Abstract
The sorption behavior of selenium onto iron-containing minerals such as goethite, ferrous oxide, magnetite, and biotite under reducing conditions were investigated by batch sorption experiments. Selenium was spiked as HSe− and Se42− in the experimental solutions and reducing conditions were maintained throughout the sorption periods. The sorption behaviors of HSe− were analyzed by the triple-layer surface complexation model with the Visual Minteq computer program. The sorption data and the model calculations suggested that the dominant sorption mechanisms of HSe− were inner-sphere surface complexation for goethite and ferrous oxide but outer-sphere surface complexation for magnetite and biotite. The intrinsic equilibrium constant of the inner-sphere surface complexation reaction of HSe− with goethite, logKintHSe = 6.0, was determined by the fitting to the experimental results. The value of the equilibrium constant for outer-sphere complexation for magnetite and biotite was estimated to be around 12.
1. Introduction
Retardation of radionuclide migration by sorption onto a host rock in natural barrier systems is one of the important factors that influence the performance of a radioactive waste disposal system. Performance assessment calculations for hypothetical high-level radioactive waste (HLW) repositories [Citation1] show that 79Se is one of the radionuclides that dominate the long-term radiological hazard. The oxidation state of Se varies from selenide [Se(−II)] to selenite [Se(IV)] and selenate [Se(VI)], depending on the redox conditions of the groundwater [Citation2]. Selenium is likely to be stable as Se(−II) under reducing conditions in deep subsurface environments, and hydroselenide, HSe−, is considered to be dominant species in groundwater [Citation1,2].
Iron-containing minerals are important sorbents of Se in rocks and soils [Citation3–10]. A considerable amount of sorption data of Se(IV) and Se(VI) onto iron-containing minerals such as goethite [Citation3,4,Citation6,Citation11–20], hematite [Citation5,6,Citation13,Citation19,Citation21], magnetite [Citation21–27], ferric oxides/oxyhydroxides [Citation12,Citation18,Citation28,29], pyrite [Citation3], biotite [Citation3], and chlorite [Citation3,Citation13] has been obtained. Both Se(IV) and Se(VI) are sorbed onto iron-containing minerals, but the sorbability of Se(IV) is generally higher than that of Se(VI). This is due to differences in the nature of their respective surface complexes. Extended X-ray absorption fine-structure (EXAFS) studies [Citation4,Citation30,Citation31] showed that Se(IV) formed a strongly bonded inner-sphere complex, whereas Se(VI) formed a mixture of outer- and inner-sphere surface complexes on ferric oxyhydroxides.
The sorption behaviors of Se(IV) and Se(VI) species onto iron-containing minerals have been analyzed by surface complexation models [Citation32–34] such as the diffuse-layer model (DLM) [Citation34–36], the constant-capacitance model (CCM) [Citation37–42], and the triple-layer model (TLM) [Citation12,Citation28,Citation30,Citation43–48]. Hayes et al. [Citation12] investigated the sorption behaviors of Se(IV) and Se(VI) onto goethite and hydrous ferric oxide (HFO) in sodium nitrate solutions by using the TLM. They found that the sorption behavior of Se(IV) was independent of the ionic strength and could be explained by inner-sphere complexation with a ferrol site (≡FeSeO3− and ≡FeSeO3−−Na+). On the other hand, the sorption of Se(VI) depending on ionic strength was explained as outer-sphere complexation (≡FeOH2+−SeO42− and ≡FeOH2+−HSeO4−). Zhang and Sparks [Citation14] analyzed the sorption phenomena of Se(IV) and Se(VI) onto goethite by using the TLM. The sorption of Se(IV) was explained as inner-sphere complexation (≡FeHSeO30 and ≡FeSeO3−) and Se(VI) was explained as outer-sphere complexation (≡FeOH2+−SeO42−). Using the sorption data from Hayes et al. [Citation12] and Zhang and Sparks [Citation14], Goldberg [Citation49] evaluated the ability of the TLM to describe the adsorption of Se(IV) and Se(VI) onto goethite, and the adsorption was found to be sensitively dependent on the surface site densities. