ABSTRACT
The large floral chambers of the Neotropical Annonaceae are pollinated by large beetles of the tribe Cyclocephalini Laporte (Melolonthidae). Previous research indicates that, depending on the geographic area, the assemblage of Cyclocephalini pollinators may differ. In this study, pollinators of Annona crassiflora Mart. were surveyed in the Cerrado of Chapada dos Guimarães, Mato Grosso, Brazil, for the first time. The analysis of 74 flowers from 24 A. crassiflora individuals revealed that the principal pollinator was Cyclocephala octopunctata Burmeister. The secondary pollinator, Cyclocephala celata Dechambre, and C. octopunctata exhibited behaviour that may promote cross pollination, visiting both female- and male-phase flowers, touching stigmas and anthers and becoming coated in pollen during anthesis. This is the first record of C. celata, a species commonly found pollinating Araceae in the Atlantic Forest, as a pollinator of an Annonaceae species in the Cerrado. Additionally, an interim phase between the female and male phases of A. crassiflora is documented here for the first time.
Introduction
The economic relevance of Coleoptera is mostly attributed to the role they play as pests in crops and stored products (Capinera Citation2001; Casari and Ide Citation2012). The last several decades have seen an increase in the number of studies concerning the role of beetles as pollinators in several plant families, many of which are economically important (Kono and Tobe Citation2007; Gottsberger Citation2012; Kishore et al. Citation2012; Meléndez and Ponce Citation2016; Lara et al. Citation2017).
Many herbivorous scarabs from the tribe Cyclocephalini, which includes nearly 500 described species, are important agricultural pests, especially in their immature stages, during which they feed on the living roots of crop plants such as soybean, sugarcane and pasture species (Casari et al. Citation1988; Santos and Ávila Citation2007; Coutinho et al. Citation2011; Nogueira et al. Citation2013; Souza et al. Citation2013, Citation2014; Albuquerque et al. Citation2014; Moore et al. Citation2018; Rodrigues et al. Citation2018). In contrast, adult Cyclocephalini rarely act as pests of agroecosystems, and they play a major role in the pollination of nearly 900 species of Neotropical plants (Moore et al. Citation2018).
Cyclocephalini are known to pollinate basal angiosperms and monocotyledons from plant families such as Magnoliaceae, Nymphaeaceae, Annonaceae and Araceae (Dieringer and Spinosa Citation1994; Dieringer et al. Citation1999; Ervik and Knudsen Citation2003; Gottsberger Citation2012; Saravy et al. Citation2021a; Torezan-Silingardi et al. Citation2021). The species-rich plant family Annonaceae is mainly pollinated by beetles of the families Nitidulidae, Staphylinidae, Curculionidae and Scarabaeidae, although there are some records of Chrysomelidae (Andrade et al. Citation1996; Ratnayake et al. Citation2007; Gottsberger Citation2012; Aragão et al. Citation2019).
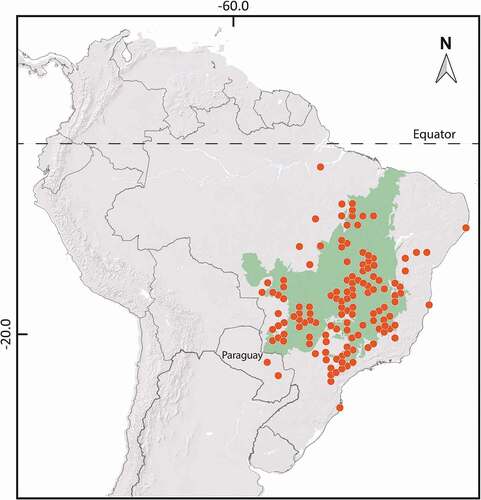
Current knowledge of the presence of Annonaceae in the Cerrado indicates the occurrence of 75 species (Mendonça et al. Citation2008). Annona crassiflora Mart., which is popularly known as araticum-do-cerrado or marolo, occurs mainly in Cerradão and Cerrado sensu stricto regions, from Ceará in the north to São Paulo in the south (Silva-Júnior et al. Citation2005; Lorenzi Citation2009). shows a distribution map for this species. Although its fruits are greatly appreciated by traditional populations of central Brazil, the high economic potential of A. crassiflora remains largely unexplored (Lorenzi Citation2009; Rezende and Malafaia Citation2012). Data on the pharmacological potential of its different plant parts is scant. However, studies have shown a high nutritious content of the fruit pulp and potential for the extraction of antioxidant compounds from the fruit peel (Cardoso et al. Citation2013). Leaf extracts of A. crassiflora have shown antioxidant activity when included in a phytocosmetic gel and in vitro anthelmintic activity (Costa Citation2017; Caldeira et al. Citation2019).
Figure 1. Global occurrence map of Annona crassiflora (araticum). Adapted from: Global Biodiversity Information Facility (GBIF Citation2021).
Gottsberger and Silberbauer-Gottsberger (Citation2006) mentioned three species of Cyclocephalini as pollinators of A. crassiflora in the Brazilian Cerrados of the states of Minas Gerais, São Paulo, Goiás and Distrito Federal: they are Cyclocephala atricapilla Mannerheim, Cyclocephala quatuordecimpunctata Mannerheim and Cyclocephala literata Mannerheim, the latter of which was found only once as a flower visitor. In a Cerrado area in the state of Goiás, Cavalcante et al. (Citation2009) found three Cyclocephala species visiting flower chambers of A. crassiflora: C. atricapilla, Cyclocephala latericia Hohne and Cyclocephala octopunctata. These are relatively large, nocturnal beetles often found in the large, thermogenic flower chambers of A. crassiflora (Gottsberger, Citation1989a, Citation1989b, Gottsberger Citation1994; Gottsberger and Silberbauer-Gottsberger Citation2006). Oliveira and Gibbs (Citation2000) cited Cyclocephala cf. atricapilla as visitors of araticum flowers in a Cerrado area of the Brasília Botanic Garden (Distrito Federal). Two species of Cyclocephalini are constant pollinators throughout the geographic range of A. crassiflora: C. atricapilla and C. quatuordecimpunctata. However, other Cyclocephala species are occasional pollinators in different geographic sites.
Given that the pollinator assemblage of A. crassiflora varies according to the geographic location despite how specialised its pollination syndrome is, this study aimed to (1) determine the floral visitors of A. crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, Mato Grosso, Brazil, and (2) identify their behaviour to determine whether it is likely to promote pollination.
Material and methods
Study area
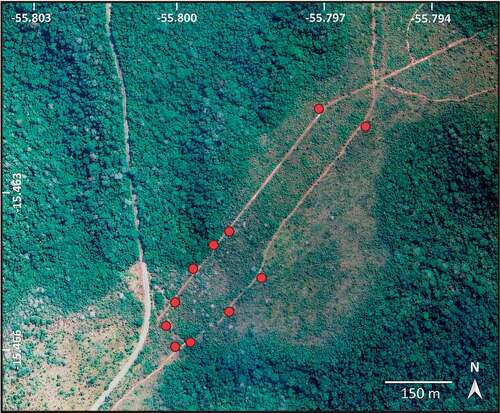
The study was carried out in two flowering seasons of A. crassiflora: during 2019, over 13 days in the months of September and October; and during the flowering event of 2020, over eight days between October and November. Both sampling periods were during the beginning of the rainy period. The study area in the Chapada dos Guimarães measured nearly 7 ha (15.27ʹS, 55.47ʹW). According to the key of Cerrado vegetation physiognomies of Ribeiro and Walter (Citation2008), the area in which the individuals of A. crassiflora were sampled was characterised as ‘Cerrado Denso’ and was bordered in the north-west by a patch of ‘Mata de Galeria Não-Inundável’ and in the south-east by a strip of ‘Mata Seca’ (). The climate in the region is type Aw (tropical with dry winter), according to the Köppen classification (Alvares et al. Citation2013). The mean annual temperature is 21.5°C, and the mean annual rainfall may reach 2100 mm at higher elevations (Vieira-Júnior et al. Citation2012). The individuals of A. crassiflora (n = 24) selected for this study were located at elevations of 774 to 822 m.
