?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The strategy of development on zeolite membrane for forward osmosis was investigated. Defect-less MFI membranes with almost the same thickness were successfully prepared on five kinds of α-Al2O3 tubular supports having different structural parameters. The water flux through these membranes could be expressed as a function of the structural parameter of support. Moreover, the effect of the Si/Al ratio in zeolite layer, which serves as an index of hydrophilicity, was examined. As a result, a membrane with a relatively hydrophilic character, having Si/Al ratio = 18.6, showed a higher water flux. Conversely, both water and salt hardly penetrated through a hydrophobic membrane (Si/Al = ∞). Reducing the structural parameter in the support layer and improving the hydrophilicity of the active layer is crucial to achieving high water-permeability of zeolite FO membrane.
1. Introduction
Forward osmosis (FO) is widely studied as a novel water treatment with low energy consumption in recent decades. FO has extensive potential applications; wastewater treatment (Goh et al. Citation2019), landfill leachate treatment (Iskander et al. Citation2017), liquid food concentration (Wenten et al. Citation2021), and pressure retarded osmosis (Chung et al. Citation2015). Previously, polymeric membranes have been widely developed for forward osmosis, such as cellulose triacetate and polyamide (Cath et al. Citation2006).
Inorganic membranes have been recently studied as alternative FO membrane due to their high thermal and chemical resistance. For example, Al2O3-TiO2 nanocomposite membrane (Azadi et al. Citation2021) and silica membrane (You et al. Citation2017) were demonstrated in FO operations. Silica membrane synthesized by a sol-gel method exhibited effective heavy metal ions rejection in acidic wastewater. Recently, we have reported the FO performance of Na-ZSM-5 zeolite membrane, which consists of a few µm-thick zeolite active layer and a few mm-thick ceramic support layer (Sakai et al. Citation2019). Our zeolite membrane displayed a very low salt flux on the basis of molecular sieving effect with high chemical stability. However, the water flux through zeolite membrane was relatively short compared with polymeric membranes, indicating a need for improvement.
In FO operation, the driving force of water permeation is the difference in osmotic pressures between feed solution (FS) and draw solution (DS). It has been widely studied that osmotic pressure, the driving force of water permeation, is significantly influenced by concentration polarization (CP) (Gruber et al. Citation2011; Bui et al. Citation2015). The CP occurring on the membrane surface and within the membrane are referred to as External CP (ECP) and Internal CP (ICP), respectively. ECP can be minimized by increasing the mass transfer rate in the feed and draw solutions. Consequently, the effect of ICP is more severe for water flux.
FO membrane was generally designed as a combination of an active layer for salt rejection and a support layer for mechanical strength. In addition, the support layer was also crucial for water permeation, as the structure of support layer greatly influences ICP. Here, the structural parameter was used to understand the relationship between the support structure and water flux. In polymeric membranes, the effect of structural parameter on water flux was extensively studied, leading to the conclusion that water flux was inversely proportional to the structural parameter (Wong et al. Citation2012; Manickam and McCutcheon Citation2017). In contrast, there are limited studies on inorganic membranes in FO operation, leaving the effect of the structural parameter of support in zeolite membrane as an open question.
Water flux through zeolite membranes is known to be influenced by the hydrophilicity of active layer in pervaporation and vapor permeation operations (Jiang et al. Citation2019; Zito et al. Citation2019). Jiang et al. synthesized CHA-type zeolite membranes with various Si/Al ratios, the index of hydrophilicity, ranging from 2.8 to 3.2. The water flux through CHA membranes increased with decreasing Si/Al ratio of the membrane in pervaporation for water/ethanol mixture. However, the relationship between the hydrophilicity of zeolite layer and water flux in FO operation should further be studied.
In this study, we prepared MFI-type zeolite membranes on porous α-Al2O3 supports having different structural parameters, and explored the effect of structural parameter on water flux through zeolite membrane. The contribution of Si/Al ratio in zeolite layer to water flux was also studied.
2. Experimental
2.1. Materials and Chemicals
5 kinds of tubular α-Al2O3 supports (SA∼SE) which had different pore size, porosity, and tortuosity were used for membrane preparation. All support was purchased from Japanese companies.
