Abstract
For the purpose of supplying agricultural water, a stationary purification system for contaminated water had been developed on the basis of the liquid–solid settling technology using flocculants. Two kinds of flocculants had been developed on the basis of preliminary tests: one that compounds iron ferrocyanide and the other that does not. With the use of this system and flocculants, a demonstration test was conducted to apply the decontamination technology on contaminated water in two swimming pools in an elementary school located at Motomiya City, Fukushima Prefecture, Japan. It is proved from the results that both the developed purification system and the flocculants can be established as a practicable decontamination technology for contaminated water: the treatment rate was 10 m3/hour and the elimination factor of radioactive materials was higher than 99%.
1. Introduction
After the accident at Fukushima Dai-Ichi nuclear power plants (NPPs), Tokyo Electric Power Company (TEPCO), sea water was used as a water coolant and injected into the reactor cores and into cooling pools of spent fuels. It had been assumed that salt concentration levels in the injected sea water would gradually rise to higher levels by evaporation itself. Therefore, one of the authors (Aritomi) insisted on the substitution of sea water with fresh water as soon as possible in order to prevent the corrosion of reactor construction materials and deterioration of the heat transfer performance capabilities, caused by increased salt content from precipitation of cooling water. In addition to this, it had been deemed necessary to develop decontamination technology for sea water in order to be utilized continuously as a coolant of melted fuels, and other contaminated radioactive materials, as an apparent coolant to be recycled or “re-cleansed” of contamination.
The chemical system study group of the Research Laboratory for Nuclear Reactors, Tokyo Institute of Technology, Japan, initiated the development of “the decontamination technology for contaminated sea water”. They found that iron ferrocyanide can be applied to the adsorption of cesium (Cs) [Citation1]. Iron ferrocyanide is the major component of Prussian blue, which had been used as pigment since the Edo period in Japan, and it had been found to be available inexpensively. Prussian blue can be obtained as a dark blue sediment by adding an excessive amount of iron ions into the cyano complex of iron, and it can be used as paint, dye, printing ink, artist's paint, etc., in the indigo blue color. It has become apparent that the decontamination factor of 1/10,000 could be realized when iron ferrocyanide is placed in contaminated water, stimulated by nonradioactive Cs, with the resultant agitated mixture causing suspended solids to be separated by filter papers into a beaker [Citation2].
In cutting asphalt pavement surfaces, using exclusive cutters, the tooth of such tools are usually cooled continuously by coolant water in order to avert “burnout” from frictional heat and the scattering of dust. As the result of a chemical analysis of collected contaminated coolant water, after such cutting and from repair work sites of such asphalt pavement roads, it has become clear that contaminated water is found with a high-level pH of hydrogen ion concentration, considerable micro particulate cinders from asphalt pavement surfaces, and many chemical substances such as suspended solids, mineral oils, and so on. The authors have jointly developed a purification system for contaminated water. In this process of development, flocculants, so-called the “ion reaction,” had been developed, and the flocculating and settling technology for the micro particles floating in the contaminated water had been created [Citation3].
By organically combining the abovementioned two findings, the purification technology for contaminated water had been developed, and the mobile purification system, which has been remodeled from that of the model for contaminated water from cutting asphalt, and the stationary purification system for contaminated water, which is capable of being carried on a track, after disassembly and re-assembly on site, had been developed.
The abovementioned decontamination technology for contaminated water was adopted in the FY2011 “Decontamination Technology Demonstration Test Activity” by the Japan Atomic Energy Agency (JAEA) [Citation4]. Twenty-five decontamination technologies were adopted in the Project (“Demonstration Activity of Decontamination in the Evacuation Zones etc. Induced by Fukushima Dai-Ichi Nuclear Power Plant Accidents”) and there were these two decontamination technologies for contaminated water among them. Another technology was to absorb radioactive contaminants by artificial zeolite blocks. The demonstration tests were conducted by applying the developed stationary purification system for contaminated water and two kinds of flocculants for contaminated pool water in an elementary school, located at Motomiya City, Fukushima Prefecture, Japan. In this paper, the demonstration test and results are reported.