Sampling of flower chamber visitors
Twenty-four blossoming individuals of A. crassiflora were randomly selected in the study area. Observations and the collection of flowers and their visitors were accomplished at the time of day in which the flowers were anthetic (i.e. from dusk until just before dawn). Flowers were considered in the female phase when the stigmatic head was covered with stigmatic exudate and in the male phase when anthers were open and detached from the receptacle. The external petals of A. crassiflora flowers delimit a semi-open flower chamber. The handling of such chambers scares off their flower visitors. Hence, flowers were hand-picked and promptly photographed. Immediately afterwards, the flowers were individually placed in labelled vials with 90% alcohol to prevent visitors’ escape. The sampled flowers were taken to the Laboratório de Ecologia e Taxonomia de Artrópodes (LETA) of the Instituto de Biociências in the Universidade Federal de Mato Grosso (UFMT). In total, the contents of the flower chambers of 74 flowers were analysed. Specimens are deposited in the Collection of Arthropods of LETA and Setor de Entomologia da Coleção Zoológica da UFMT.
The behavioural characteristics of visiting insects were considered appropriate for pollination when they (1) were found inside the floral chambers, (2) were found inside the floral chambers in either the female phase or the male phase, which favours cross pollination and/or (3) pollen adhered to the flower visitors’ bodies.
Thermogenesis measurements
During nine days in September and October 2019, the floral thermogenesis of A. crassiflora flowers was assessed on 71 flowers from 14 individuals. The temperature was taken by inserting a digital thermal sensor device (GTH 175 PT, Germany) in the floral chamber until it touched the base of the petals’ inner surface. The obtained value was then subtracted from the ambient temperature to calculate thermogenesis in degrees Celsius.
Imaging procedures
Digital photographic field records were obtained with a Sony NEX-F3 camera. Photographs of the material examined in the laboratory were obtained with a Leica CH-94335 microscope camera attached to a Leica L2 stereomicroscope (Leica Microsystems) and were captured with Leica Application Suite v. 3.8 software. When necessary to highlight particular aspects of the plant structures and/or their visitors, images were obtained via multifocal sequences, stacked and grouped with Adobe Photoshop 2020 v. 21.0.2 software.
Taxonomic analysis
Non-coleopteran floral visitors of the subphylum Hexapoda were identified at the order level (with the exception of Hymenoptera, belonging to Formicidae, and Hemiptera, belonging to Aphidae). Flower visitors of the order Coleoptera were identified to the lowest taxonomic level possible. Photographs were sent to taxonomists of Scarabaeoideae (sensu Cherman and Morón Citation2014). The classification in higher taxonomic categories of Coleoptera follows Bouchard et al. (Citation2011). The familial and subfamilial classification of scarabs followed Cherman and Morón Citation2014. The species-level classification of Scarabaeidae followed Endrödi (Citation1985). The classification of Curculionidae at the supra-species level was in accordance with Alonso-Zarazaga and Lyal (Citation1999).
Results
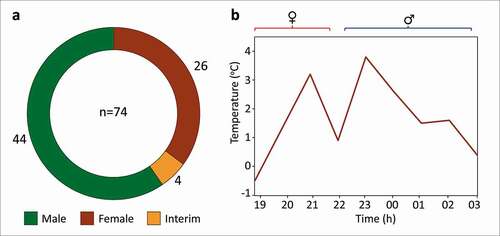
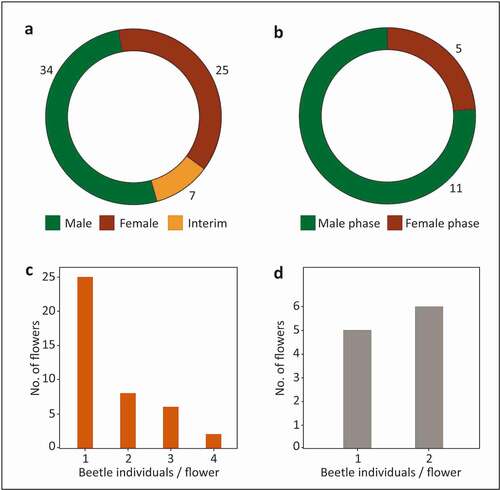
Flower buds of Annona crassiflora may take up to four months to fully develop (). On the day of anthesis, the pendant flower chambers open up during the day, and the colour of the external petals changes from a greener tone to a more ferruginous hue (). However, anthesis only starts during the evening, when the flowers (n = 26, ) enter the female phase (7.30pm–11.00pm), which is perceived based on the presence of a sticky, transparent, glossy stigmatic exudate covering the entire surface of the gynoecium ().
Figure 3. Some steps in the floral cycle of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) Developing flower buds photographed in early August, almost two months before the flowering season of A. crassiflora. (b) Open flower chamber photographed in the morning before the onset of anthesis. (c) Flower entering the female phase, photographed from below during crepuscule. Note the sticky, transparent, glossy substance on the gynoecium (the stigmatic exudate; arrow). Petals were spread open to show internal structures.

There appears to be a brief interim phase in anthesis during which flowers (n = 4, ) are neither in the female phase nor in the male phase. Depending on the tree, this phase may occur between approximately 10.00pm and 11.00pm. This stage is perceivable when the stigmatic head is either darkened and/or detached from the receptacle but anthers are not yet open (). Flowers in the male phase were found from 8.45pm to 3.50am (n = 44, ). The beginning of the male phase is marked by the opening of anthers and their detachment from the receptacle (). After 3 h, corollas start dropping as a whole unit, marking the end of anthesis.
Figure 4. Some steps in the floral cycle of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) Interim in the floral cycle. Note that the stigmatic head is detached from the receptacle (arrow). Petals were spread open to show the flower chamber interior. (b) Flower found on the ground in the morning close to anthesis, showing the aspect of the flower during the male phase, with detached stamens filling the floral chamber.

Figure 5. Aspects of floral biology of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) Number of sampled flowers (n = 74) according to the floral phase. Note the greater number of male flowers collected in the field relative to the female-phase flowers sampled. (b) Floral thermogenesis during one floral cycle. Note that there are two heat production peaks during the night, one at approximately 7.00pm, when the flowers are in the female phase, and another at approximately 11.00pm, when they are in the male phase. During the interim phase, heat production diminishes without ceasing altogether.
In the course of anthesis, flowers give off a sweet, fruity scent with a faint background of acetone. This smell is enhanced by floral thermogenesis, which starts during the female phase and intensifies during the male phase. During the interim, however, between the female and male phases, floral heat production decreases. After 3 h, corollas start dropping as a whole unit, marking the end of anthesis ().
As shown in and , among the 119 visitors collected from the anthetic flowers of A. crassiflora, 74 belonged to the order Coleoptera. The remainder, as demonstrated in , were insect members of the orders Thysanoptera (n = 12), Diptera (n = 8, all in larval stages), Hymenoptera (n = 4, all from the family Formicidae) and Hemiptera (n = 3). The individuals found in the flowers of A. crassiflora came from only two families of Coleoptera (): Melolonthidae (n = 82 individuals) and Curculionidae (n = 10 individuals). From the former taxon, only two species were collected, Cyclocephala octopunctata (n = 66; ) and Cyclocephala celata Dechambre (n = 16; ), and all of these specimens were found inside the floral chambers during anthesis (; ).
Table 1. List of floral visitors of 74 anthetic Annona crassiflora flowers in a Cerrado area in the municipality of Chapada dos Guimarães, MT. Ni = total number of individuals in each morphospecies found on the flowers. Nf (%) = percentage of flowers in which each listed morphospecies was found. Ni/f = average number of individuals of each morphospecies per flower. PFC (%) = percentage of each morphospecies individual found inside floral chambers. All other specimens were found in the alcohol samples.