Sodium aluminate (Na2O, 33 wt%; Al2O3, 37 wt%; Kanto Chemical Co.), colloidal silica (ST-S, Nissan Chemical Ind. Ltd.), sodium hydroxide (97%, Kanto Chemical), tetraethylorthosilicate (TEOS, 98 wt%, Merck Co.), tetrapropylammonium hydroxide (TPAOH, 1.0 M, Sigma-Aldrich Co.) and ethanol (99.5 wt%, Kanto Chemical Co.) were used for membrane preparations. All chemicals were directly used without further purification.
2.2. Membrane Preparation
ZSM-5 and silicalite-1 membranes were synthesized by a secondary growth method. ZSM-5, aluminosilicate MFI-type zeolite, membranes were synthesized on 5 kinds of tubular α-Al2O3 supports to evaluate the effect of the structural parameter of porous support on water flux. Moreover, a membrane with silicalite-1, all silica MFI-type zeolite, was synthesized on a support to study the influence of Si/Al ratio. Both ZSM-5 and silicalite-1 are kinds of MFI-type zeolite, characterized by a micropore with 0.55 nm diameter in their framework.
ZSM-5 membranes were prepared on the outer surface of tubular support, SA-SE. Seed crystals were loaded on the outer surface by a dip-coating method. The seeded support was immersed into a synthesis solution and hydrothermally treated at 453 K for 12 h. A synthesis solution having a molar composition of Al2O3: 240SiO2: 53.3Na2O: 8000H2O was prepared by mixing sodium aluminate, colloidal silica, sodium hydroxide, and distilled water. After the hydrothermal treatment, the membrane was washed with boiling water and dried at 383 K for 12 h before use.
Silicalite-1 membrane was prepared on support, SD. The composition of the synthesis solution prepared by the mixing of TEOS, TPAOH, distilled water, and ethanol was 25SiO2: 1.5(TPA)2O: 1650H2O: 200EtOH. The temperature and period of hydrothermal treatment were 363 K and 120 h, respectively. After drying, the membrane was calcined at 773 K for 8 h to remove the organic structure-directing agent, TPA cation. All other protocols for membrane preparation were the same as that of ZSM-5 membrane.
Details of the preparation procedures of ZSM-5 (Sakai et al. Citation2019) and silicalite-1 (Sakai et al. Citation2020) membranes were described elsewhere.
2.3. N2 Permeation Test for Support
N2 permeances through various supports were evaluated by the unary system permeation tests. N2 was fed to the outer surface of support at 0.4 MPa. The permeate side was kept at atmospheric pressure. The flow rate of N2, QN2 [m3 s−1], was measured using a dry gas meter (DC-2c, SINAGAWA). The N2 permeance, πN2 [mol m−2 s−1 Pa−1], was calculated by the following EquationEq. (1)(1)
(1) ;
(1)
(1)
where A, P, R, and T represent membrane area [m2], pressure [Pa], gas constant [Pa m3 mol−1 K−1], and temperature [K], respectively. ΔP is the pressure difference between the feed and permeate sides [Pa].
2.4. FO Measurement for Zeolite Membrane
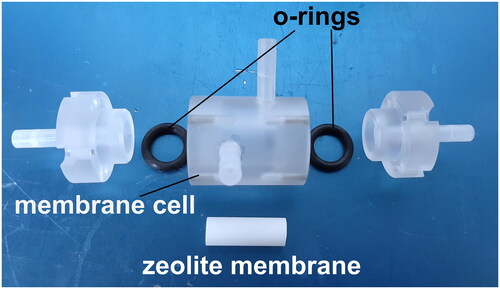
Zeolite membrane was fixed in a membrane cell with O-rings, as shown in . The effective membrane area was 6.28 × 10−4 m2. The membrane temperature was controlled at 333 K using a water bath. 5 wt% of NaCl aqueous solution and distilled water were used as the draw solution (DS) and the feed solution (FS), respectively. DS and FS were circulated the outside and inside of the tubular membranes by a peristaltic pump (AL-DS mode). Each flow rate was adjusted at 300 mL min−1. To minimize the influence of ECP, the Re numbers of DS and FS were set above 12,000 to ensure turbulent flow in both DS and FS during the FO operation.