2. Development of stationary purification system for contaminated water
2.1 Stationary purification system for contaminated water
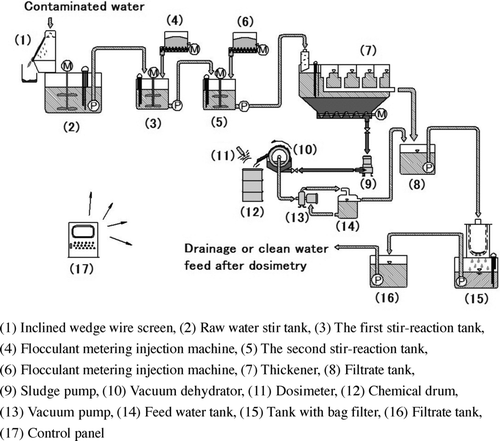
The stationary purification system for contaminated water had been developed by partially remodeling a stationary purification system developed for the treatment of turbid water generated from cutting asphalt pavement, as discussed in the previous introductory section. The system shown in is composed of an inclined wedge wire screen, a raw water stir tank, a stir reaction tank, a thickener, and a filtration tank. Contaminated pool water is pumped up to (1) an inclined wedge wire screen (a 0.4-mm stainless steel wedge wire screen), where varying sized particles, bigger than 0.4 mm in diameter are removed, and then they are injected into (2) a raw water stir tank that is equipped with a water level indicator. Upon injecting raw water into the tank, and concurrently, the water being stirred to prevent settling of suspended solids, the controlling equipment in the raw water tank signals that the water level has reached a certain level. Then, the water is sent to (3) the first stir reaction tank. As raw water feeding pumps are designed to effect injection of raw water into two sets of stir reaction tanks, utilizing electrically operated valves via the operation of either a single unit or two units, these pumps are able to operate continuously. Upon high water level being attained in the first stir reaction tank, the flocculant (0.1% by weight, adjusted according to the properties of the contaminated water) is injected through (4) the flocculant metering injection machine. After the water has been stirred continuously for about 5 minutes, it is pumped to (5) the second stir reaction tank. Similarly, the flocculant (0.1% by weight, adjusted according to properties of the contaminated water) is injected in the second stir reaction tank through (6) the flocculant metering injection machine. Then, the water is stirred continuously for about 5 minutes. It was revealed in these demonstration tests that these two stages can reduce the amount of flocculants inputted, and the amount of stirring time to less than that of a single stage during the development. This is the reason why two-stage stir reaction tanks are necessary. The treated water in the second stir reaction tank flows to (7) a thickener with a pump that is able to produce fluid flow without disturbing the condition of the aggregates. The treated water in this thickener is separated into solid and liquid components, because of the difference in specific gravities between water and aggregates. The clear supernatant water shall be contained first in (8) the filtration tank, then transferred to (15) a tank with a bag filter of 10 μm through a pump that can produce fluid flow; the water with suspended solids is filtered there, and subsequently, the water is pumped to a purification water tank that has a capacity of 12 m3. The water is contained there in the tank until the time when it can be verified that its measured radioactivity by a dosimeter meets the effluent standard. The water can then be released, after verification that it has satisfied the standard by an inspection. The aggregates, settled at the bottom of (7) the thickener, as concentrated and contaminated mud, with the portion of water being approximately 80–90%, are pumped to (10) the solid–liquid separator, so-called “Oliver-type vacuum dehydrator” by (9) a pump capable of fluid migration without disturbing properties of the aggregates. After discriminating solid and liquid components by the separator, solidification would be effected into the following two types of solids: organic bodies, such as algae, with a solid component and water content about 50–60%, and inorganic bodies, such as silt, with a solid component and water content about 30–40%. Since this dehydrated sludge is radioactive, it would be contained and stored in (12) a stainless steel drum for chemicals, after being placed in an excellent impermeable bag, after measurement of its radioactivity by (11) a dosimeter. The water, filtrated by the vacuum dehydrator, is pumped to (14) the feed water tank to be used as priming water, and subsequently, the overflow water would be transferred to (8) the filtrate tank, and then to the tank with a bag filter through a pump capable of fluid migration. A control board (17) controls the whole system.
