ABSTRACT
The genus Muricea, a highly speciose gorgonian coral group in the Tropical Eastern Pacific (TEP), is commonly found on shallow rocky reefs including Machalilla National Park (MNP), El Pelado Marine Reserve (REMAPE) and Galápagos Marine Reserve (GMR). Here, we report the presence of M. hebes, M. echinata and M. robusta, which have been not previously reported at the REMAPE area and along the Ecuadorian region. These new records for Muricea hebes (Verril, 1864) in Ecuador broaden the known geographical distribution of these species across the tempered waters of California and Mexico, and the Tropical Eastern Pacific, belonging to Panama and Ecuador. Muricea robusta (Verril, 1864) was previously recorded in Mexico and Colombia, whereas M. echinata (Verril, 1866) was only found in Panama. This report contributes to increasing the knowledge of marine diversity in Ecuador, and broadens the previously recorded geographic distribution of the genus Muricea throughout the TEP.
Introduction
The only amphi-American and aposymbiotic group of octocorals is Muricea (Cnidaria: Plexauridae) (Sánchez Citation2016). This genus is usually abundant in shallow waters of the Tropical Eastern Pacific (TEP), in areas exposed to moderate water motion, commonly inhabiting rocky reefs or rocky caves and less commonly found on sandy bottoms (Breedy and Guzman Citation2016; Steiner et al. Citation2018). This group has a broad depth distribution, where most of the species thrive in euphotic shallow waters between 5 and 30 m, and two species inhabit the mesophotic zone between 30 and 220 m, one in the Caribbean Sea and the other in the Gulf of Mexico (Sánchez et al. Citation2019). Furthermore, the wide geographic distribution of the genus encompasses the TEP (Breedy and Guzman Citation2016) and the Atlantic Ocean, specifically the Caribbean Sea, the Gulf of Mexico, and part of the South Atlantic Ocean of Brazil (Marques and Castro Citation1995).
This wide distribution is feasible for Muricea and octocorals because they are considered mixotrophic organisms. Octocorals acquire energy through autotrophic means, via symbiont photosynthesis, and through additional heterotrophic behaviour, allowing them to successfully settle in different habitats. For instance, it is well known that octocorals are efficient filter feeders (Fabricius et al. Citation1995; Fabricius and Klumpp Citation1995; Oppen et al. Citation2005) by maximising nutrient acquisition where the concentration of light and plankton can be often limiting (Lewis Citation1982; Sánchez Citation2016). As an aposymbiotic genus, some species possess common symbiotic dinoflagellate algae, and some do not have that symbiont. This aposymbiotic status represents an interesting ecological issue that requires further research. The mutualistic relationship is mainly with photosynthetic dinoflagellates belonging to the Symbiodinaceae genus Breviolum (Oppen et al. Citation2005; LaJeunesse et al. Citation2018; Lau et al. Citation2019), as in M. echinata, M. laxa, M. muricata and M. atlantica. On the other hand, the mesophotic species M. pendula in the Gulf of Mexico and all the species in the Pacific Ocean, including M. echinata, M. crassa, M. plantaginea, M. purpurea, M. squarrosa, M. austera and Muricea sp., as well as endemic species of Ecuador such as M. galapagensis (Breedy and Guzman, Citation2016; Steiner et al. Citation2018), are azooxanthellate (Sánchez et al. Citation2019).
Along the TEP (Breedy and Guzman Citation2016) and specifically in the Equatorial Front, Muricea species are abundant and widely distributed in shallow rocky reefs. The El Pelado Marine Reserve (REMAPE), declared a Marine Protected Area (MPA) under the Marine Reserve category in 2012, is one of the richest localities for Muricea species. Muricea corals prefer the surrounding rocky reefs and can significantly shape the boundary layer in coral reef and rocky reef habitats, sharing an ecological setting living in sympatry, and exposed to fluctuations in salinity and temperature (Fiedler and Lavín Citation2017; Steiner et al. Citation2018) due to the mix of currents including the northern end of the Humboldt current. To assess the octocoral biodiversity of this zone, in 2018 we explored these rocky reef formations at El Pelado Islet and collected several octocoral species from multiple genera, including Muricea sp., Pacifigorgia sp., Leptogorgia sp. and Carijoa sp. Here, we report the first records of M. hebes, M. robusta and M. echinata for the Ecuadorian coast and update a checklist with all the octocoral samples collected in the study area.