The water flux was calculated using the changes in the volume of DS and FS. The salt flux, JS, was calculated using the change of Na concentration in FS before and after the FO experiment. The concentrations of Na and Cl were analyzed by an ion electrode (Orion Dual Star, Thermo Scientific).
For all FO tests, excess amounts of FS and DS were used, resulting in almost no concentration difference before and after the experiment.
3. Results and Discussion
3.1. Evaluation of Structural Parameters of Tubular a-Al2O3 Supports
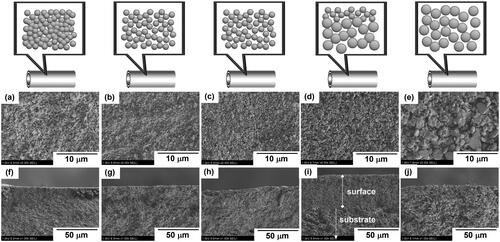
5 kinds of tubular α-Al2O3 supports which had different pore sizes, porosities, and tortuosities were used for membrane preparation. Each support exhibited uniform dimensions, with a diameter of 10 mm, a length of 30 mm, and a thickness 1.5 mm. provides the schematic diagrams of their structures and typical FE-SEM images of these supports. SA, SB, SC, and SE had symmetric structures, possessing uniform pore-structure throughout the entire support. In contrast, SD, had an asymmetrical structure, i.e. the outer layer had narrow pore and the inner layer had large pore. As seen in , the outer surface layer with a thickness of 40 µm was observed in SD.
Figure 2. Typical FE-SEM images of porous α-Al2O3 support. Surface of (a)SA, (b)SB, (c)SC, (d) SD and (e)SE; Cross-section of (f)SA, (g)SB, (h)SC, (i) SD and (j)SE.
The structural parameters, S [m], of symmetric supports were defined as the following EquationEq. (2)(2)
(2) ;
(2)
(2)
where τ, L and e represent tortuosity [−], thickness [m], and porosity [−], respectively. The porosities of each support which were evaluated by a mercury intrusion method were provided by the support suppliers. To evaluate the tortuosity in support, a N2 permeation test was carried out. Under the conditions of the permeation test, the mean free path of N2 was 17 nm, sufficiently smaller than the pore sizes of porous supports. Therefore, we used the following EquationEq. (3)
(3)
(3) on the basis of Poiseuille flow principles to calculate tortuosity (EL-Bourawi et al. Citation2007);
(3)
(3)
where r and µ are the pore radius of support [m] and viscosity [Pa s] of N2, respectively. We evaluated the N2 permeance through each support, and then calculated the tortuosity and structural parameters.
In the case of asymmetric support, SD, the surface and substrate layer had the same structures as SB and SE, respectively. Thus, the structural parameter of SD was estimated by the following EquationEq. (4)(4)
(4) ;
(4)
(4)
where Lsubstrate and Lsurface are the thicknesses of substrate and surface layer determined by microscopic observation.
lists the structural properties of each support. N2 permeance naturally increased with increasing pore size and porosity from SA to SE.
Table 1. Properties of porous α-Al2O3 supports.
The N2 permeance through SE, with a pore size of 0.7 mm and a porosity of 48%, was 19.7 × 10−6 mol m−2 s−1 Pa−1, a value more than 10-fold greater than that through SA with a pore size of 0.14 mm and a porosity of 27%. From the results of N2 permeation tests, the structural parameter of each support was calculated within the range of 4.53–6.38 mm. The order of structural parameters of the supports was as follows; SA > SB > SC > SD > SE.
3.2. Membrane Characterization
and present the XRD patterns and typical FE-SEM images of ZSM-5 membranes prepared on SA–SE. Silicalite-1 membrane prepared on SD was also depicted. Si/Al ratios displayed in were detected by SEM-EDS.
Figure 3. XRD patterns of prepared membranes. ZSM-5 membrane on (a) SA, (b) SB, (c) SC, (d) SD and (e) SE; (f) Silicalite-1 membrane on SD.