This system is responsive to a wide range of radio density and water quality by adjusting the contents and compound ratio of the flocculants.
The installed condition of the system is as shown in . This system is capable of running continuously by operation of one of the two stir-reaction tanks, alternating between the two sets of tanks. By such alternating operations, efficiency is improved and the amount of contaminated water at 10 m3/hour is made possible. The treatment amount of this system is at the rated operation of 10 m3/hour, and its optimization can be improved by adjusting the contents of the flocculants and the stirring time of the tank.
The power consumption of this system is very low and is operational with an electric generator of 45 kVA; it is operational without an external electric source. Although the system used in the demonstration tests had been manufactured with carbon steel, if the construction material of system is changed to SUS304 instead of carbon steel, its durability would be improved, and the time required for maintenance could be reduced considerably.
2.2 Flocculants
In this paper, “flocculant” means a powder that has flocculating and precipitating functions when put in water mixed with suspended solids.
The flocculants used in this study are a mixture of several powder blends: about 30wt% aluminum sulfate, about 18wt% silica sand, about 12wt% calcium oxide, about 5wt% activated carbon, and 35wt% others, and are based upon the ion reaction N developed previously. The composition and compound ratio are different dependent upon the conditions of the contaminated water to be purified. Flocculants are excellent in adsorbing the suspended solids in water, and have the characteristics to re-aggregate in the case where the aggregates dissipate. As they are inorganic agents, they have the characteristics of being less subject to radiation damage and to re-elution in the adsorbed substances. In addition, flocculants have very little variation in pH and show alkalinity. For this reason, it is not necessary to neutralize the water's pH at the time when the treated water is released. The effective applicable range of flocculants to water quality is so wide that flocculants’ capacity to maintain aggregation efficacy in all kinds of water in general in the environment is applicable even to sea water.
In the demonstration test, a system had been developed with the purpose of capturing both the ionized radioactive Cs and the Cs existing in the suspended solids by compounding iron ferrocyanide that selectively adsorbs Cs. This reaction, is called as “ion reaction NF” by compounding ion ferrocyanide. Since the preliminary test, the quantity of compounding iron ferrocyanide had been set at 20% by weight. Where the radioactive Cs did not ionize in water by absorbing blue-green algae or by adhering to fine soil, the “ion reaction N,” whereby the iron ferrocyanide is not compounded, had also been used.
3. Demonstration test procedures
3.1 Preliminary experiment
The preliminary experiment had been implemented using pool water that was to be decontaminated. By placing 200mL of pool water in a beaker, the most suitable additive amount was sought by visual confirmation of the flocculating and settling situation, and 0.05%, 0.1%, and 0.2% of the two kinds of flocculants (one that compounded with iron ferrocyanide, and the other that did not) were added. It had been confirmed that radioactive materials had been removed by analyzing the radionuclide concentration in the pool water – as a suspected liquid, flocculated with the most suitable amount and filtered. The measurement of the radionuclide concentration and the radionuclide analysis were conducted by a germanium semiconductor detector. Additionally, in the case of the flocculant that compounded with iron ferrocyanide, the amount of elution in the suspected liquid was also analyzed.
In the preliminary test, the elimination factor of radioactive materials was higher than 99%, as shown in the results of the preliminary test in . In the case of the flocculant that did not compound iron ferrocyanide, 10.3 Bq/L of 134Cs was detected, but neither a trace of water-soluble Cs existed nor it could be determined whether it fell within the detection limit value variation. Furthermore, the release of cyanogens from the iron ferrocyanide had been confirmed.