Materials and methods
Study area
During 2018, we collected colonies from all the gorgonian coral species that we found by scuba diving at four stations around El Pelado Islet at the REMAPE area in the province of Santa Elena. The collecting stations were Laberinto, La Pared, Bajo 40 and Acuario. During the collection, all species were labelled and photographed ( and ).
Table 1. Data set of the octocorals collected from the El Pelado Equatorial Front (October 2018).
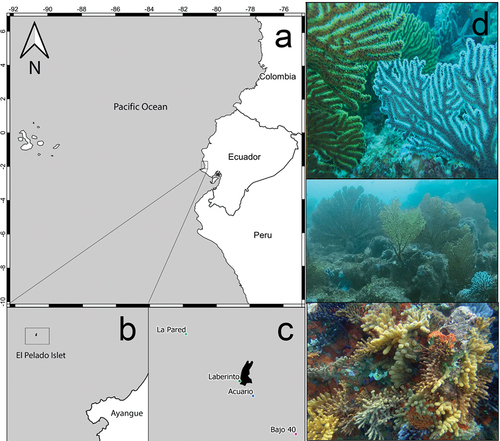
Figure 1. (a–c) Map of the sampling stations. (CENAIM-ESPOL, Bajo 40, Laberinto, Acuario, La Pared). Map credits: Divar Castro – CENAIM. (d) In situ rocky reefs of El Pelado which these species inhabit in sympatry. Exsitu Photos by D.C. Vergara and Juan A. Sánchez. Insitu Photos by Rubén Abad and Karla. B. Jaramillo.
Sampling description
The field and laboratory methodology used followed that described by Breedy and Guzmán (Citation2003), with minor modifications. At the laboratory, the species identification and morphological descriptions (Supplementary material, Table S1) were achieved using voucher subsamples at the National Center of Aquaculture and Marine Investigations (CENAIM-ESPOL) repository.
Description
After their collection in October 2018, the soft coral samples were stored. During the data management process, the names of the specimens were checked with the World Register of Marine Species (WoRMS), the data set was prepared according to the Darwin Core standard at the CENAIM-ESPOL repository, and finally, the data were compared with previous reports and publications of octocoral species, focusing on standardised morphological feature guides to the relevant genera.
Geographic coverage
Description
El Pelado Islet is located 6950.11 m from the shore, near the San Pedro and Ayangue localities in the El Pelado Marine Reserve (REMAPE) on the Ecuadorian coastline in the province of Santa Elena, Ecuador. Samples were collected from four rocky reefs – Bajo 40, Laberinto, La Pared and Acuario () – with a mean temperature of 22–24°C and a salinity of 33.5–34 Practical Salinity Units (PSU).
Coordinates
Bajo 40: -1.919°S, -80.804°W
Laberinto: -1.901°S, -80.835°W
La Pared: -1.899°S, -80.840°W
Acuario: -1.904°S, -80.820°W
Results
Description
The taxonomic coverage of the data set is limited to the soft coral assemblages of the study area. A list of the species included in the data set, also indicating the field code, museum code, locality and collection date, is given in .