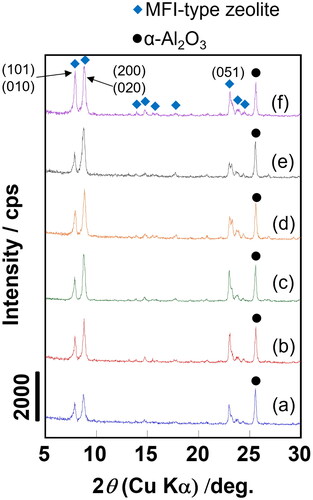
Figure 4. Typical FE-SEM images of prepared zeolite membranes. Surface and cross-section of ZSM-5 membrane on (a,g)SA, (b,h)SB, (c,i)SC, (d,j) SD and (e,k)SE. Surface (f) and cross-section (l) of silicalite-1 membrane on SD.

We only observed the diffraction peaks attributed to MFI-type zeolite and α-Al2O3 in , suggesting that all membranes had pure MFI-type structures. MFI-type zeolite membranes occasionally exhibit the unique orientations of crystal layer. Furthermore, the permeation and separation properties were affected by a crystal orientation (Lai et al. Citation2003; Choi et al. Citation2006; Zhou et al. Citation2014). It is notable that there is not much difference in crystal orientation among membranes, as the intensity ratios of each peak were almost the same among the samples. Consequently, we believe that the effect of crystal orientation on membrane performance is negligible.
Continuous layers of zeolite crystals were observed in the surface images (as shown in ) and the surfaces of support layers were fully covered with zeolite layer. All membranes possessed almost the same membrane thickness, ca. 2 µm, as determined from cross-section views. The Si/Al ratios of ZSM-5 membranes fell within a narrow range, 18.6–19.7. In silicalite-1 membrane, Al was undetectable via SEM-EDS, indicating that Si/Al ratio of this membrane was infinity.
The molecular sieving properties of these membranes was evaluated by a separation test for n-hexane/2,2-dimethylbutane, serving as an indicator for the defect quantity in MFI-type zeolite membrane (refer to Table S1). The separation factors exceeding 100 for these membranes suggested that all ZSM-5 and silicalite-1 membranes had very few defects.ZSM-5 membranes with nearly identical thicknesses and Si/Al ratios, and very few defects were successfully prepared on different supports. Thus, the effect of structural parameter of support on water flux is able to be estimated by using these ZSM-5 membranes. Furthermore, the influence of Si/Al ratio of zeolite layer can be evaluated by comparing ZSM-5 and silicalite-1 membranes prepared on the same support.
3.3. Effect of Structural Parameters on Water Flux in FO Operation
presents the relationships between the structural parameters of tubular supports and water flux in ZSM-5 membrane. The water flux changed from 0.12 to 1.62 L m−2 h−1 for different supports. In contrast, the salt fluxes through ZSM-5 membranes were very low, < 25 mg m−2 h−1 in all cases. Since ZSM-5 membranes were successfully prepared with less defects on each support as described above, thereby impeding salt penetration by a molecular sieving effect. The molecular sieving property of ZSM-5 membrane in FO operation was thoroughly discussed in our previous report (Sakai et al. Citation2019).
Figure 5. Water and salt fluxes through (a) ZSM-5 membranes and (b) polymeric membranes a function of structural parameters of support. Closed and open keys represented water and salt flux, respectively. In ○, SA; □, SB; ▵, SC; ◊, SD; ▿, SE. In ○, Al2O3; □, polysulfone; ▵, polyphenylsulfone; ◊, polyether sulfone; ▿, polyvinylidene fluoride.
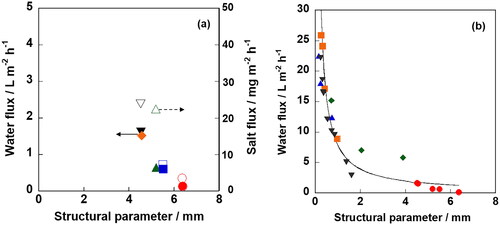
Here, we discuss the effect of structural parameter on water flux through ZSM-5 membrane. Clearly, the water flux could be expressed as a function of the structural parameter of support according to the following theoretical equation (Loeb et al. Citation1997; Cath et al. Citation2006; Yasukawa et al. Citation2014);
(5)
(5)
where, A, B and D represent the water permeability, the solute permeability and the diffusion coefficient of the solute.