Table 1 Results of preliminary tests
3.2 Demonstration test at the site
For pool water of about 300 m3 at an elementary school in Motomiya City, the elimination test of radioactive materials had been conducted utilizing the flocculant that compounded the iron ferrocyanide of 20% in ratio by weight (ion reaction NF).
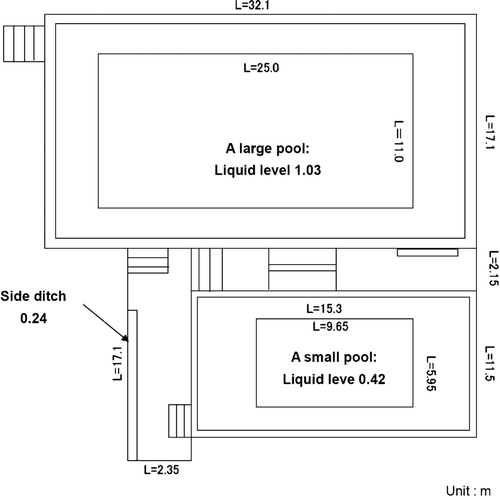
The treatment plant used the stationary purification system for contaminated water described above. The targets of the decontamination work were both large and small pool water, as shown in .
The operation procedures of the purification system for contaminated water in the demonstration test are as follows:
| 1. | To add 0.1% by weight of the flocculant to raw water in which contaminants were coarsely removed by an inclined wedge wired screen in the first and second stir reaction tanks, respectively; | ||||
| 2. | then, to stir them in their respective tanks for 5 minutes, and to adsorb and flocculate radioactive materials in the water; | ||||
| 3. | after that, to pump the water to the thickener, and to precipitate the aggregate by the difference in specific gravities between the water and the aggregate; and | ||||
| 4. | finally, to filter the clear upper portion by a bag filter of 10 μm. | ||||
This filtrated water is the purified one. The treated water (about 10 m3) was impounded in the storage tanks, and the radionuclide concentration in the water in each tank was measured. In addition, a part of the treated water was re-used as washing water for the high-pressure washing, which will be reported in a series of this paper.
The drainage process of the treated water, after the removal of radioactive materials, had been conducted by confirming the effluent conditions with Fukushima Prefecture by obtaining the consent of local water-rights owners and by submitting measurement results for radionuclide and cyanogenic compound concentration levels in the treated water.
As contaminants, which has been generated in purifying contaminated water and coarsely removed from the water, and the sludge at the bottom of the thickener are radioactive, all of such substances were stored in stainless steel chemical drums.
4. Results of the demonstration test and considerations
The radionuclide concentration in raw water, treated in the demonstration test, was in the range of 34–1116 Bq/L, which was a total amount of 134Cs and 137Cs, as presented in Table and . It increased as the treatment of the pool water progressed from the upper surface to the bottom and as the amount of suspended solids increased. The reason of such increased radionuclide concentration levels is that most of such suspended solids was algae, such as blue-green algae, and it absorbed radioactive Cs.
The decontaminated water (about 10 m3) was stored in a filtrate tank and its radionuclide concentration was measured to obtain permission for drainage. The results for the treated clean water are as follows: The radionuclide concentration levels in all 25 samples were below the measurement limit value. The elimination factor of radioactive materials was higher than 99%. The measurement of radionuclide concentrations had been conducted by a germanium semiconductor detector, and its measurement time was 2000 seconds. The measured limit value was almost below 10 Bq/L. Two kinds of flocculants were used: one that compounded iron ferrocyanide and the other that did not. As the radionuclide concentrations were below the measurement limit value using the two kinds of flocculants, it has been confirmed that the pool water under the demonstration test contained less ionized Cs: Radioactive Cs was either absorbed by algae, such as green-blue algae, or adhered to finely grained soil suspended at the bottom of the pool, and therefore, it was not in an ionized state in the pool water.
When the cyanogen complex is used as an adsorbent of radioactive Cs, the liberation of cyanogens is a pending problem. In the case where the iron ferrocyanide compounding ratio was 20%, its content did not exceed the drainable standard of 0.5 mg/L, as shown in . Its highest concentration, however, reached 0.44 mg/L, and it is difficult to increase the compounding ratio of iron ferrocyanide more than 20%.