Taxa included
We present a quantitative assessment of Alcyonaceae communities from the southern TEP. A total of 25 species were collected: 11 species of Muricea (M. austera, M. crassa, M. echinata, M. fruticosa, M. plantaginea, M. purpurea, M. robusta, M. squarrosa, Muricea sp., M. californica and M. hebes); five species of Pacifigorgia (P. adamsii, P. irene, P. firma, P. rubicunda and Pacifigorgia sp.); five species of Leptogorgia (L. alba, L. cuspidata, L. pumila, L. obscura and L. taboquilla); two species of Heterogorgia (H. hickmani and Heterogorgia sp.); Psammogorgia arbuscula; and Carijoa riisei. Moreover, we describe the features of the three species newly reported in this study (Table S1 and ).
Figure 2. Underwater in situ record of Muricea hebes colony with (a) the white extended polyps, and (b) M. hebes with the polyps contracted. (c) Ex situ dry colony of M. hebes. (d) Location of colonies inside a cavern at the Bajo 40 site. (e) Close-up of the small tubular calyxes in a branch. Exsitu Photos by D.C. Vergara and Juan A. Sánchez. Insitu Photos by Rubén Abad and Karla. B. Jaramillo.
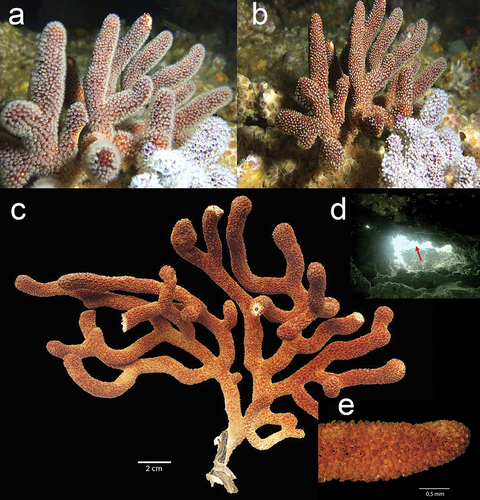
Figure 3. Underwater in situ record of Muricea echinata: (a) full colony with extended white polyps and close-up of the polyps at La Pared site. (b) Ex situ dry colony of M. echinata. (c) Close-up of the calyxes in a bifurcated branch. Exsitu Photos by D.C. Vergara and Juan A. Sánchez. Insitu Photos by Rubén Abad and Karla. B. Jaramillo.
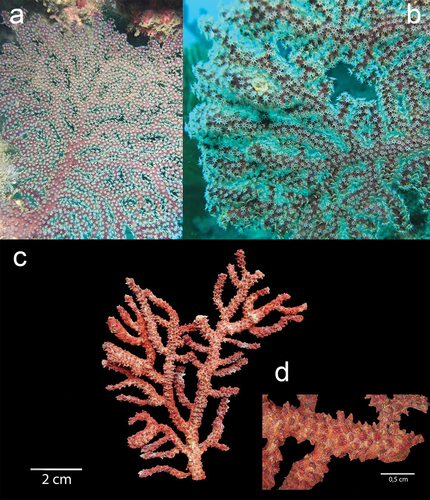
Figure 4. Underwater in situ record of Muricea robusta: (a) full colony with extended yellow polyps and close-up of the polyps in Bajo 40 site; and (b) with the polyps inside the colony. (c) Ex situ dry colony of M. robusta. (d) Close-up of the large orange calyxes in a branch. Exsitu Photos by D.C. Vergara and Juan A. Sánchez. Insitu Photos by Rubén Abad and Karla. B. Jaramillo.