In , the effects of structural parameter on water flux through various organic support and α-Al2O3 support were compared (Tiraferri et al. Citation2013; Zhong et al. Citation2013; Emadzadeh et al. Citation2014; Wang and Xu Citation2015; Rastgar et al. Citation2017; Zhang et al. Citation2017; Park et al. Citation2018; Zhang et al. Citation2018; Kim and Heldman Citation2021; Kahrizi et al. Citation2022). It is noted that the experimental conditions and the water permeability coefficients in the active layer vary significantly, making it challenging to directly compare the different types of plots. However, the relationship between the structural parameter and the water flux could be represented in a simple form by a single curve, not only just for zeolite membranes but also for polymeric membranes. The equation of the regression curve in was represented as y = 5.36 x−1 (R2 = 0.936).
The structural parameters of support for polymeric membranes were reported to be in the range of 0.08–1.6 mm, which is an order of magnitude smaller than those of α-Al2O3 supports used in this study. A zeolite membrane with the structural parameter of 1 mm would yield a water flux exceeding 10 L m−2 h−1, as evaluated from the results of .
Recently, some zeolite membranes were successfully synthesized on α-Al2O3 hollow fiber support (Min et al. Citation2019; Yang et al. Citation2019; Araki et al. Citation2021), which had minimal thickness. The structural parameters of these hollow fibers were calculated from the reported values of porosity, thickness, pore size, and N2 or H2 permeance. lists the reported properties and calculated structural parameters of hollow fiber supports. These α-Al2O3 hollow fibers exhibited small structural parameters of 0.20 and 0.65 mm, which are significantly lower than those of the tubular supports used in this study, suggesting that these hollow fiber supports were well-suited for zeolite membranes for FO operations. By comparing and , we found that the thickness of support was the most critical factor for structural parameters because there were not much differences in porosity and tortuosity.
Table 2. Properties of hollow fiber α-Al2O3 supports.
Zeolite membranes achieve minimal salt flux by a molecular sieving effect, which remain unaffected by a structural parameter in support. As a result, a large water flux and a small salt flux can be obtained simultaneously by the preparation of zeolite membranes on hollow fiber.
In this study, we have successfully demonstrated that the structural parameter is one of the important factors in water flux. An important factor in support structure not included in the structural parameter is pore size. The effect of the pore size of support on the water flux is a subject for future study.
3.4. Effect of Si/Al Ratio in MFI-Type Zeolite Membrane on Water Flux
The effect of Si/Al ratio in zeolite layer on water flux was studied by comparing ZSM-5 (Si/Al of 18.6) and silicalite-1 (Si/Al of infinity) membranes synthesized on the same support, SD. As listed in , the water and salt fluxes through ZSM-5 membrane were 1.52 L m−2 h−1 and 15.3 mg m−2 h−1, respectively, whereas both water and salt hardly penetrated through silicalite-1 membrane; water flux < 0.0 L m−2 h−1, salt flux < 2.0 mg m−2 h−1.
Table 3. Water and salt fluxes through ZSM-5 and Silicalite-1 membranes.
As the Al content in zeolite framework increases, the framework becomes hydrophilic, leading to that zeolite membranes with a low Si/Al ratio should be suitable for dehydration operation. Jiang et al. studied that the effect of Si/Al ratio in CHA-type zeolite membrane on water flux in pervaporation (Jiang et al. Citation2019). They reported that water flux through CHA membrane increased as Si/Al ratio decreased within the range of 2.8–3.2.
Silicalite-1, all-silica MFI, is well known as very hydrophobic zeolite. Its strong hydrophobic character was applied to ethanol/water separation (Elyassi et al. Citation2016; Ueno et al. Citation2017). Because silicalite-1 membrane had a relatively strong interaction with ethanol due to its hydrophobicity, ethanol preferentially penetrated through the membrane in spite of its bulky size compared with water. On the other hand, ZSM-5 membrane with a small value of Si/Al exhibited water selectivity from the mixture of water/alcohol (Zhou et al. Citation2005; Li et al. Citation2016). Their results suggested that even zeolites with the same framework exhibited markedly different water fluxes and selectivities depending on their hydrophilicity.
