ABSTRACT
The new zodariid genus Spinozodium gen. nov. is described, comprising two species from Tajikistan: the type species S. denisi (Spassky, Citation1938) comb. nov. (ex. Zodarion Walckenaer, 1826) and the new species S. khatlonicum sp. nov.; the female of the type species, displaying highly reduced receptacles, is described for the first time. Both species are illustrated and their distributions are mapped, and keys to the Middle Asian zodariid subfamilies and genera are provided.
http://www.zoobank.org/urn:lsid:zoobank.org:pub:E70AB248-A42C-4822-82E9-88C16C93781B
Introduction
Zodariidae Thorell, 1881 is a large family of spiders, currently comprising 1249 extant species in 89 genera and five subfamilies worldwide (Jocqué Citation1991; WSC Citation2022). In Tajikistan, nine species in four genera and two subfamilies of Zodariidae have been reported so far (Mikhailov Citation2021; Zamani and Marusik Citation2022): Lachesana Strand, 1932 (one species) (Lachesaninae Jocqué, Citation1991), Parazodarion Ovtchinnikov, Ahmad and Gurko, 2009 (one species), Zodariellum Andreeva and Tyshchenko, 1968 (six species) and Zodarion Walckenaer, 1826 (one species) (Zodariinae Thorell, 1881). Recently, all zodariid species from Tajikistan previously assigned to Zodarion were found to belong to Zodariellum, except for one species, ‘Zodarion’ denisi Spassky, Citation1938 (Zamani and Marusik Citation2022). This species was originally described on the basis of two male specimens collected in Muminabad, southern Tajikistan (Spassky Citation1938). Further male and juvenile material from southern and western Tajikistan was later reported by Spassky (Citation1952), Andreeva and Tystshenko (Citation1968) and Andreeva (Citation1976), and the holotype was re-illustrated by Marusik and Koponen (Citation2001). We had the opportunity to examine a series of zodariid material recently collected in Tajikistan, encompassing not only the aforementioned species and its undescribed female, but also a closely related species new to science. Both species possess unique characters in their copulatory organs which in our opinion warrants the designation of a new genus. The new genus and the two species are (re)described herein.
Material and methods
Specimens were photographed using a Canon EOS 7D camera attached to an Olympus SZX16 stereomicroscope, and a JEOL JSM-5200 scanning electron microscope, at the Zoological Museum of the University of Turku. Digital images were montaged using CombineZP and edited using CorelDRAW graphic design software. The map was prepared using SimpleMappr (Shorthouse Citation2010). Lengths of leg segments were measured on the dorsal side and are listed as: total length (femur, patella, tibia, metatarsus, tarsus). All measurements are in millimetres.
The term ‘Middle Asia’ is used for the region comprising Central Asian republics of the former Soviet Union, namely Kazakhstan, Uzbekistan, Kyrgyzstan, Turkmenistan and Tajikistan.
Abbreviations
Eyes: ALE ‒ anterior lateral eye, AME ‒ anterior median eye, PLE ‒ posterior lateral eye, PME ‒ posterior median eye. Male palp: RTA ‒ retrolateral tibial apophysis.
Depositories
MMUE – Manchester Museum of the University of Manchester, United Kingdom (D.V. Logunov); SMNH – Steinhardt Museum of Natural History, Tel Aviv, Israel (S.L. Zonstein); ZMMU – Zoological Museum of Moscow State University, Russia (K.G. Mikhailov).
Family Zodariidae Thorell, 1881
1. Large (>10 mm) with spiny legs and long cheliceral fangsLachesaninae (comprising a single genus in the region: Lachesana)
- Small (<10 mm), legs spineless, cheliceral fangs short ......................... Zodariinae
Key to the subfamilies occurring in Middle Asia
Subfamily Zodariinae Thorell, 1881
1. Six eyes ................................................................. Trygetus Simon, 1882 (one species)
- Eight eyes 2
2. Cymbium with tutaculum and deep diverticulum, RTA with 1 or 2 transversal outgrowth(s), copulatory ducts screwed and convergings Zodariellum (14 species)
- Different3
3. RTA with spine-like setae, epigyne with septum and distinct arch-shaped anterior hood .................................................. Spinozodium gen. nov. (two species)
- RTA without spine-like setae, epigyne lacking septum4
4 Male palpal tibia with only RTA, RTA longer than tibia, embolus filamentous, cymbium lacking depression, epigynal fovea wider than long Parazodarion (one species)
- Male palpal tibia with two apophyses, RTA not longer than tibia, cymbium with shallow baso-dorsal depression, embolus not filamentous, epigynal fovea longer than wide ................ Acanthinozodium Denis, 1966 (one species)
Key to the genera of Zodariinae occurring in Middle Asia
Genus Spinozodium gen. nov.