Figure 6. Quantitative data on the visitation of Annona crassiflora flowers in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) Number of coleopteran and non-coleopteran visitors of anthetic flowers. (b) Number of insect flower visitors sorted by order. (c) Number of beetles visiting anthetic flowers, classified at the family level. (d) Number of beetles visiting anthetic flowers classified at the species and morphospecies levels.
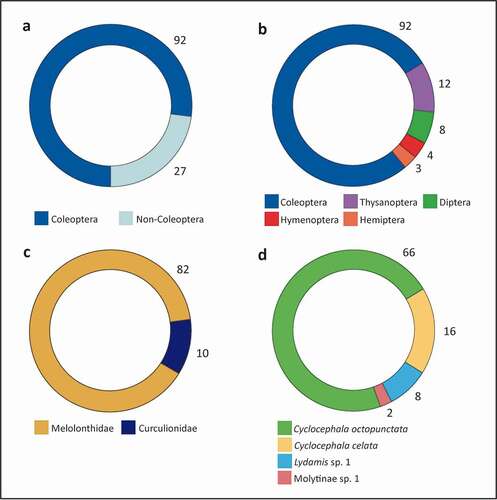
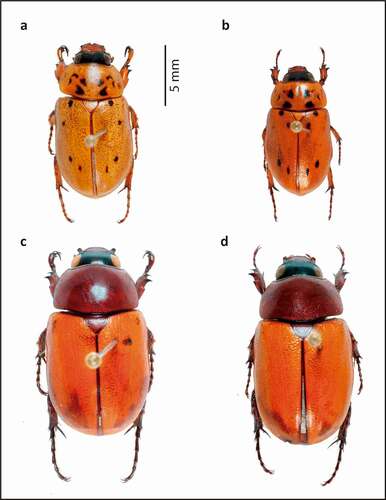
Figure 7. Pollinators of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) Dorsal habitus of Cyclocephala octopunctata (male). (b) Dorsal habitus of Cyclocephala octopunctata (female). (c) Dorsal habitus of Cyclocephala celata (male). (d) Dorsal habitus of Cyclocephala celata (female).
The three most significant taxa collected on the flowers of A. crassiflora were C. octopunctata, C. celata and Lydamis sp. 1 Pascoe. All individuals of both Cyclocephala species were found within the floral chambers (). However, it was unclear whether, at the time of flower collection in the field, the small individuals of Lydamis sp. 1 were located inside the flower chambers. These specimens were unknowingly collected during the nocturnal field work. The Lydamis sp. 1 specimens were identified only in the laboratory, when the alcohol containing the samples was observed under a stereomicroscope (). Additionally, the two identified specimens of Molytinae sp. 1 (Curculionidae) were found outside the floral chambers, feeding on the adaxial surface of the outer petals.
The two most abundant species found in the flower chambers of A. crassiflora, C. octopunctata and C. celata, exhibited very similar behaviour during visitation. They were present in both female- and male-phase flowers (), fed on the basal lobes of the inner petals, and touched the androecium as well as the gynoecium with their large bodies (). In some cases, more than one individual was present in the same floral chamber (). Individuals of both Cyclocephala species, whose activity was observed only during the night, when the flowers of A. crassiflora are anthetic, were seen moving about inside the floral chambers with their elytra covered in pollen (). Under the stereomicroscope, tetrads of conspecific pollen were found to be adhered not only to the elytra of Cyclocephala but also to their conspicuous ventral setae. The sticky stigmatic exudate produced during the female phase of the flowers can mediate the adherence of pollen tetrads to the smooth surfaces of the beetles’ bodies ().
Figure 8. Cyclocephala octopunctata visiting flowers of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) A male individual found inside the floral chamber during the female phase. Note the darkening gynoecium, characteristic of an advanced female phase. (b) A specimen covered in pollen found in a male-phase flower. (c) Two individuals inside a female-phase flower. Note that their heads are directed towards the base of the inner petals, where basal alimentary lobes are located. (d) One individual feeding on one basal lobe of an inner petal in a female-phase flower.
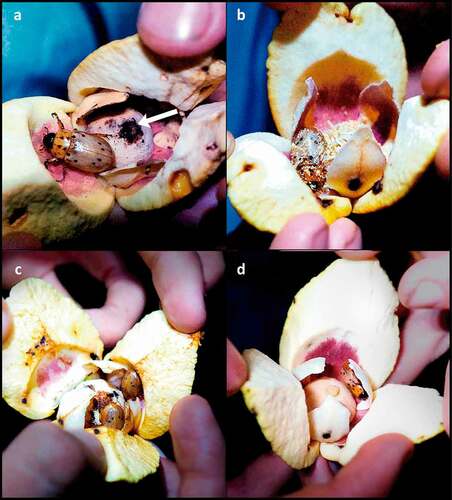
Figure 9. Cyclocephala celata visiting flowers of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) Here, the elytra (arrow) of one individual of C. celata are visible inside a female-phase flower. (b) Individuals of C. celata covered in pollen exiting a male-phase flower. (c) Two individuals of C. celata covered in pollen inside a recently fallen corolla that was picked from the ground beneath an A. crassiflora tree. Note that their heads are directed towards the base of the petals, where the nutritious basal lobes are located. (d) This individual of C. celata had just escaped from inside a corolla that was found on the ground under an A. crassiflora individual on the day following flower anthesis.
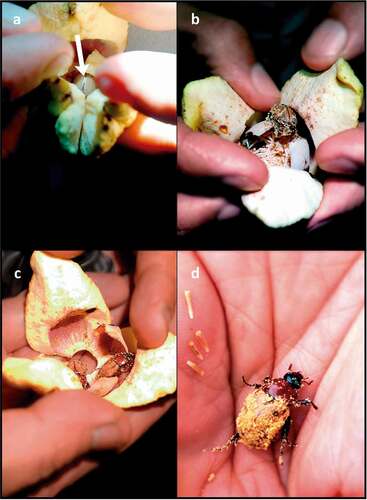
Figure 10. Quantitative data on the visitation of Annona crassiflora flowers by both Cyclocephala species sampled in this study. (a) Number of individuals of Cyclocephala octopunctata (n = 66) classified according to the anthesis phase of the flowers in which they were found. (b) Number of individuals of Cyclocephala celata (n = 16) classified according to the anthesis phase of the flowers in which they were found. (c) Distribution of the number of individuals of C. octopunctata per sampled flower (n = 41 flowers). (d) Distribution of the number of individuals of C. celata per sampled flower (n = 11 flowers).
At the end of anthesis, the thick gamopetalous corolla drops as a whole unit, which provides Cyclocephala individuals with shelter from desiccation and predators throughout the day after anthesis (, ). Two post-anthetic flowers were found on the ground beneath the crowns of two A. crassiflora trees at approximately 10.00am, each associated with an individual of C. celata covered with pollen. A third post-anthetic flower was collected in the afternoon after anthesis and was surprisingly still attached to the pedicel; this flower contained two individuals of C. octopunctata, covered with pollen ().
Figure 11. Individuals of Cyclocephalini found in post-anthetic flowers of Annona crassiflora in a Cerrado area in the municipality of Chapada dos Guimarães, MT. (a) A specimen of Cyclocephala celata from a flower found on the ground under the tree crown in the morning after anthesis. Note the pollen tetrads adhered to the tibial and tarsal setae of the right mesothoracic leg. (b) Individuals of C. octopunctata collected in the morning after anthesis from a flower still attached to the pedicel.
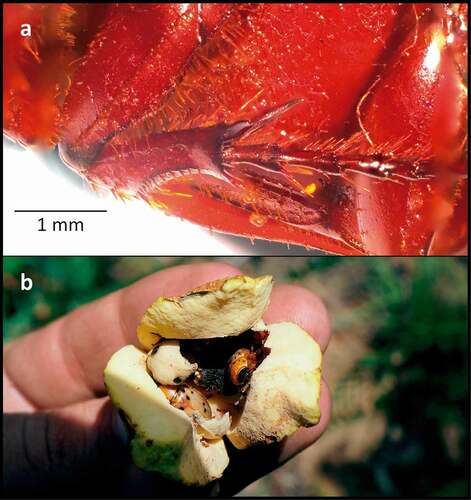
The relatively ample floral chambers allow Cyclocephalini beetles to encounter conspecifics foraging in the same flower (; ). Up to four specimens of C. octopunctata were found inside single floral chambers of A. crassiflora (). More than one individual of C. celata was found inside six floral chambers (). We concluded that within the study area, both sampled species of Cyclocephala (C. octopunctata and C. celata) are pollinators of A. crassiflora.
Discussion
Protogyny is a common condition of plant species pollinated by beetles, regardless of the pollinators’ circadian rhythms. Cantharophilous flowers and inflorescences whose anthesis is diurnal show protogynous flower cycles, not only in Annonaceae species but also in palms (Oliveira et al. Citation2003; Gottsberger Citation2012; Paulino-Neto Citation2014a). Protogyny in nocturnal blossoms, such as A. crassiflora, has been described in water lilies, aroids and Annonaceae, especially within the genus Annona (Ervik and Knudsen, Citation2003; Silberbauer-Gottsberger et al. Citation2003; Paulino-Neto Citation2014a, 2014b; Costa et al. Citation2017).
During the female phase, the stigmatic exudate () of Annonaceae seems to play a threefold role in pollination: it serves as an alimentary resource for beetles and a pollen germination medium, and facilitates pollen adhesion to pollinators’ bodies (Lau et al. Citation2017b). For example, although the indumentum of C. octopunctata, the principal pollinator of A. crassiflora in our study area, is less dense than that of C. celata, pollen adheres to the smooth surfaces of the former, probably with the aid of the sticky stigmatic exudate (, , ). Further tests need to be carried out to evaluate the two other possible roles that the stigmatic exudate may play in the pollination of A. crassiflora: as an alimentary resource for beetles and as a pollen germination medium.
During the male phase, however, Annonaceae flowers offer pollen, which is produced in massive quantities, as an alimentary reward for beetles (Gottsberger Citation2012). In a Cerrado area in the municipality of Marilândia, in the state of Mato Grosso, Costa et al. (Citation2017) observed Cyclocephala species feeding on stamens still attached to the receptacle, providing evidence of pollinivory. In the field, during our nocturnal observations, when insect-visited male flowers were removed from the trees, beetles were found immersed in the pollen and stamens, such that even the accidental ingestion of pollen could not be prevented. However, to our knowledge there has been no reported gut content analysis of Cyclocephala that visit Annona spp. flowers searching for pollen traces, and the present study did not undertake such an endeavour. Gut content analyses need to be accomplished to confirm pollen ingestion by the Cyclocephalini that visit A. crassiflora in our study areas.
Some rewards are present during the flower cycle (and even afterwards): for instance, the flower chamber offers the Cyclocephalini beetles shelter from desiccation and predators and a mating site, even after the flower drops, which was verified in the field by the observation of both C. octopunctata and C. celata inside post-anthetic floral chambers collected from the ground (, ). The relatively large size of the floral chamber also allows conspecific beetles to aggregate and mate, which was verified by the numerical distribution of individuals per flower chamber (). Furthermore, the basal lobes on the abaxial surface of the inner petals provide constant alimentary rewards for Cyclocephalini during and even after the floral cycle. Gottsberger and Webber (Citation2018) verified that these nutritious tissues are rich in starch and lipids in five species of Amazonian Annonaceae from four genera, all of which are pollinated by Cyclocephalini. Nutritious tissue at the base of the inner petals in Annonaceae has also been reported in species pollinated by small, diurnal beetles (Olesen Citation1992).
The role of thermogenesis in cantharophilous blossoms has been the subject of controversial debate regarding whether it functions solely as a means of attraction or also provides resources (Meeuse and Raskin Citation1988; Seymour and Schultze-Motel Citation1997; Seymour and Shultze-Motel Citation1998; Dieringer et al. Citation1999; Seymour et al. Citation2003, Citation2009; Rands and Whitney Citation2008). There has been a consensus in the literature regarding the concept of floral heat production as a means of attracting pollinators, which suggests that heat serves to spread odour over longer distances, especially during the night (Gottsberger Citation1990; Seymour and Schultze-Motel Citation1997; Küchmeister et al. Citation1998; Dieringer et al. Citation1999). There is even a hypothesis that the thermogenicity of one Annonaceae species, Xylopia championii Hook. f. and Thomson, may be perceived by infrared (IR) organs of beetles, but this remains strictly conjectural, since such organs have not been confirmed in the taxa of this species’ pollinators (Ratnayake et al. Citation2007). Dieringer et al. (Citation1999) concluded that the heat produced by the protogynous flowers of Magnolia tamaulipana A. Vázquez (Magnoliaceae) did not affect the endothermy cost of its Cyclocephalini pollinator. In contrast, Seymour et al. (Citation2009) discovered that the scarabs that pollinated Philodendron solimoesense A.C. Sm. (Araceae) did not exhibit thermoregulation inside the floral chambers, thus avoiding the costs of endothermy.
It becomes quite obvious when considering blossoms pollinated by nocturnal scarabs across different plant families that there is remarkable evolutionary convergence towards thermogenicity in phylogenetically distant taxa. Such convergence might have occurred due to the twofold roles that thermogenesis may play as both an odour intensifier and an energetic reward for pollinators (Bernhardt Citation2000; Seymour et al. Citation2003, Citation2009).
In the present study, thermogenicity in A. crassiflora was shown to be intermittent. There were two peaks of heat production: one during the female phase and another, slightly longer and more intense, peak during the male phase, between 11.00pm and 12.00am (). These results are not entirely consistent with those of Gottsberger (Citation1989a), who found that the heat production peak of A. crassiflora flowers occurred during the female phase between 7.00pm and 8.00pm. This difference may be due to the climatic conditions of the region of the study, in the municipality of Botucatu, in the state of São Paulo. The author pointed out that thermogenesis in A. crassiflora seems to be temperature-dependent and that the conditions under which the flowers reach higher temperatures are more likely to occur in northern regions of the Cerrado, such as our study area. The heat production peak in the male-phase flowers of A. crassiflora in the Cerrado of Chapada dos Guimarães may therefore be explained by the lower decline in ambient temperature during late night relative to the cooler Cerrados in the state of São Paulo.
Thermogenesis is a common feature of cantharophilous blossoms and also occurs in other Annonaceae genera. Küchmeister et al. (Citation1998) observed thermogenesis events in both female- and male-phase flowers of Amazonian Anaxagorea spp., the most basal genus within Annonaceae. The three Anaxagorea species analysed in their study show diurnal anthesis and are pollinated by small Staphylinidae and Nitidulidae. In the same study, these authors also detected thermogenesis in two Duguetia (Annonaceae) species pollinated by Cyclocephalini. These species present nocturnal anthesis, in accordance with their pollinators’ active period, in a two-day flowering cycle, with a long interim between the two nights of anthesis and heat production events in both male and female flowers (Küchmeister et al. Citation1998).
Regarding the dichogamy observed in A. crassiflora in the Cerrado of Chapada dos Guimarães, more male-phase flowers were collected, which reflects the fact that the male phase is longer rather than sampling effort bias (). The short interim phase was accordingly the least sampled in the field (). Unlike the present study, Gottsberger (Citation1989a, Citation1989Citationb) and Silberbauer-Gottsberger and Gottsberger (Citation2006) did not detect an interim phase in A. crassiflora flowers. Long interim phases of approximately one day were found in Annona spp. with a two-day flowering rhythm in the Cerrado, such as Annona coriacea Mart. and Annona cornifolia A. St.-Hil. (Gottsberger Citation1989a; Silberbauer-Gottsberger and Gottsberger, Citation2006; Costa et al. Citation2017), but this was not observed in A. crassiflora. The present study is the first to describe an interim phase in A. crassiflora, which highlights the role that dichogamy plays in avoiding self-pollination.