Owing to strong hydrophobicity, silicalite-1 membrane inhibited water permeation in FO operation. As a result, we concluded that hydrophilic zeolite, i.e. a zeolite with a low Si/Al ratio, was suitable for water permeable membrane in FO operations.
4. Conclusions
ZSM-5 membranes having almost the same thickness, Si/Al ratio, and orientation were synthesized on various tubular α-Al2O3 supports, exhibiting very few defects. Water flux through zeolite membrane was expressed as a function of the structural parameter of support, suggesting that employing supports with small structural parameters, such as hollow fibers, significantly increases water flux. The relationship between water flux and structural parameter for polymeric membranes and zeolite membranes can be expressed in a simplified manner with the same curve. In contrast, salt flux remained affected by support structure due to the molecular sieving effect of zeolite layer. Hydrophilicity of the zeolite layer, studied by comparing ZSM-5 and silicalite-1 membranes, revealed that a hydrophilic zeolite layer contributed to improved water flux in FO operations.
Finally, we concluded that the reducing structural parameter in support layer and enhancing the hydrophilicity of active layer are crucial for developing highly water-permeable zeolite FO membranes.
| Nomenclature | ||
| L | = | support thickness [m] |
| ππ | = | permeance [mol m−2 s−1 Pa−1] |
| P | = | pressure [Pa] |
| Q | = | flow rate [mol s−1] |
| r | = | pore size of support [m] |
| R | = | gas constant [kg m3 mol−1 K−1] |
| S | = | structural parameter [m] |
| T | = | temperature [K] |
| α | = | separation factor [-] |
| ε | = | porosity [-] |
| µ | = | viscosity [Pa s] |
| τ | = | tortuosity [-] |
Supplemental Material
Download MS Word (28.5 KB)Disclosure Statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Araki S, Yamashita R, Li K, Yamamoto H. 2021. Preparation and gas permeation properties of all-silica CHA zeolite hollow fiber membranes prepared on amorphous-silica hollow fibers. J Membr Sci. 634:119338. doi: 10.1016/j.memsci.2021.119338.
- Azadi F, Karimi-Jashni A, Zerafat MM, Saadat S. 2021. Fabrication, optimization, and performance of a novel double-skinned Al2O3. TiO2 ceramic nanocomposite membrane for forward osmosis application. Environ Technol Innov. 22:101423. doi: 10.1016/j.eti.2021.101423.
- Bui N-N, Arena JT, McCutcheon JR. 2015. Proper accounting of mass transfer resistances in forward osmosis: Improving the accuracy of model predictions of structural parameter. J Membr Sci. 492:289–302. doi: 10.1016/j.memsci.2015.02.001.
- Cath TY, Childress AE, Elimelech M. 2006. Forward osmosis: principles, applications, and recent developments. J Membr Sci. 281:70–87. doi: 10.1016/j.memsci.2006.05.048.
- Choi J, Ghosh S, Lai Z, Tsapatsis M. 2006. Uniformly a-oriented MFI zeolite films by secondary growth. Angew Chem Int Ed Engl. 45:1154–1158. doi: 10.1002/anie.200503011.
- Chung T-S, Luo L, Wan CF, Cui Y, Amy G. 2015. What is next for forward osmosis (FO) and pressure retarded osmosis (PRO). Sep Purif Technol. 156:856–860. doi: 10.1016/j.seppur.2015.10.063.
- EL-Bourawi MS, Khayet M, Ma R, Ding Z, Li Z, Zhang X. 2007. Application of vacuum membrane distillation for ammonia removal. J Membr Sci. 301:200–209. doi: 10.1016/j.memsci.2007.06.021.
- Elyassi B, Jeon MY, Tsapatsis M, Narasimharao K, Basahel SN, Al-Thabaiti S. 2016. Ethanol/water mixture pervaporation performance b-oriented silicalite-1 membranes made by gel-free secondary growth. AIChE J. 62:556–563. doi: 10.1002/aic.15124.