Type species
Zodarion denisi Spassky, Citation1938 from Tajikistan.
Etymology
A combination of spino-, referring to the characteristic spine-like setae on the retrolateral tibial apophysis of the male palp, and -zodium, a common ending for zodariid genera; gender neuter.
Diagnosis
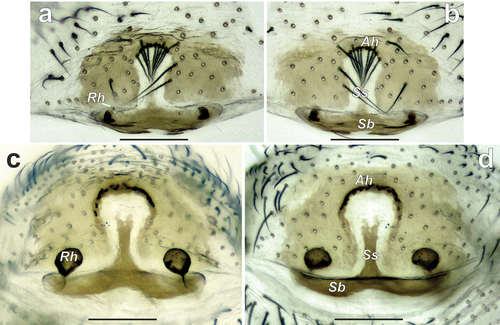
The males of the new genus differ from those of the other Zodariinae genera occurring in Middle Asia by having spine-like setae on RTA ( and ) (vs lacking). The females of Spinozodium gen. nov. differ from those of the other Zodariinae genera by having a distinct epigynal septum and distinctly sclerotised anterior hood bearing setae that cover the anterior half of fovea ( and ) (vs lacking both septum and sclerotised anterior hood with setae).
Description
Small: males 2.4–2.6 long, carapace 1.2–1.42 long; females 3.1–3.6 long, carapace 1.5–1.65 long. Carapace either uniformly light brown or with darker cephalic region. Legs and palps uniformly coloured. Abdomen blackish with light median band in posterior 2/3, band either continuous with angled lateral branches or composed of roughly triangular to diamond-shaped spots; lateral sides and venter pale. Leg formula 4132 or 4123.
Male palp. Tibia with only RTA; RTA long, about ½ of cymbium length, longer than tibia, anterior half bent ventrally, proximal half with around a dozen spine-like setae, distal half slender, spineless and more heavily sclerotised than proximal half; cymbium 2 times longer than wide, without diverticulum or tutaculum, but with longitudinal fold (Cf) in S. denisi (); cymbial trichobothrium as in ; bulb oval to almost round; tegular apophysis (Ta) about 1.5 times longer than wide, located in anterior half of bulb, with either very short claw-like or straight process (Tp), and retrolateral lobe (Tr); conductor (Co) small, located at 12 o’clock position; embolus broad, originating from about 6 o’clock position, terminal part with small membranous process (Ep), opening of sperm duct (Os) located on anterior and close to tip of embolus.
Epigyne. Plate wider than long, with distinct fovea and septum; fovea ampullate, with distinctly sclerotised anterior hood (Ah); anterior hood bearing row of long, converging setae partly covering anterior half of fovea; stem of septum (Ss) thin, base of septum (Sb) more than 2 times wider than stem; base of septum bent antero-dorsally and barely visible in intact epigyne; receptacles (Re) separated by 4–7 diameters, each consisting of a base and a globular head (Rh).
Composition
Two species: Spinozodium denisi comb. nov. and S. khatlonicum sp. nov.
Distribution
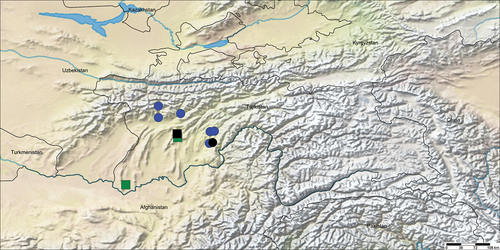
Known only from Tajikistan; although the distributions of the two currently known species overlap, they are not known to occur sympatrically ().
Spinozodium denisi (Spassky, Citation1938), comb. nov.