Sweet, fruity smells, such as those given off by A. crassiflora during anthesis, have been reported in other Annona species pollinated by Cyclocephalini, such as Annona tomentosa R.E. Fr., Annona aurantiaca Barb. Rodr., A. coriacea and A. cornifolia, all of which are found in the Cerrado (Gottsberger Citation1989a, Citation1989b; Gottsberger and Silberbauer-Gottsberger Citation2006; Mendonça et al. Citation2008). Similar smells have been described in Annona species pollinated by small beetles, such as Annona squamosa L., a very economically important species pollinated by Nitidulidae (Gottsberger Citation1989a; Kishore et al. Citation2012), and Amazonian species Annona glabra L., pollinated by small Chrysomelidae (Gottsberger, Citation1999).
Curiously, although Gottsberger (Citation1989a) described the floral scent of A. crassiflora as ‘disagreeable’, Gottsberger and Silberbauer-Gottsberger (Citation2006) mentioned further nuances acquired by the fruity bouquet when floral temperature rises, describing it as predominantly ‘unpleasant and cyanide-like’. The perception recorded in the present study was that the background smell that intensifies during thermogenesis is reminiscent of acetone. These discrepancies are the result of the subjectivity of scent perception by researchers, indicating the need to carry out scent extraction and chemical analyses of blossoms to assess their sensorial effects on pollinators and other evolutionary implications.
Plant evolutionary convergence towards the production of fruity floral scents in Cyclocephalini-pollinated angiosperms is worth noting when the floral bouquet of unrelated genera of Annonaceae and other angiosperm families is taken into account. Cantharophilous Magnolia species tend to emit fruity scents, as observed in Magnolia schiedeana Schltdl. and Magnolia ovata (St.- Hil.) Spreng., whose scent has been described by Gottsberger et al. (Citation2012) as ‘fruit-like, reminiscent of apples and/or cherimoya (Annona cherimola Mill.) and melon fruits’ (Gottsberger Citation1989a, Citation1989Citationb; Dieringer and Espinosa Citation1994; Gottsberger et al. Citation2012). Regarding the role of the background acetone or ‘cyanide-like’ scent, the question arises of whether it plays the role of a sexual pheromone, as suggested by Schatz (Citation1990) when reviewing aroid scent emissions, or deters visitation by less efficient or pillaging visitors, an evolutionary mechanism envisioned in a review by Schiestl and Johnson (Citation2013).
After scent emission draws pollinators close to flowers, visual cues seem to be the ultimate attractant to plant reproductive parts (Pellmyr and Patt Citation1986; Ervik et al. Citation1999). Similar to most blossoms pollinated by nocturnal beetles, A. crassiflora flowers possess pale corollas, with light yellow inner and outer petals (Pellmyr and Patt Citation1986; Bernhardt Citation2000; Gottsberger and Silberbauer-Gottsberger Citation2006; Gottsberger et al. Citation2012). In distinct contrast, the outer petals bear semicircular purple spots on their basal half (). The pigment from these spots was immediately solubilised when petals were placed in 90% alcohol. Similar spots have been found in floral chambers from Annonaceae visited not only by nocturnal Cyclocephalini but also by diurnal beetles such as A. squamosa, Anaxagorea dolichocarpa Sprague and Sandwith, and Xylopia aromatica (Lam.) Mart. (Bernhardt Citation2000; see fig. 3a in Kishore et al. Citation2012 for A. squamosa; fig. 1b in Gottsberger Citation2012 for A. dolichocarpa; fig. 5d in Aragão et al. Citation2019 for X. aromatica, and fig. 3 in Saravy et al. Citation2021b). Given the scarcity of knowledge on the interaction between these visual floral signals and the coleopteran sensorial system, one can only speculate about the effect this arrangement (reddish-purple-over-yellow in A. crassiflora flowers) has on the perception of beetles.
Unlike the other two Annonaceae species present in the study area, Cardiopetalum calophyllum Schltdl. and Xylopia aromatica (Lam.) Mart., in which dozens of anthetic flowers occur on each individual every day, relatively few flowers of A. crassiflora blossom each day on each tree, rendering the sampling task a more reasonable endeavour. The flower chambers of A. crassiflora are semi-open, which allows beetles mobility from flower to flower. Therefore, assessing the visitation rate by counting the number of visited flowers at any given time of anthesis would provide a misleading notion of the total percentage of flowers visited on a tree during one night. The paucity of blossoming flowers on each tree on each night (rarely over 10 flowers) also constrains their collection. For example, by collecting flowers both with and without beetles, we would prevent the floral scent from attracting beetles to the trees and remaining therein. Moreover, beetle permanence on trees also depends on the presence of shed corollas. Therefore, only flowers with beetles were collected so that we could more accurately estimate the taxonomic composition of flower visitors.
Quantitative data on flower visitors during the nocturnal anthesis of A. crassiflora flowers show the prevalence of visitations by Coleoptera, with a residual presence of other orders of Insecta (; ). Despite the extraordinary diversity of the order Coleoptera, it is difficult to draw robust conclusions regarding the specialisation of the relationship between the beetles sampled in the present study and A. crassiflora flowers due to undersampling and a lack of research on natural history and taxonomic constraints, especially in the Cerrado (Pinheiro et al. Citation1998; McKenna et al. Citation2015; Monné and Costa Citation2021). Nevertheless, members of only two families of beetles (Melolonthidae and Curculionidae) were present in anthetic flowers (; ). When inferior taxonomic categories are put in perspective, specialisation emerges even more clearly: within these two families, only four species from three genera of beetles were found (; ).
The two identified Cyclocephala species differ greatly in their morphology and behaviour from the two weevil species (). In addition to being present in female and male flowers, both C. celata and C. octopunctata were found in relatively large numbers inside floral chambers (; ), touching the stigmas and open anthers. In male-phase flowers, Cyclocephala individuals coated in pollen were observed (, , ). Both species are relatively large and possess dense vestitures (especially C. celata, on its ventral surface), to which pollen can adhere (). Regarding the weevil species, Lydamis sp. 1 was seen frequently in the field on flower buds during the day, engaged in drilling holes, mating and ovipositing, which indicates that it probably shows only diurnal activity. It is therefore likely that the Lydamis sp. 1 individuals collected in anthetic flowers during the night were only sheltering inside them. Furthermore, no pollen loads were detected on Lydamis sp. 1 or Molytinae sp. 1. In conclusion, in the Cerrado in our study area, the putative pollinators of A. crassiflora were identified as C. celata and C. octopunctata, which indicates a specialised pollinator–plant relationship (Johnson and Steiner Citation2000).
A review undertaken by Moore and Jameson (Citation2013) estimated that at least 97 species of Cyclocephalini were associated with flowers of at least 191 plant species from 17 families, especially Magnoliales and monocots. Associations with dicots were also reported therein, but most of them were of destructive rather than symbiotic in nature (Moore and Jameson Citation2013). Distant families such as Magnoliaceae and Araceae have developed analogous structures that attract and shelter Cyclocephalini, referred to as ‘floral chambers’ in both cases (Gibernau Citation2003; Gottsberger et al. Citation2012). Convergent evolution seems to drive not only analogous floral features, such as odour and colour, as previously observed, but also size and shape, even though these characteristics are formatted by different constraints in phylogenetically distant taxa. In Araceae, for instance, the Cyclocephalini-bearing floral chamber is formed by a constriction of the spathe, delimiting a basal space in the inflorescence where beetles remain concealed during anthesis (Gottsberger Citation1990; Gibernau et al. Citation1999; Gibernau Citation2003). In the genus Magnolia (Magnoliaceae) and in water lilies (Nymphaeaceae), which belong to the order Magnoliales, flower chambers visited by Cyclocephalini are delimited by the closing of petals (Prance and Arias Citation1975; Prance Citation1980; Ervik and Knudsen Citation2003; Gottsberger Citation2012).
