials. Apidologie, 32(2), 139–148. https://doi.org/10.1051/apido:2001119
- Fakhimzadeh, K. (2001b). The effects of powdered sugar Varroa control treatments on Apis mellifera colony development. Journal of Apicultural Research, 40(3-4), 105–109. https://doi.org/10.1080/00218839.2001.11101058
- Fakhimzadeh, K. (2001c). Acute impact on the honey bee (Apis mellifera) after treatment with powdered sugar and C02 for the control of Varroa destructor. American Bee Journal, 141(11), 817–820.
- Fakhimzadeh, K., Ellis, J. D., & Hayes, J. W. (2011). Physical control of Varroa mites (Varroa destructor): the effects of various dust materials on Varroa mite fall from adult honey bees (Apis mellifera) in vitro. Journal of Apicultural Research, 50(3), 203–211. https://doi.org/10.3896/IBRA.1.50.3.04
- Fries, I., Imdorf, A., & Rosenkranz, P. (2006). Survival of mite infested (Varroa destructor) honey bee Apis mellifera) colonies in a Nordic climate. Apidologie, 37(5), 564–570. https://doi.org/10.1051/apido:2006031
- Giacomelli, A., Pietropaoli, M., Carvelli, A., Iacoponi, F., & Formato, G. (2016). Combination of thymol treatment (Apiguard ®) and caging the queen technique to fight Varroa destructor. Apidologie, 47(4), 606–616. https://doi.org/10.1007/s13592-015-0408-4
- Gregorc, A., & Sampson, B. (2019). Diagnosis of Varroa mite (Varroa destructor) and sustainable control in honey bee (Apis mellifera) colonies – a review. Diversity, 11(12), 243. https://doi.org/10.3390/d11120243
- Gregorc, A., Knight, P. R., & Adamczyk, J. (2017). Powdered sugar shake to monitor and oxalic acid treatments to control Varroa mites (Varroa destructor Anderson and Trueman) in honey bee (Apis mellifera) colonies. Journal of Apicultural Research, 56(1), 71–75. https://doi.org/10.1080/00218839.2017.1278912
- Guichard, M., Dainat, B., & Dietemann, V. (2023). Prospects, challenges, and perspectives in harnessing natural selection to solve the “Varroa problem” of honey bees. Evolutionary Applications, 16(3), 593–608. https://doi.org/10.1111/eva.13533
- Haber, A. I., Steinhauer, N. A., & vanEngelsdorp, D. (2019). Use of chemical and nonchemical methods for the control of Varroa destructor (Acari: Varroidae) and associated winter colony losses in U.S. beekeeping operations. Journal of Economic Entomology, 112(4), 1509–1525. https://doi.org/10.1093/jee/toz088
- Harbo, J. R., & Harris, J. W. (1999). Selecting honey bees for resistance to Varroa jacobsoni. Apidologie, 30(2-3), 183–196. https://doi.org/10.1051/apido:19990208
- Le Conte, Y., Ellis, M., & Ritter, W. (2010). Varroa mites and honey bee health: Can Varroa explain part of the colony losses? Apidologie, 41(3), 353–363. https://doi.org/10.1051/apido/2010017
- Lee, K., Moon, R. D., Burkness, E. C., Hutchison, W. D., & Spivak, M. (2010). Practical sampling plans for Varroa destructor (Acari: Varroidae) in Apis mellifera (Hymenoptera: Apidae) colonies and apiaries. Journal of Economic Entomology, 103(4), 1039–1050. https://doi.org/10.1603/EC10037
- Macedo, P. A., Wu, J., & Ellis, M. D. (2002). Using inert dusts to detect and assess Varroa infestations in honey bee colonies. Journal of Apicultural Research, 41(1-2), 3–7. https://doi.org/10.1080/00218839.2002.11101062
- Mattila, H. R., & Otis, G. W. (2000). The efficacy of Apiguard against Varroa and tracheal mites, and its effect on honey production: 1999 trial. American Bee Journal, 140(12), 969–973.