()
Figure 1. Habitus of Spinozodium denisi (a, b) and S. khatlonicum sp. nov. (c, d), dorsal view. (a, c) females; (b, d) males. Scale bars = 0.5 mm.

Zodarion denisi Spassky, Citation1938: 580, figure 5 (♂).
Zodarium [sic!] denisi: Spassky Citation1952: 193. – Andreeva and Tystshenko Citation1968: 688, figure 6 (♂).
Zodarion denisi: Andreeva Citation1976: 37, figure 44 (♂). – Marusik and Koponen Citation2001: 46, figures 28–31 (♂).
Material examined
TAJIKISTAN: Dushanbe: 2♂ (MMUE), env. of Dushanbe, Hissar Mt. ridge, 20 km of Varzob Hway, Gusgarf Vil., exposed northern slopes with Acer litter and cliffs, 8 May 2015 (Yu. M. Marusik); 4♂ 4♀ (ZMMU), env. of Dushanbe, Hissar Mt. ridge, 48 km of Varzob Hway, Gusgarf Vill., 38.555, 68.482, 1530 m, exposed southern slopes with Juglans litter and under stones, 7 May 2015 (Yu. M. Marusik, M. Saidov); 3♂ 1♀ (MMUE), env. of Dushanbe, Varzob valley, surroundings of Varzob Lake, 38.399, 68.482, 1043 m, pine litter, 3 May 2015 (Yu. M. Marusik); 1♂ (SMNH), Hissar Mt. Range, Ramit Res., Darai Holmon (Kholmon) creek gorge, 38.756, 69.304, 1370 m, 2 May 2015 (S.L. Zonstein); Khatlon Region: 2♂ (ZMMU), env. of Khovaling, Obimazar River, 38.3490, 69.9699, 1413 m, gravelly river shores with some bushes, 27 April 2015 (Yu. M. Marusik); 7♂ 1♀ (ZMMU), Khovaling Dist., opposite to Alakosim Kishlak, 38.359333, 70.064200, 1840 m, 28 April 2015 (Yu. M. Marusik).
Diagnosis
The male of this species differs from that of its congener by (1) the tip of RTA bent at a different angle (see ), (2) straight, spine-like and longer process of tegular apophysis (Tp) located prolaterally (vs claw-like, shorter and located anteriorly), and (3) having teeth (Tt) on the anterior edge of the tegular apophysis (vs no teeth). The female of S. denisi differs from that of its congener by (1) the shorter anterior hood and subparallel margins of fovea (vs wider hood and posteriorly converging lateral margins of fovea; see ), (2) relatively shorter base of septum and (3) smaller head of receptacle (see ).
Description
Male. Habitus as in . Total length 2.60. Carapace 1.42 long, 0.99 wide. Eye sizes: AME: 0.10, ALE: 0.08, PME: 0.07, PLE: 0.07. Carapace, sternum, chelicerae, labium and maxillae yellowish. Carapace with irregular darker markings. Legs yellowish, without annulations. Abdomen dorsally dark greyish with pale beige median stripe, ventrally pale beige. Spinnerets uniformly pale beige. Measurements of legs: I: 3.97 (1.05, 0.42, 0.84, 0.97, 0.70), II: 3.73 (1.02, 0.45, 0.74, 0.92, 0.60), III: 3.74 (1.00, 0.45, 0.73, 0.96, 0.60), IV: 5.03 (1.28, 0.47, 1.16, 1.44, 0.68).
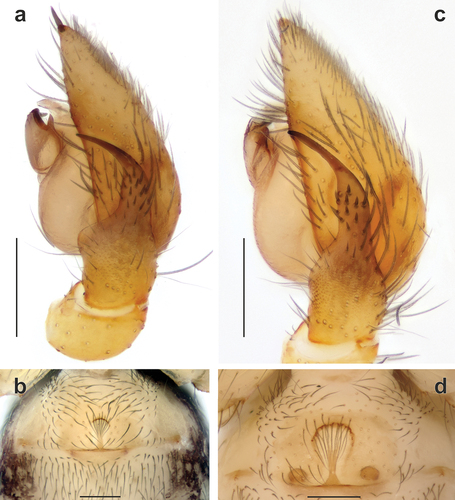
Palp as in , and ; terminal half of RTA bent ventrally; cymbium with shallow fold (Cf) opposing RTA; bulb oval, tegular apophysis (Ta) with straight, spine-like process (Tp), anterior edge of tegular apophysis with fine teeth (Tt).