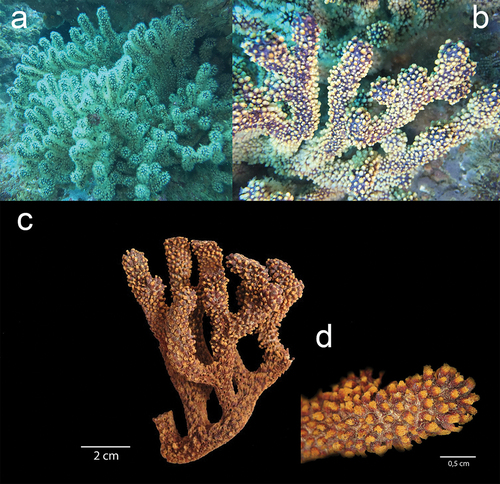
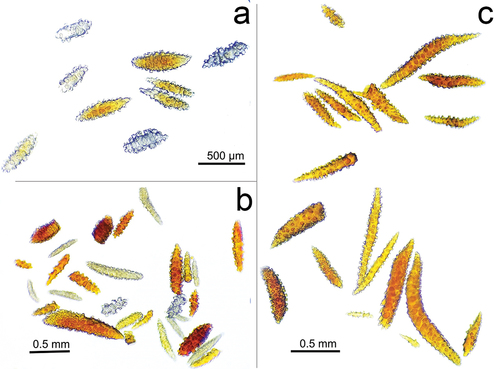
Figure 5. Sclerites of the three species (a) Muricea hebes, (b) Muricea robusta and (c) Muricea echinata collected at El Pelado Islet (REMAPE area). Sclerite Photos by Rubén Abad and Karla. B. Jaramillo.
Muricea hebes (Verrill, 1864)
The newly recorded specimens of M. hebes () were collected at 15 m depth inside a small crevice in the Bajo 40 mound. The in situ colony was dark reddish-orange with white polyps while the ex situ colony presented a yellowish-brown colour with a finger-like colony shape. The colony is 16 cm tall and 20 cm wide with an irregularly dichotomous branching pattern. Branches are flattened and 5–9 mm in diameter, and the unbranched terminal ends measure up to 4 cm. The coenenchyma tissue is thick and brownish in colour. Calyxes are arranged closely at branchlets and they are located all around the branches, up to 1.2 mm long and 1 mm wide. This species was previously described by Breedy as a yellowish-brown colony with a finger-like colony shape and an irregularly dichotomous branching pattern of 3.2 cm length of the unbranched terminal branchlet. The coenenchyma tissue is thick, 1–1.8 calyx height at branches, and there is a close and slightly imbricate calyx arrangement at branchlets. The calycular sclerites are white, whereas the coenenchymal sclerites are pale yellow calycular and coenenchymal (Table S1). Colour: pale yellow. Previously described as unilateral spinose spindles (uss) (Breedy and Guzman Citation2016). Muricea hebes was previously reported in Mexico, at Pájaros Island, Mazatlan Bay, Sinaloa; in Cabo Pulmo, Gulf of California; and in the Pearl Islands, Panamá (Breedy and Guzman Citation2016).
Muricea echinata (Verril, 1866)
The new record of M. echinata was collected () from La Pared at 15 m depth, embedded in coralline algae, on rocky substrates. The in situ colony was dark reddish to dark brown with white polyps ()), while the ex situ colony ()) presented a reddish-brown colour with a bushy colony shape. The colony is 10 cm tall and 8 cm wide, with an irregular lateral branching pattern extended in one plane. Branches are flattened, 4–6 mm in diameter, and the unbranched terminal ends measure up to 3 cm. The coenenchyma tissue is thin and reddish in colour. Calyxes are located all around the branches and they are prominent, up to 1.5 mm long and 1 mm wide ()). The arrangement at bracelets is close. The colonies were previously described by Breedy and Guzman (Citation2016) as having a colour that varies to a deeper orange hue and with an irregular branching pattern spreading in a single plane. Unbranched terminal ends reach up to 30 mm long. The colonies measure 8.5–10 cm tall and 7–14 cm wide. The branches are thinner at the base and a little ticker at the tips (Table S1). Calyxes are located all around the branches and they are prominent, up to 3 mm long and covered with large spindles with sharp ends. Coenenchyma is thin and composed basically of the calyx sclerites; they are orange and light brown, and the larger ones are darker (). This species was previously reported in Pájaros Island in Panamá.