Table 2 Radionuclide concentration in raw water (unit: Bq/L)
Table 3 Cyanogen solubility in cleaned water (unit: mg/L)
As the ionized Cs in the environmental water is extremely small amount, it is considered that the iron ferrocyanide compounding ratio 20% is excessive. To resolve this problem, it is necessary to conduct a simple Cs removal test on the contaminated water to be decontaminated. The suitable compounding ratio could be determined from the results of a simple test. From the abovementioned results of the demonstration test, the operation results of the stationary purification system for contaminated water are summarized in .
Table 4 Treatment performance of the purification system for contaminated water
It is believed that the radionuclides released due to accident at nuclear power plants are of a wide variety. Most of the radioactive contamination is from iodine (I) and cerium (Ce), which are comparably easy to diffuse, and it is an undeniable fact that the other radionuclides do also scatter. In the demonstration test, an analysis had been applied to cobalt (Co) and manganese (Mg), which are easy to measure given the time constraint. The result of the analysis is presented in . From this, it was not deemed necessary to consider I nuclides, undergoing their half-lives undetected. Cs nuclides were clearly the intended target when conducting decontamination activity at Motomiya City. Thus, the decontamination technology utilizing iron ferrocyanide as an adsorbent was sufficient.
Table 5 Measurement results for 60Co and 54Mn (unit: Bq/L)
As to 90St, it has not been analyzed, given time constraints, and the difficulty in detecting its radioactivity due to its beta decay; this is left for future tasks for resolution.
The sediments, flocculated by the treatment of contaminated water, are extracted from the bottom portion of the thickener and form the dehydrated sludge. Where the radionuclide concentration levels in the dehydrated sludge are below 8000 Bq/kg, it would be possible to landfill at the final disposal site. From this demonstration test, even with radionuclide concentration levels in contaminated water being as low as 34–1116 Bq/L, the resultant dehydrated sludge, which had been concentrated, shows radionuclide concentration levels of 29,100–77,200 Bq/kg, as shown in , and this makes it impossible to landfill.
Table 6 Radionuclide concentration in dehydrated sludge (unit: Bq/kg)
In addition, the results of dissolution tests of cyanogenic compounds, based on waste elution tests as per the Environment Agency's announcement no. 13 in FY 1977, are presented in . According to the table, 20% of the iron ferrocyanide was contained, and the amount of elution of cyanogenic compounds would be 67 mg/L, substantially exceeding the elution standard of 1 mg/L, and thus becoming a “specially controlled waste”. Therefore, additional testing was conducted on ion reaction FN using pool water, where compounding rates of iron ferrocyanide were 0.2% and 0.01%, respectively, and rates of dissolution of cyanogenic compounds in the dehydrated sludge were examined.
Table 7 Elution test results for ferrocyanic compounds in dehydrated sludge
The result is presented in Table . Even by reducing the compounding rate to 0.01%, the rate of dissolution of cyanogenic compounds becomes 1.4 mg/L and still exceeds the elution standard. From these results aforementioned, it is necessary to use the ion reaction FN for the decontamination of contaminated water when the ionized radioactive Cs does exist in the water. In such a case, it is necessary to establish the technology to eliminate the cyanogenic component in the purified water and the technology to dissolve the cyanogenic component in the dehydrated sludge.
Measurements on the safety of the dehydrated sludge have been attempted. Where radioactive materials were re-eluted, methods of storage such as total and complete water shielding should be explored. Processes have been conducted to add the dehydrated sludge of one-tenth (1/10) in ratio by weight to distilled water and to vibrate the mixture for 6 hours. Subsequently, the suspect liquid was determined, which was obtained by filtering the supernatant liquid with membrane filters of 0.45 μm. The suspect liquid was measured by a germanium semiconductor detector for 2000 seconds. A Marinelli beaker was used as the measuring vessel. As a result, the re-elution of Cs was not observed, as shown in . The long-term changes in the characteristics of the dehydrated sludge were not yet clear, but it was found that short-term stability was secured. From this, it can be concluded that there are no problems in storing the dehydrated sludge in impermeable bags and chemical drums for the time being, at least.