Figure 2. Male palps of Spinozodium denisi (a–c) and S. khatlonicum sp. nov. (d–f). (a, d) ventral view; (b, e) prolateral view; (c, f) dorsal view. Scale bars = 0.2 mm.
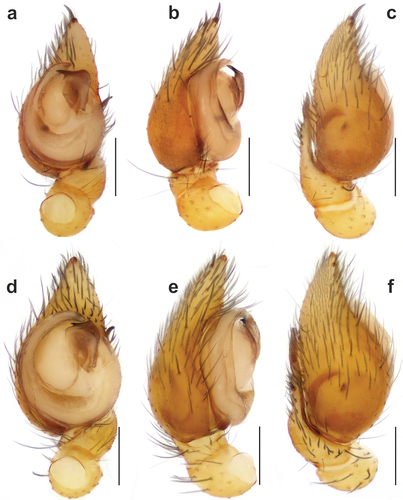
Figure 3. Scanning electron microscopy images of the male palps of Spinozodium khatlonicum sp. nov. (a–c) and S. denisi (d–g). (a, e) detail of embolus and tegular apophysis, anteroventral view; (b, d) ventral view; (c, f) retrolateral view; (g) cymbial trichobothrium. Scale bars = 0.1 mm, unless otherwise stated. Abbreviations: Cf – cymbial fold, Co – conductor, Em – embolus, Ep – process of embolus tip, Os – opening of the sperm duct, Ta – tegular apophysis, Tp – process of tegular apophysis, Tr – retrolateral arm of tegular apophysis, Tt – teeth of tegular apophysis.
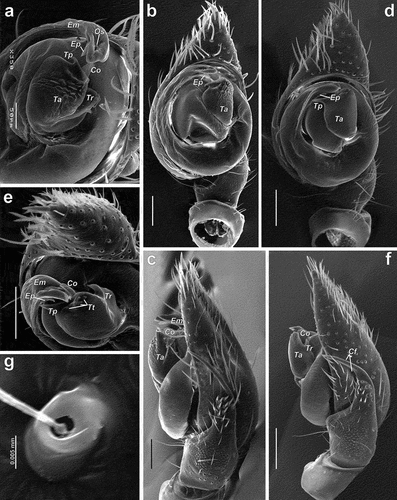
Figure 4. Spinozodium denisi (a, b) and S. khatlonicum sp. nov. (c, d). (a, c) male palp, retrolateral view; (b, d) intact epigyne, ventral view. Scale bars = 0.2 mm.
Female. Habitus as in . Total length 3.60. Carapace 1.50 long, 1.05 wide. Eye sizes: AME: 0.10, ALE: 0.09, PME: 0.08, PLE: 0.08. Colouration as in male. Measurements of legs: I: 4.24 (1.10, 0.47, 0.88, 1.06, 0.73), II: 3.78 (1.00, 0.46, 0.77, 0.95, 0.60), III: 4.05 (1.07, 0.49, 0.76, 1.13, 0.60), IV: 5.51 (1.38, 0.50, 1.27, 1.62, 0.74).
Epigyne as in and ; anterior hood short and arched; margins of fovea subparallel (); septal stem (Ss) about 2 times shorter than septal base (Sb); receptacles separated by about 7 diameters and each bearing a small head (Rh).
Distribution
Known only from Dushanbe and Khatlon regions, western to south-central Tajikistan (). Ovtsharenko and Fet (Citation1980) and Krivokhatsky and Fet (Citation1981) reported this species from Turkmenistan, although these records were later attributed to Parazodarion raddei (Simon, 1889) (sub Zodarion) by Mikhailov and Fet (Citation1994).
Comments
The female of this species is described here for the first time.
Spinozodium khatlonicum sp. nov.