Muricea robusta (Verril, 1864)
The M. robusta colonies were collected at Bajo 40 at 15 m depth. Colonies have yellow polyps with purple soft tissue around them ()), while the ex situ colony presented a brownish-orange colour and a bushy colony shape ()). The colony measures 11 cm tall and 5 cm wide with an irregular dichotomous branching pattern. Stems are slightly flattened, 11 mm in diameter and the unbranched terminal ends measure up to 7 cm. The coenenchyma tissue is thick and reddish-brown in colour. Calyxes are closely arranged at branchlets and are located all around the branches, up to 1 mm long and 0.8 mm wide. The calycular and coenenchymal sclerites are light orange and of a light type, with a mean size of 0.64 × 0.26 mm for calycular spindles and 0.15 × 0.05 mm for anthocodial sclerites ().
Discussion
This report contributes to increasing the knowledge of marine diversity in Ecuador, and broadens the previously recorded geographic distribution of the genus Muricea throughout the TEP (Breedy and Guzman Citation2016). Previously, the REMAPE area included eight reported Muricea species (Steiner et al. Citation2018), whereas we were able to collect 11 Muricea species, with different morphotypes of some species (i.e., orange Muricea plantaginea), as well as other gorgonian species (i.e. Pacifigorgia sp., Leptogorgia sp. and Heterogorgia sp.) with more than one morphotype per species (i.e., orange and white Leptogorgia). In the TEP three Muricea species were sampled, in The Gulf of California, Mexico, and Panama (M. hebes); in Mexico, Costa Rica and Panama (M. echinata); and in Mexico and Colombia (M. robusta) (Breedy and Guzman Citation2016), expanding Muricea’s tropical southern distribution to Ecuador (El Pelado Islet). Thus, our study has increased the known geographic distribution of the genus.
Furthermore, we contribute recent morphological descriptions of the newly recorded species. We present descriptions and photographs of in situ and ex situ colonies, facilitating future Muricea identification in situ for researchers performing scuba diving, and identification ex situ when researchers evaluate morphological differences using museum specimens. Moreover, sclerite descriptions were registered due to their phenotypic relevance; sclerites and spicules are known to be important morphological traits for octocoral identification.
Finally, we recommend an ongoing census of rocky coastal ecosystems, together with deeper explorations in mesophotic environments, to continue identifying and registering new gorgonian records, as well as recurrent invasive species such as the octocoral Carijoa riisei. We highlight the remarkable importance of tracking and recording the gorgonian marine diversity of shallow and mesophotic environments in tropical regions.
Temporal coverage
Notes
The field trips were conducted from 17 to 19 October 2018. The data set was created in October 2018. Some specimens could not be identified to the species level, and these samples will be analysed in the future for further genetic publications.
Usage rights
We suggest Creative Commons BY license if possible.
Data resources
Data package title
List of 25 species collected, 11 species belonging to Muricea, five species of Pacifigorgia, five species of Leptogorgia, two species of Heterogorgia, Psammogorgia arbuscula and the invasive species Carijoa riisei, collected at El Pelado Islet (REMAPE area).
Number of data sets
One.
Data set name
Checklist of octocorals in the Equatorial Front. doi:10.6084/m9.figshare.15183828.
Data format version
1.0.
Description
The data presented here correspond to the occurrences of deep-sea octocorals from the Equatorial Front and are based on identifying the collected specimens to the species level where possible.
Authors’ contribution
DCV-F and JAS contributed to the conceptualisation of the study. DCV-F, RA and ASS contributed to the methodology and sampling. DCV-F, RA and KJ conducted morphological analyses. DCV-F and RA wrote the original draft. KJ and ASS contributed to writing, editing and reviewing the manuscript. JAS, JR and DCV-F helped in the acquisition of funding. JAS and JR supervised the study. All authors read and approved the final manuscript.
TNAH-OA_21-200-File007.docx
Download MS Word (19.1 KB)Acknowledgements
We thank the members of CENAIM-ESPOL, Ecuador, for their valuable guidance and help during the field trip; and the BIOMMAR laboratory members for their support and useful comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/00222933.2022.2063081.