Among the 15 species of Cyclocephala that have been catalogued in the Cerrado, only two were collected in our study area: C. octopunctata and C. celata (Gonçalves et al. Citation2020). In the Cerrado, Annonaceae flower chambers with nocturnal anthesis are visited by a relatively constant group of Cyclocephala species, regardless of their geographical distance from each other. For instance, C. atricapilla is the principal pollinator of A. crassiflora and A. coriacea in Cerrado areas located in the states of São Paulo, Maranhão, Minas Gerais, Goiás and Mato Grosso and the Federal District (Gottsberger Citation1989b, Citation1990, Citation1994; Oliveira and Gibbs Citation2000; Silberbauer-Gottsberger et al. Citation2003; Gottsberger and Silberbauer-Gottsberger Citation2006; Cavalcante et al. Citation2009; Paulino-Neto Citation2014b; Costa et al. Citation2017).
In the present study, the most abundant Cyclocephala species in the Cerrado area of Chapada dos Guimarães collected inside the floral chambers of A. crassiflora was C. octopunctata (; ), which is also known to be associated with another Annona species from the Cerrado, Annona coriacea (Costa et al. Citation2017). The present study provides the first record of C. octopunctata as the main pollinator of A. crassiflora and the first investigation of the associated pollinator fauna of this plant species in the Cerrado of Chapada dos Guimarães, MT.
The secondary pollinator of A. crassiflora in Chapada dos Guimarães, C. celata, is very rare in lists of associations between beetles and Annonaceae (; ). However, associations between this scarab and aroids have been described in the literature (Maia et al. Citation2010; Moore and Jameson Citation2013). The only record of C. celata as a flower visitor of Annonaceae comes from an introduced species that was probably native to Central America (Annona muricata L., locally known as graviola) in a study performed at a commercial orchard in the state of Pernambuco surrounded by a native fragment of Atlantic rainforest. There are thus far two accounts of C. celata as a pollinator of two species of Araceae in Atlantic rainforests in Pernambuco, which is very suggestive of the possibility of cooption of native scarab species by introduced cantharophilous crops, despite their phylogenetically distant relationships (Junqueira et al. Citation1996; Maia et al. Citation2010; Parizotto and Grossi Citation2019). The cooption of native beetle species by introduced cantharophilous plants of economic importance has been reported in one Annona hybrid in Australia and oil palms in tropical America (Blanche and Cunninghum Citation2005; Meléndez and Ponce Citation2016). Hence, the maintenance of local Cyclocephala populations through habitat conservation might prove financially beneficial, especially in cases where pollination deficiency causes a reduction in productivity, as observed on Annona spp. plantations around the globe (Pinto Citation2005). Although there is only one dubious available record of C. celata occurrence in the Cerrado, the present study provides the first record of this species as a pollinator of an Annonaceae (Gonçalves et al. Citation2020).
Acknowledgements
We thank Maria Eduarda Basso, Ítalo Rondon, André Luiz Santiago Soares, Filipe F. de Deus, Natanael Rosário Alves da Silva, Fernando Zaiden, Nicholas Grohnert and Valéria Schmidt for laboratory and field assistance. Sérgio Ide (IB/USP), Fernando Vaz-de-Mello (IB/UFMT) and Brett C. Ratcliffe (University of Nebraska State Museum) kindly confirmed the identification of Cyclocephala species. We also appreciated the use of special photographic equipment at Rogério Rossi (IB/UFMT). We also thank Enymar Ataide, who kindly hosted us on his property during our preliminary investigations, and Lindomar Nascimento e Silva (Fundação Educacional Presbiteriana do Buriti). Ana Silvia Tissiani helped with the figures. The study was conducted under the Brazilian SISBIO permit number 267603 issued to MIM.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Albuquerque LSC, Souza TB, Dalia ACD, Iannuzzi L. 2014. New biological and immature morphological records of the masked chafer, Cyclocephala paraguayensis. J Insect Sci. 14:101. doi:10.1673/031.014.101.
- Alonso-Zarazaga MA, Lyal CHC. 1999. A world catalogue of families and genera of Curculionoidea (Excepting Scolytidae and Platypodidae). Barcelona: Entomopraxis.
- Alvares CA, Stape JL, Sentelhas PC, Gonçalves JLM, Sparovek G. 2013. Köppen’s climate classification map for Brazil. Meteorol Z. 22:711–728. doi:10.1127/0941-2948/2013/0507.
- Andrade BM, Oliveira-Filho AT, Soares AR. 1996. Pollination and breeding system of Xylopia brasiliensis Sprengel (Annonaceae) in South Eastern Brazil. J Trop Ecol. 12:313–320. doi:10.1017/S0266467400009482.
- Aragão DS, Costa CBN, Nascimento VT. 2019. Biologia floral, fenologia reprodutiva e polinização de Xylopia aromatica (Lam.) Mart. (Annonaceae) em uma área de Cerrado no oeste da Bahia. Paubrasilia. 2:17–26. doi:10.33447/paubrasilia.v2i1.17.
- Bernhardt P. 2000. Convergent evolution and adaptive radiation of beetle-pollinated angiosperms. Plant Syst Evol. 222:293–320. doi:10.1007/BF00984108.
- Blanche R, Cunningham SA. 2005. Rain forest provides pollinating beetles for atemoya crops. J Econ Entomol. 98:1193–1201. doi:10.1603/0022-0493-98.4.1193.
- Bouchard P, Bousquet Y, Davies AE, Alonso-Zarazaga MA, Lawrence JF, Lyal CHC, Newton AF, Reid CAM, Scmitt M, Ślipiński A, et al. 2011. Family-group names in Coleoptera (Insecta). ZooKeys. 88:1–972.
- Caldeira MAM, Morais-Costa F, Macêdo KM, Martins-Júnior VS, Pereira DEA, Soares ACM, Martins IP, Lopes IMG, Braga FC, Oliveira NJF, et al. 2019. Anthelmintic activity of Annona crassiflora leaves against Haemonchus contortus: part 1: in vitro inhibition of the hatchability and larval development. Medicina Veterinária. 13:184–191. doi:10.26605/medvet-v13n2-3067.
- Capinera JL. 2001. Handbook of vegetable pests. California: Academic Press.
- Cardoso LM, Oliveira DS, Bedetti SF, Martino HSD, PinheiroSant’Ana HM. 2013. Araticum (Annona crassiflora Mart.) from the Brazilian Cerrado: chemical composition and bioactive compounds. Fruits. 68:121–134. doi:10.1051/fruits/2013058.
- Casari SA, Vani SA, and Casari-Chen SA 1988. Larvas de Coleoptera do Brasil. São Paulo: Museu de Zoologia/Universidade de São Paulo; p. 115–128.
- Casari SA, Ide S. 2012. Coleoptera Linnaeus, 1758. In: Albertino JA, Melo GAR, Carvalho CJB, Casari AS, Constantino R, editors. Insetos do Brasil – diversidade e taxonomia. Ribeirão Preto: holos Editora; p. 454–535.
- Cavalcante TRM, Naves RV, Franceschinelli RV, Da Silva RP. 2009. Polinização e formação de frutos em araticum. Bragantia. 68:13–21. doi:10.1590/S0006-87052009000100002.
- Cherman MA, Morón MA. 2014. Validación de la familia Melolonthidae Leach, 1819 (Coleoptera: Scarabaeoidea). Acta Zool Mex. 30:201–220. doi:10.21829/azm.2014.301139.
- Costa GP. 2017. Estudo da atividade antioxidante de folhas e polpa de Annona crassiflora Mart. para utilizar como fitocosmético. Master Thesis. Universidade Estadual Paulista “Júlio de Mesquita Filho” UNESP.
- Costa MS, Silva RJ, Paulino-Neto HF, Ferreira MJB. 2017. Beetle pollination and flowering rhythm of Annona coriacea Mart. (Annonaceae) in Brazilian Cerrado: behavioral features of its principal pollinators. PLoS ONE. 12:e0171092. doi:10.1371/journal.pone.0171092.
- Coutinho GV, Rogrigues SR, Cruz EC, Abot AR. 2011. Bionomic data and larval density of Scarabaeidae (Pleurosticti) in sugarcane in the central region of Mato Grosso do Sul, Brazil. Rev Bras Entomol. 55:389–395. doi:10.1590/S0085-56262011005000038.