()
Type material
Holotype ♂ (ZMMU), TAJIKISTAN: Khatlon Region: Dangara Dist., Sanglok Mt. range, above Shar-Shar Pass, 38.298950, 69.226633, 1700–2060 m, 29 April 2015 (Yu.M. Marusik). Paratypes: 5♂ 9♀ (ZMMU), same data as the holotype; 4♂ 2♀ (ZMMU), Dangara dist., Sanglok Mt. range, above Shar-Shar Pass, 38.218183, 69.238033, 1362 m, 30 April 2015 (Yu.M. Marusik); 3♂ 1♀ (MMUE), Shaartuz Dist., Khushody, 37.091320, 68.041950, 378 m, edge of sandy desert, 20.04.2015 (Yu.M. Marusik).
Etymology
The specific epithet refers to the type locality.
Diagnosis
The male of this species differs from that of S. denisi by (1) the tip of RTA bent at a different angle (see ), (2) smaller and claw-like process of tegular apophysis (Tp) located anteriorly (vs larger, spine-like, and located prolaterally), and (3) having small ridges on the anterior edge of tegular apophysis (vs having teeth instead of ridges). The female of the new species differs from that of the type species by (1) the converging lateral margins of fovea (vs subparallel; see ), (2) relatively wider base of septum and (3) larger head of receptacle (see ).
Description
Male (Holotype)
Habitus as in . Total length 2.40. Carapace 1.20 long, 0.93 wide. Eye sizes: AME: 0.11, ALE: 0.08, PME: 0.07, PLE: 0.08. Carapace, sternum, chelicerae, labium and maxillae yellowish. Carapace with irregular darker markings. Legs yellowish, without annulations. Abdomen dorsally dark greyish with pale beige median stripe, ventrally pale beige. Spinnerets uniformly pale beige. Measurements of legs: I: 3.88 (1.10, 0.36, 0.90, 0.82, 0.70), II: 3.68 (1.00, 0.40, 0.80, 0.90, 0.58), III: 3.64 (0.90, 0.45, 0.73, 0.96, 0.60), IV: 5.09 (1.36, 0.45, 1.14, 1.46, 0.68).
Palp as in , and ; terminal half of RTA bent ventrally; bulb almost round; tegular apophysis (Ta) with small claw-like process (Tp), anterior part of tegular apophysis with transversal broken ridges ().
Female
Habitus as in . Total length 3.65. Carapace 1.65 long, 1.20 wide. Eye sizes: AME: 0.13, ALE: 0.11, PME: 0.09, PLE: 0.09. Colouration as in male. Measurements of legs: I: 4.85 (1.30, 0.45, 1.05, 1.30, 0.75), II: 4.32 (1.20, 0.45, 0.90, 1.10, 0.67), III: 4.54 (1.25, 0.46, 0.90, 1.25, 0.68), IV: 6.36 (1.70, 0.55, 1.50, 1.83, 0.78).
Epigyne as in and ; anterior hood arched; foveal lateral margins converging posteriorly (); heads of receptacles (Rh) visible through integument (); septal base (Sb) about 3 times wider than septal stem (Ss); head of receptacle larger than base of receptacle, separated by about 4 diameters.
Distribution
Known only from the listed localities in Khatlon region, south-western Tajikistan ().
Discussion
Considering the results of this paper, there are 10 species of four genera of Zodariidae known from Tajikistan, of which one genus (Spinozodium gen. nov.) and four species – S. denisi, S. khatlonicum sp. nov., Zodariellum tadzhikum (Andreeva and Tyshchenko, 1968), Z. testaceofasciatum (Spassky, 1941) – are currently known from this country only. Furthermore, following Zamani and Marusik (Citation2021, Citation2022) and the current results, Zodarion sensu stricto appears to be restricted to the eastern Mediterranean; those species currently considered in this (clearly polyphyletic) genus occurring outside of this range should most likely be transferred to other genera, ideally within a large-scale taxonomic revision of the group. Furthermore, a phylogenetic analysis aiming to elucidate the relationship of Spinozodium gen. nov. to other genera of Zodariinae should be conducted; the genus does not seem to bear a close relationship with Zodariellum, Acanthinozodium or any species groups of Zodarion sensu lato.