- McMenamin, A. J., & Genersch, E. (2015). Honey bee colony losses and associated viruses. Current Opinion in Insect Science, 8, 121–129. https://doi.org/10.1016/j.cois.2015.01.015
- Nazzi, F., & Le Conte, Y. (2016). Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annual Review of Entomology, 61(1), 417–432. https://doi.org/10.1146/annurev-ento-010715-023731
- Neumann, P., & Carreck, N. L. (2010). Honey bee colony losses. Journal of Apicultural Research, 49(1), 1–6. https://doi.org/10.3896/IBRA.1.49.1.01
- Noble, N. I., Stuhl, C., Nesbit, M., Woods, R., & Ellis, J. D. (2021). A comparison of Varroa destructor (Acari: Varroidae) collection methods and survivability in in vitro rearing systems. Florida Entomologist, 104(1), 13–17. https://doi.org/10.1653/024.104.0103
- O’Neal, R. J., & Waller, G. D. (1984). On the pollen harvest by the honey bee (Apis mellifera L.) near Tucson, Arizona (1976-1981). Desert Plants, 6, 81–109.
- Peng, Y. S., Fang, Y., Xu, S., & Ge, L. (1987). The resistance mechanism of the Asian honey bee Apis cerana Fabr, to an ectoparasitic mite Varroa jacobsoni Oud. Journal of Intervebrate Pathology, 49(1), 54–60. https://doi.org/10.1016/0022-2011(87)90125-X
- Pettis, J. S., & Jadczak, T. (2005). Detecting coumaphos resistance in Varroa mites. American Bee Journal, 145(12), 967–970.
- Pettis, J. S., Kochansky, J., & Feldlaufer, M. F. (2004). Larval Apis mellifera L. (Hymenoptera: Apidae) mortality after topical application of antibiotics and dusts. Journal of Economic Entomology, 97(2), 171–176. https://doi.org/10.1093/jee/97.2.171
- Pritchard, D. J. (2016). Grooming by honey bees as a component of Varroa resistant behavior. Journal of Apicultural Research, 55(1), 38–48. https://doi.org/10.1080/00218839.2016.1196016
- Ramirez, W. B. (1987). VII Brazil Congress: The latest on Varroa jacobsoni. New Letter for Beekeepers in Tropical and Subtropical Countries, 10, 11.
- Ramirez, W. B. (1989). Can Varroa mites be controlled with “dust”? Apiacta, 24, 3–6.
- Ramirez, W. B., & Malavasi, J. G. (1991). Conformation of the ambulacrum of Varroa jacobsoni oudemans (Mesostigmata: Varroidae): A grasping structure. International Journal of Acarology, 17(3), 169–173. https://doi.org/10.1080/0164795910863903
- Rinkevich, F. D. (2020). Detection of amitraz resistance and reduced treatment efficacy in the Varroa mite, Varroa destructor, within commercial beekeeping operations. PloS One, 15(1), e0227264. https://doi.org/10.1371/journal.pone.0227264
- Rosenkranz, P., Aumeier, P., & Ziegelmann, B. (2010). Biology and control of Varroa destructor. Journal of Invertebrate Pathology, 103 (Suppl.1), S96–S119. https://doi.org/10.1016/j.jip.2009.07016
- SAS 9.4 (2016). SAS Institute, Cary, North Carolina USA.
- Stanimirovic, Z., Aleksic, N., Stevanovic, J., Cirkovic, D., Mirilovic, M., Djelic, N., & Stojic, V. (2011). The influence of pulverised sugar dusting on the degree of infestation of honey bee colonies with Varroa destructor. Acta Veterinaria, 61(2-3), 309–325. https://doi.org/10.2298/AVB1103309S
- Stevanovic, J., Stanimirovic, Z., Lakic, N., Djelic, N., & Radovic, I. (2012). Stimulating effect of sugar dusting on honey bee grooming behaviour. Entomologia Experimentalis et Applicata, 143(1), 23–30. https://doi.org/10.1111/j.1570-7458.2012.01231.x
- Teal, P. E. A., Duehl, A. J., & Carroll, M. J. (2014). Methods for attracting honey bee parasitic mites. U.S. Patent Number 8,647,615 B1, February 11, 2014.
- Traynor, K. S., Mondet, F., de Miranda, J. R., Techer, M., Kowallik, V., Oddie, M. A. Y., Chantawannakul, P., & McAfee, A. (2020). Varroa destructor: a complex parasite, crippling honey bees worldwide. Trends in Parasitology, 36(7), 592–606. https://doi.org/10.1016/j.pt.2020.04.004
- Wang, Q., Xu, X., Zhu, X., Chen, L., Zhou, S., Huang, Z. Y., & Zhou, B. (2016). Low-temperature stress during capped brood stage increases pupal mortality, misorientation and adult mortality in honey bees. PloS One, 11(5), e0154547. https://doi.org/10.1371/journal.pone.0154547