Table 8 Elution test results for dehydrated sludge (unit: Bq/L)
5. Conclusions
The followings are clarified from this demonstration test:
| 1. | The stationary purification system for contaminated water successfully achieved the processing capacity of 10 m3/hour of contaminated water. | ||||
| 2. | In preliminary tests, flocculants compounding iron ferrocyanide (ion reaction NF) and not compounding (ion reaction N), suitable for the decontamination of contaminated pool water, were prepared and applied to the purification system for contaminated water. As a result, the radionuclide concentration level in the pool water of about 300 m3 decreased to a level below the measured limit value (10 Bq/L) and visibility through the treated water increased by more than 1 m. | ||||
| 3. | Even with the use of the flocculant that did not compound iron ferrocyanide (ion reaction N), the contaminated pool water was decontaminated below the measured limit value. After 9 months since the accident of the Fukushima Dai-Ichi NPPs, it could now be determined that not only Cs in pool water had been adsorbed onto green-blue algae or had adhered to fine-grained soil but also ionized Cs in the impounded pool water was nearly nonexistent. | ||||
| 4. | When the flocculant that compunded iron ferrocyanide (ion reaction NF) was used, cyanogens were found in the treated water after decontamination of the contaminated water. However, the compounding ratio of iron ferrocyanide decreased to less than 20%; such flocculant was able to reduce the concentration of cyanogens below the drainable standard (0.5 mg/L). | ||||
| 5. | In the case of pool water, it is important to control the radionuclide concentration level in raw water for the treatment of contaminated water, because the radionuclide concentration level in the upper part of the pool is not relatively high. However, the radionuclide concentration level in suspended solids, settling to the lower part and forming sludge at the bottom, is relatively high. | ||||
| 6. | The retention capacity of the contaminated water to retain Cs in the dehydrated sludge, after separation of solids and liquids, is high. As for cyanogens, if the compounding ratio of iron ferrocyanide is set at 0.2% by weight, then cyanogenic concentration in the elutes can be reduced almost to the standard level. | ||||
From the abovementioned conclusions, it is proved that the developed stationary purification system for contaminated water using flocculants can be established as a practicable one.
Acknowledgements
This study has been adopted and carried out as the “Decontamination Technology Demonstration Test Activity” in FY 2011 conducted by the Japan Atomic Energy Agency (JAEA) as a part of the project “Demonstration Activity of Decontamination in the Evacuation Zones etc. Induced by Fukushima Dai-Ichi Nuclear Power Plant Accidents” commissioned by the Cabinet Office.
Notes
Note: ND means nondetection.
Note: Values within parentheses represent detection limit value.
Note: Values within parentheses represent detection limit value.
Note: Values within parentheses represent detection limit value.
Note: Detection limit value: 0.1 mg/L.
Note: Values within parentheses represent detection limit value.
References
- Benes , J and Kyrs , M . 1963 . The isolation of caesium-137 from liquid radioactive fall-out . Anal Chim Acia , 29 : 564 – 568 .
- Takeshita , K and Ogata , T . 2012 . Application of ion exchange technique to decontamination of polluted water generated by Fukushima nuclear disaster . J Ion Exchange , 23 : 1 – 5 . [in Japanese].
- Takanashi , J , Hosobuchi , S and Aritomi , M . 2009 . Study on water treatment system for cutting asphalt road (1st report, Development of water treatment system for cutting asphalt road) . Trans. JSME , 75B : 2504 – 2510 . in Japanese
- JAEA . 2012 . Report of the results of the decontamination model projects Internet http://www.jaea.go.jp/fukushima/decon04/english/3-2%20Decontamination_Technology_Demonstration_Test_Project-2.pdf