- Dieringer G, Spinosa SJE. 1994. Reproductive ecology of Magnolia schiedeana (Magnoliaceae), a threatened cloud forest tree species in Veracruz, Mexico. Bull Torrey Bot Club. 121:154–159. doi:10.2307/2997167.
- Dieringer G, Cabrera RL, Lara M, Loya L, Reyes-Castillo P. 1999. Beetle pollination and floral thermogenicity in Magnolia tamaulipana (Magnoliaceae). Int J Plant Sci. 160:64–71. doi:10.1086/314099.
- Endrödi S. 1985. The Dynastinae of the world. Budapest: Akadémiai Kiadó.
- Ervik F, Tollsten L, Knudsen JT. 1999. Floral scent chemistry and pollination ecology in phytelephantoid palms (Arecaceae). Plant Syst Evol. 217:279–297. doi:10.1007/BF00984371.
- Ervik F, Knudsen JT. 2003. Water lilies and scarabs: faithful partners for 100 million years? Biol J Linn Soc. 80:539–543. doi:10.1046/j.1095-8312.2003.00258.x.
- GBIF. 2021. GBIF: the global biodiversity information facility. Available at: https://www.gbif.org. Acessad 2021 Mar 15.
- Gibernau M, Barabé D, Cerdan P, Dejean A. 1999. Beetle pollination of Philodendron solimoesense (Araceae) in French Guiana. Int J Plant Sci. 160:1135–1143. doi:10.1086/314195.
- Gibernau M. 2003. Pollinators and visitors of aroid inflorescences. Aroideana. 26:66–83.
- Gonçalves JA, Grossi PC, Togni PHB, Oliveira CM, Frizzas MR. 2020. The genus Cyclocephala Dejean (Coleoptera: Scarabaeidae: Dynastinae) in Brazil: diversity and spatio‑temporal distribution. J Insect Conserv. 24:547–559. doi:10.1007/s10841-020-00230-6.
- Gottsberger G. 1989a. Comments on flower evolution and beetle pollination in the genera Annona and Rollinia. Plant Syst Evol. 167:189–194. doi:10.1007/BF00936405.
- Gottsberger G. 1989b. Beetle pollination and flowering rhythm of Annona spp. (Annonaceae) in Brazil. Plant Syst Evol. 167:165–187. doi:10.1007/BF00936404.
- Gottsberger G. 1990. Flowers and beetles in the South American tropics. Bot Acta. 103:360–365. doi:10.1111/j.1438-8677.1990.tb00175.x.
- Gottsberger G. 1994. As anonáceas do cerrado e a sua polinização. Rev Bras Biol. 54:391–402.
- Gottsberger G 1999. Pollination and evolution in neotropical Annonaceae. Plant Species Biol. 14:143–152. doi:10.1046/j.1442-1984.1999.00018.x
- Gottsberger G, Silberbauer-Gottsberger I. 2006. In the evening when the beetles come: pollination in Annonaceae and Philodendron. In: Gottsberger G, Silberbauer-Gottsberger I, editors. Life in the Cerrado: a South American tropical seasonal vegetation. Vol. II. Pollination and seed dispersal. Ulm: Reta Verlag; p. 138–158.
- Gottsberger G. 2012. How diverse are Annonaceae with regard to pollination? Bot J Linn Soc. 169:245–261. doi:10.1111/j.1095-8339.2011.01209.x.
- Gottsberger G, Silberbauer-Gottsberger I, Dötterl S. 2012. Pollination ecology of Magnolia ovata may explain the overall large flower size of the genus. Flora. 207:107–118. doi:10.1016/j.flora.2011.11.003.
- Gottsberger G, Webber AC. 2018. Nutritious tissue in petals of Annonaceae and its function in pollination by scarab beetles. Acta Bot Bras. 32:279–286. doi:10.1590/0102-33062017abb0356.
- Johnson SD, Steiner KE. 2000. Generalization versus specialization in plant pollination systems. Tree. 15:140–143. doi:10.1016/s0169-5347(99)01811-x.
- Junqueira NTV, Cunha MM, Oliveira MAS, Pinto ACQ. 1996. Graviola para exportação: aspectos fitossanitários. Brasília: Embrapa-SPI.
- Kishore K, Shukla AK, Babu N, Sarangi DN, Patanayak S. 2012. Pollination biology of Annona squamosa L. (Annonaceae): evidence for pollination syndrome. Sci Hortic. 144:212–217. doi:10.1016/j.scienta.2012.07.004.
- Kono M, Tobe H. 2007. Is Cycas revoluta (Cycadaceae) wind- or insect-pollinated? Am J Bot. 94:847–855. doi:10.3732/ajb.94.5.847.
- Küchmeister H, Webber AC, Silberbauer-Gottsberger I, Gottsberger G. 1998. A polinização e sua relação com a termogênese em espécies de Arecaceae e Annonaceae da Amazônia Central. Acta Amazon. 28:217–245. doi:10.1590/1809-43921998283245.
- Lara CE, Díez MC, Restrepo Z, Núñez LA, Moreno F. 2017. Flowering phenology and flower visitors of the Macana Palm Wettinia kalbreyeri (Arecaceae) in an Andean montane forest. Rev Mex Biodivers. 88:106–112. doi:10.1016/j.rmb.2017.01.001.
- Lau JYY, Pang CC, Ramsden L, Saunders RMK. 2017b. Stigmatic exudate in the Annonaceae: pollinator reward, pollen germination medium or extragynoecial compitum? J Integr Plant Biol. 59:881–894. doi:10.1111/jipb.12598.
- Lorenzi H. 2009. Árvores Brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. Vol. 2. Nova Odessa: Instituto Plantarum.
- Maia ACD, Schlindwein C, Navarro DMAF, Gibernau M. 2010. Pollination of Philodendron acutatum (Araceae) in the Atlantic Forest of northeastern Brazil: a single scarab beetle species guarantees high fruit set. Int J Plant Sci. 171:740–748. doi:10.1086/654846.
- McKenna DD, Wild AL, Kanda K, Bellamy CL, Beutel RG, Caterino MS, Farnum CW, Hawks DC, Ivie MA, Jameson ML, et al. 2015. The beetle tree of life reveals that Coleoptera survived end Permian mass extinction to diversify during the Cretaceous terrestrial revolution. Syst Entomol. 40:835–880. doi:10.1111/syen.12132.
- Meeuse BJD, Raskin I. 1988. Sexual reproduction in the arum lily family, with emphasis on thermogenicity. Sex Plant Reprod. 1:3–15. doi:10.1007/BF00227016.
- Meléndez MR, Ponce WP. 2016. Pollination in the oil palms Elaeis guineensis, E. oleifera and their hybrids (OxG), in tropical America. Pesqui Agropecu Bras. 46:102–110. doi:10.1590/1983-40632016v4638196.
- Mendonça RC, Felfili JF, Walter BMT, Silva-Júnior MC, Filgueiras TS, Nogueira PE, Fagg CF. 2008. Flora vascular do Bioma Cerrado – checklist com 12.356 espécies. In: Sano SM, Almeida SP, Ribeiro JF, editors. Cerrado - Ecologia e Flora. Vol. 2. Brasília: Embrapa Informação Tecnológica; p. 421–1279.
- Monné ML, Costa C 2021. Coleoptera in catálogo taxonômico da fauna do Brasil. PNUD. Available at: http://fauna.jbrj.gov.br/fauna/faunadobrasil/223. Acessed 2021 May 04.
- Moore MR and Jameson ML. (2013). Floral Associations of Cyclocephaline Scarab Beetles. J. Insect Sci, 13(100):1–43. doi: 10.1673/031.013.10001
- Moore MR, Cave RD, Branham MA. 2018. Synopsis of the cyclocephaline scarab beetles (Coleoptera, Scarabaeidae, Dynastinae). ZooKeys. 745:1–99.