One of the interesting findings reported here is the newly described female of S. denisi. Unlike that of its sister species S. khatlonicum sp. nov., the female of S. denisi possesses highly reduced and almost vestigial receptacles, which is a rare character in spiders. It is possible that S. denisi displays a unique mating behaviour which has resulted in the reduced size of these structures. A well-known case of atrophied receptacles in spiders has been documented for Harpactea sadistica Řezáč, Citation2008 (Dysderidae). Unlike other spiders, in which the eggs are fertilised simultaneously with oviposition, the male of H. sadistica performs traumatic insemination and fertilisation occurs in the ovaries, with eggs developing as embryos before oviposition (Řezáč Citation2008, Citation2009). Therefore, we strongly encourage conducting a comparative study on the mating behaviour of S. denisi and S. khatlonicum, as such research is likely to unveil similar interesting and rarely documented aspects of spider biology.
Acknowledgements
YM is thankful to Seppo Koponen (Turku, Finland) for arranging his stay in Turku, and Sergei L. Zonstein (Tel Aviv, Israel) for organising their joint expedition to Tajikistan in 2015. We are grateful to two anonymous reviewers for their valuable comments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Andreeva EM. 1976. Pauki Tajikistana. Dushanbe; p. 196. [in Russian].
- Andreeva EM, Tystshenko VP. 1968. Materials on the fauna of spiders (Aranei) of Tadjikistan. II. Zodariidae Zool Zh. 47:684–689. [in Russian].
- Jocqué R. 1991. A generic revision of the spider family Zodariidae (Araneae). Bull Am Mus Nat Hist. 201:1–160.
- Krivokhatsky VA, Fet V. 1981. The characteristics of distribution of spiders of Badkhyz in the spring period. Proc Acad Sci Turkmen SSR Ser Biol Sci. 1:45–51. [in Russian].
- Marusik YM, Koponen S. 2001. Spiders of the family Zodariidae from Mongolia (Arachnida: Araneae). Reichenbachia. 34:39–48.
- Mikhailov KG. 2021. Advances in the study of the spider fauna (Aranei) of Russia and adjacent regions: a 2017 update. Invertebr Zool. 18(1):25–35. doi:10.15298/invertzool.18.1.03.
- Mikhailov KG, Fet V. 1994. Fauna and zoogeography of spiders (Aranei) of Turkmenistan. In: Fet V, Atamuradov KI, editors. Biogeography and ecology of Turkmenistan. Dordrecht: Kluwer Academic Publisher; p. 499–524.
- Ovtsharenko VI, Fet V. 1980. Fauna and ecology of spiders (Aranei) of Badhyz (Turkmenian SSR). Entomol Obozr. 59(2):442–447. [in Russian].
- Řezáč M. 2008. Description of Harpactea sadistica n. sp. (Araneae: Dysderidae) - a haplogyne spider with reduced female genitalia. Zootaxa. 1698(1):65–68. doi:10.11646/zootaxa.1698.1.5.
- Řezáč M. 2009. The spider Harpactea sadistica: co-evolution of traumatic insemination and complex female genital morphology in spiders. Proc R Soc B. 276(1668):2697–2701. doi:10.1098/rspb.2009.0104.
- Shorthouse DP. 2010. SimpleMappr, an online tool to produce publication-quality point maps. [ accessed 2022 Apr 18]. http://www.simplemappr.net.
- Spassky SA. 1938. Araneae palaearcticae novae. II. Festschrift Embrik Strand. 4:573–582. [in Russian].
- Spassky S. 1952. Pauki Turanskoi zoogeograficheskoi provincii. Entomol Obozr. 32:192–205.
- WSC. 2022. World Spider Catalog. Version 23.0. Bern: Natural History Museum. [ accessed 2022 Jun 26]. http://wsc.nmbe.ch.
- Zamani A, Marusik YM. 2021. Revision of the spider family Zodariidae (Arachnida, Araneae) in Iran and Turkmenistan, with seventeen new species. ZooKeys. 1035:145–193. doi:10.3897/zookeys.1035.65767.
- Zamani A, Marusik YM. 2022. New taxonomic considerations in Zodariellum Andreeva & Tyshchenko, 1968 (Araneae: Zodariidae), with notes on the presence of cymbial diverticulum in different zodariid genera. Zootaxa. 5178(2):161–177. doi:10.11646/zootaxa.5178.2.3.