- Emadzadeh D, Lau WJ, Matsuura T, Rahbari-Sisakht M, Ismail AF. 2014. A novel thin film composite forward osmosis membrane prepared from PSf–TiO2 nanocomposite substrate for water desalination. Chem Eng J. 237:70–80. doi: 10.1016/j.cej.2013.09.081.
- Goh P S, Ismail A F, Ng B C, Abdullah M S. 2019. Recent progresses of forward osmosis membranes formulation and design for wastewater treatment. Water 11:2043. doi: 10.3390/w11102043.
- Gruber MF, Johnson CJ, Tang CY, Jensen MH, Yde L, Hélix-Nielsen C. 2011. Computational fluid dynamics simulations of flow and concentration polarization in forward osmosis membrane systems. J Membr Sci. 379:488–495. doi: 10.1016/j.memsci.2011.06.022.
- Iskander SM, Zou S, Brazil B, Novak JT, He Z. 2017. Energy consumption by forward osmosis treatment of landfill leachate for water recovery. Waste Manag. 63:284–291. doi: 10.1016/j.wasman.2017.03.026.
- Jiang J, Peng L, Wang X, Qiu H, Ji M, Gu X. 2019. Effect of Si/Al ratio in the framework on the pervaporation properties of hollow fiber CHA zeolite membranes. Micropor Mesopor Mater. 273:196–202. doi: 10.1016/j.micromeso.2018.07.015.
- Kahrizi M, Gonzales PR, Kong L, Matsuyama H, Lu P, Lin J, Zhao S. 2022. Significant roles of substrate properties in forward osmosis membrane performance: A review. Desalination. 528:115615. doi: 10.1016/j.desal.2022.115615.
- Kim W-J, Heldman DR. 2021. A mathematical estimation of the structural parameter for prediction of Forward Osmosis (FO) performance. J Water Process Eng. 39:101719. doi: 10.1016/j.jwpe.2020.101719.
- Lai Z, Bonilla G, Diaz I, Nery JG, Sujaoti K, Amat MA, Kokkoli E, Terasaki O, Thompson RW, Tsapatsis M, et al. 2003. Microstructural optimization of a zeolite membrane for organic vapor separation. Science. 300:456–460. doi: 10.1126/science.1082169.
- Li L, Yang J, Li J, Wang J, Lu J, Yin D, Zhang Y. 2016. High performance ZSM-5 membranes on coarse macroporous α-Al2O3 supports for dehydration of alcohols. AIChE J. 62:2813–2824. doi: 10.1002/aic.15234.
- Loeb S, Titelman L, Korngold E, Freiman J. 1997. Effect of porous support fabric on osmosis through a Loeb-Sourirajan type asymmetric membrane. J. Membr. Sci. 129:243–249. doi: 10.1016/S0376-7388(96)00354-7.
- Manickam SS, McCutcheon JR. 2017. Understanding mass transfer through asymmetric membranes during forward osmosis: a historical perspective and critical review on measuring structural parameter with semi-empirical models and characterization approaches. Desalination. 421:110–126. doi: 10.1016/j.desal.2016.12.016.
- Min B, Yang S, Korde A, Kwon YH, Jones CW, Nair S. 2019. Continuous zeolite MFI membranes fabricated from 2D MFI nanosheets on ceramic hollow fibers. Angew Chem Int Ed Engl. 58:8201–8205. doi: 10.1002/anie.201903554.
- Park MJ, Gonzales RR, Abdel-Wahab A, Phuntsho S, Shon HK. 2018. Hydrophilic polyvinyl alcohol coating on hydrophobic electrospun nanofiber membrane for high performance thin film composite forward osmosis membrane. Desalination. 426:50–59. doi: 10.1016/j.desal.2017.10.042.
- Rastgar M, Shakeri A, Bozorg A, Salehi H, Saadattalab V. 2017. Impact of nanoparticles surface characteristics on pore structure and performance of forward osmosis membranes. Desalination. 421:179–189. doi: 10.1016/j.desal.2017.01.040.
- Sakai M, Kaneko T, Sasaki Y, Sekigawa M, Matsukata M. 2020. Formation process of columnar grown (101)-oriented silicalite-1 membrane and its separation property for xylene isomer. Crystals. 10:949. doi: 10.1016/j.jwpe.2019.100864.