- Nogueira GAL, Rodrigues SR, Tiago EF. 2013. Biological aspects of Cyclocephala tucumana Brethes, 1904 and Cyclocephala melanocephala (Fabricius, 1775) (Coleoptera: Scarabaeidae). Biota Neotrop. 13:86–90. doi:10.1590/S1676-06032013000100009.
- Olesen JM. 1992. Flower mining by moth larvae vs. pollination by beetles and bees in the cauliflorous Sapranthus palanga Annonaceae) in Costa Rica. Flora. 187:9–15. doi:10.1016/S0367-2530(17)32201-6.
- Oliveira PE, Gibbs PE. 2000. Reproductive biology of woody plants in a Cerrado community of Central Brazil. Flora. 195:311–329. doi:10.1016/S0367-2530(17)30990-8.
- Oliveira MSP, Couturier G, Beserra P. 2003. Biologia da polinização da palmeira tucumã (Astrocaryum vulgare Mart.) em Belém, Pará, Brasil. Acta Bot Bras. 17:343–353. doi:10.1590/S0102-33062003000300002.
- Parizotto DR, Grossi PC. 2019. Revisiting pollinating Cyclocephala scarab beetles (Coleoptera: Melolonthidae: Dynastinae) associated with the soursop (Annona muricata. Annonaceae). Neotrop. Entomol. 48:415–421. doi:10.1007/s13744-018-0647-y.
- Paulino-Neto HF. 2014a. Polinização por besouros. In: Rech AR, Agostini K, Oliveira PE, Machado IC, editors. Biologia da polinização. Rio de Janeiro: projeto Cultural. p. 259–276.
- Paulino-Neto HF. 2014b. Polinização e biologia reprodutiva do araticum-liso (Annona coriacea Mart.: Annonaceae) em uma área de Cerrado paulista: implicações para fruticultura. Rev Bras Frutic. 36:132–140. doi:10.1590/S0100-29452014000500016.
- Pellmyr O, Patt JM. 1986. Function of olfactory and visual stimuli in pollination of Lysichiton americanum (Araceae) by a staphylinid beetle. Madroño. 33:47–54.
- Pinheiro F, Diniz IR, Kitayama K. 1998. Comunidade local de Coleoptera em Cerrado: diversidade de espécies e tamanho do corpo. An Soc Entomol Bras. 27:543–550. doi:10.1590/S0301-80591998000400006.
- Pinto ACQ. 2005. Agronomy. In: Williams JT, Smith RW, Hughes A, Haq N, Clement CR, editors. Annona species. Southampton: International Centre for Underutilised Crops, University of Southampton; p. 72–123.
- Prance GT, Arias JR. 1975. A study of the floral biology of Victoria amazonica (Poepp.) Sowerby (Nymphaeaceae). Acta Amazon. 5:109–139. doi:10.1590/1809-43921975052109.
- Prance GT. 1980. A note on the pollination of Nymphaea amazonum. Mart. & Zucc. (Nymphaeaceae). Brittonia. 32:505–507. doi:10.2307/2806159.
- Rands SA, Whitney HM. 2008. Floral temperature and optimal foraging: is heat a feasible floral reward for pollinators? PLoS ONE. 3:e2007. doi:10.1371/journal.pone.0002007.
- Ratnayake RMCS, Gunatilleke IAUN, Wijesundara DSA, Saunders RMK. 2007. Pollination ecology and breeding system of Xylopia championii (Annonaceae): curculionid beetle pollination, promoted by floral scents and elevated floral temperatures. Int J Plant Sci. 168:1255–1268. doi:10.1086/521689.
- Rezende ML, Malafaia GC. 2012. A cadeia produtiva do marolo na região Sul de Minas Gerais. Revista Economia & Gestão. 12:49–63.
- Ribeiro JF, Walter BMT. 2008. As principais fitofisionomias do Bioma Cerrado. In: Sano SM, Almeira SP, Ribeiro JF, editors. Cerrado: ecologia e Flora. Vol. 1. Brasília: Embrapa Informação Tecnológica; p. 151–212.
- Rodrigues SR, Barbosa CAF, Fuhrmann J, Amaro RA. 2018. Mating behavior and description of immature stages of Cyclocephala melanocephala (Fabricius, 1775) (Coleoptera: Scarabaeidae: Dynastinae), identification key and remarks on known immatures of Cyclocephalini species. Rev Bras Entomol. 62:205–219. doi:10.1016/j.rbe.2018.07.001.
- Santos B, Ávila CJ. 2007. Aspectos bioecológicos de Cyclocephala forsteri Endrödi, 1963 (Coleoptera: Melolonthidae) no Estado do Mato Grosso do Sul. Rev Agric. 82:298–303.
- Saravy FP, Marques MI, Schuchmann K-L. 2021a. Coleopteran pollinators of Annonaceae in the Brazilian Cerrado - A review. Diversity. 13:438. doi:10.3390/d13090438.
- Saravy FP, Marques MI, Schuchmann K-L. 2021b. Diversity of insect flower visitors of Xylopia aromatica (Magnoliales, Annonaceae) in a Brazilian savanna. Diversity. 13:661. doi:10.3390/d13120661.
- Schatz GE. 1990. Some aspects of pollination biology in Central American forests. In: Bawa KS, Hadley M, editors. Reproductive ecology of tropical forest plants. Paris: UNESCO/The Parthenon Publishing Group; p. 69–84.
- Schiestl FP, Johnson SD. 2013. Pollinator-mediated evolution of floral signals. Trends Ecol Evol. 28:307–315. doi:10.1016/j.tree.2013.01.019.
- Seymour RS, Schultze-Motel P. 1997. Heat-producing flowers. Endeavour. 21:125–129. doi:10.1016/S0160-9327(97)80222-0.
- Seymour RS, Schultze-Motel P. 1998. Physiological temperature regulation by flowers of the sacred lotus. Philos Trans R Soc. 353:935–943. doi:10.1098/rstb.1998.0258.
- Seymour RS, White CR, Gibernau M. 2003. Heat reward for insect pollinators. Nature. 426:243–244. doi:10.1038/426243a.
- Seymour RS, White CR, Gibernau M. 2009. Endothermy of dynastine scarab beetles (Cyclocephala colasi) associated with pollination biology of a thermogenic arum lily (Philodendron solimoesense). J Exp Biol. 212:2960–2968. doi:10.1242/jeb.032763.
- Silberbauer-Gottsberger I, Gottsberger G, Webber AC. 2003. Morphological and functional flower characteristics of New and Old World Annonaceae with respect to their mode of pollination. Taxon. 52:701–718. doi:10.2307/3647345.
- Silva-Júnior MC, Dos Santos GC, Nogueira PE, Munhoz CBR, Ramos AE. 2005. 100 Árvores do Cerrado: guia de Campo. Brasília: Rede de Sementes do Cerrado.
- Souza TB, Maia ACD, Albuquerque LSC, Ianuzzi L. 2013. The life of Cyclocephala celata Dechambre, 1980 (Coleoptera: Scarabaeidae: Dynastinae) in captivity with descriptions of the immature stages. J Nat Hist. 48:5–6.
- Souza TB, Maia ACD, Albuquerque CMR, Iannuzzi L. 2014. Description of Cyclocephala distincta Burmeister (Coleoptera: Scarabaeidae: Dynastinae: Cyclocephalini) immatures and identification key for third instars of some Cyclocephala species. Zootaxa. 3872:180–186. doi:10.11646/zootaxa.3872.2.4.
- Torezan-Silingardi HM, Silberbauer-Gottsberger I, Gottsberger G. 2021. Pollination ecology: natural history, perspectives and future directions. In: Torezan-Silingardi HM, Del-Claro K, editors. Plant-animal interactions: source of biodiversity. Cham: Springer Nature; p. 119–174.
- Vieira-Júnior HT, Moraes JM, de Paula TLF. 2012. Chapada dos Guimarães (MT). In: Schobbenhaus C, Da Silva CR, editors. Geoparques do Brasil: propostas. Vol. 1. Rio de Janeiro: CPRM; p. 283–316.