Additional information
Funding
References
- Breedy O, Guzmán HM. 2003. Octocorals from Costa Rica: the genus Pacifigorgia (Coelenterata: Octocorallia: Gorgoniidae). Zootaxa. 281:1–60.
- Breedy O, Guzman H. 2016. A revision of the genus Muricea Lamouroux, 1821 (Anthozoa, Octocorallia) in the eastern Pacific. Part II. ZooKeys. 581:1–69. doi:10.3897/zookeys.581.7910
- Fabricius KE, Benayahu Y, Genin A. 1995. Herbivory in asymbiotic soft corals. Science. 268(5207):90–92. doi:10.1126/science.268.5207.90
- Fabricius K, Klumpp D. 1995. Widespread mixotrophy in reef-inhabiting soft corals:the influence of depth, and colony expansion and contraction on photosynthesis. Mar Ecol Prog Ser. 125:195–204. doi:10.3354/meps125195
- Fiedler PC, Lavín MF. 2017. Oceanographic conditions of the Eastern Tropical Pacific. In: Glynn PW, Manzello DP, Enochs IC, editors. Coral reefs of the Eastern Tropical Pacific: persistence and loss in a dynamic environment. Springer Netherlands; p. 59–83. doi:10.1007/978-94-017-7499-4_3
- LaJeunesse TC, Parkinson JE, Gabrielson PW, Jeong HJ, Reimer JD, Voolstra CR, Santos SR. 2018. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr Biol. 28:2570–2580. doi:10.1016/j.cub.2018.07.008
- Lau YW, Poliseno A, Kushida Y, Quéré G, Reimer J. 2019. The classification, diversity and ecology of shallow water octocorals. doi:10.1016/B978-0-12-409548-9.12109-8
- Lewis JB. 1982. Feeding behaviour and feeding ecology of the Octocorallia (Coelenterata: Anthozoa). J Zool. 196(3):371–384. doi:10.1111/j.1469-7998.1982.tb03509.x
- Marques ACSJ, Castro CB. 1995. Muricea (Cnidaria, Octocorallia) from Brazil, with description of a new species. Bull Mar Sci. 56(1):161–172.
- Oppen MJHV, Mieog JC, Sánchez CA, Fabricius KE. 2005. Diversity of algal endosymbionts (Zooxanthellae) in octocorals: the roles of geography and host relationships. Mol Ecol. 14(8):2403–2417. doi:10.1111/j.1365-294X.2005.02545.x
- Sánchez JA. 2016. Diversity and Evolution of Octocoral Animal Forests at Both Sides of Tropical America. In: Rossi S., Bramanti L., Gori A., Orejas Saco del Valle C., editors. Marine Animal Forests. Springer, Cham. 111–143. doi:10.1007/978-3-319-17001-5_39-1
- Sánchez JA, Dueñas LF, Rowley SJ, Gonzalez-Zapata FL, Vergara DC, Montaño-Salazar SM, Calixto-Botía I, Gómez CE, Abeytia R, Colin PL, et al. 2019. Gorgonian corals. In: Loya EY, Puglise KA, Bridge TCL, editors. Mesophotic coral ecosystems. Springer International Publishing; p. 729–747. doi:10.1007/978-3-319-92735-0_39
- Sánchez JA. 2016. Diversity and evolution of octocoral animal forests at both sides of tropical America. In: Rossi S, Bramanti L, Gori A, Del Valle COS, editors. Marine animal forests. Springer International Publishing; p. 1–33. doi:10.1007/978-3-319-17001-5_39-1
- Steiner SCC, Riegl B, Lavorato A, Rodríguez J. 2018. Community structure of shallow water Alcyonacea (Anthozoa: Octocorallia) from the southern Tropical Eastern Pacific. Ecol Res. 33(2):457–469. doi:10.1007/s11284-018-1567-3