- Sakai M, Seshimo M, Matsukata M. 2019. Hydrophilic ZSM-5 membrane for forward osmosis operation. J. Water Process Eng. 32:100864. doi: 10.3390/membranes11060382.
- Tiraferri A, Yip NY, Straub AP, Castrillon SR-V, Elimelech M. 2013. A method for the simultaneous determination of transport and structural parameters of forward osmosis membranes. J Membr Sci. 444:523–538. doi: 10.1016/j.memsci.2013.05.023.
- Ueno K, Negishi H, Okuno T, Saito T, Tawarayama H, Ishikawa S, Miyamoto M, Uemiya S, Sawada Y, Oumi Y. 2017. High-performance silicalite-1 membranes on porous tubular silica supports for separation of ethanol/water mixtures. Sep Purif Technol. 187:343–354. doi: 10.1016/j.seppur.2017.06.071.
- Wang Y, Xu T. 2015. Anchoring hydrophilic polymer in substrate: an easy approach for improving the performance of TFC FO membrane. J Membr Sci. 476:330–339. doi: 10.1016/j.memsci.2014.11.025.
- Wenten IG, Khoiruddin K, Reynard R, Lugito G, Julian H. 2021. Advancement of forward osmosis (FO) membrane for fruit juice concentration. J Food Eng. 290:110216. doi: 10.1016/j.jfoodeng.2020.110216.
- Wong MCY, Martinez K, Ramon GZ, Hoek EMV. 2012. Impacts of operating conditions and solution chemistry on osmotic membrane structure and performance. Desalination. 287:340–349. doi: 10.1016/j.desal.2011.10.013.
- Yang S, Kwon YH, Koh D-Y, Min B, Liu Y, Nair S. 2019. Highly selective SSZ-13 zeolite hollow fiber membranes by ultraviolet activation at near-ambient temperature. Chem Nano Mat. 5:61–67. doi: 10.1002/cnma.201800272.
- Yasukawa M, Higa M, Matsuyama H. 2014. Development of analysis method for forward osmosis hollow fiber membrane performance. Bull Soc Sea Water Sci. 68:94–101. doi: 10.11457/swsj.68.94.
- You S, Lu J, Tang CY, Wang X. 2017. Rejection of heavy metals in acidic wastewater by a novel thin-film inorganic forward osmosis membrane. Chem Eng J. 320:532–538. doi: 10.1016/j.seppur.2004.12.016.
- Zhang X, Shen L, Guan C-Y, Liu C-X, Lang W-Z, Wang Y. 2018. Construction of SiO2@MWNTs incorporated PVDF substrate for reducing internal concentration polarization in forward osmosis. J Membr Sci. 564:328–341. doi: 10.1016/j.memsci.2018.07.043.
- Zhang X, Shen L, Lang W-Z, Wang Y. 2017. Improved performance of thin-film composite membrane with PVDF/PFSA substrate for forward osmosis process. J Membr Sci. 535:188–199. doi: 10.1016/j.memsci.2017.04.038.
- Zhong P, Fu X, Chung T-S, Weber M, Maletzko C. 2013. Development of thin-film composite forward osmosis hollow fiber membranes using direct sulfonated polyphenylenesulfone (sPPSU) as membrane substrates. Environ Sci Technol. 47:7430–7436. doi: 10.1021/es4013273.
- Zhou L, Wang T, Nguyen QT, Li J, Long Y, Ping Z. 2005. Cordierite-supported ZSM-5 membrane: Preparation and pervaporation properties in the dehydration of water–alcohol mixture. Sep Purif Technol. 44:266–270. doi: 10.1016/j.seppur.2004.12.016.
- Zhou M, Korelskiy D, Ye P, Grahn M, Hedlund J. 2014. A uniformly oriented MFI membrane for improved CO2 separation. Angew Chem Int Ed Engl. 53:3492–3495. doi: 10.1002/anie.201311324.
- Zito PF, Brunetti A, Caravella A, Drioli E, Barbieri G. 2019. Water vapor permeation and its influence on gases through a zeolite-4A membrane. J Membr Sci. 574:154–163. doi: 10.1016/j.memsci.2018.12.065.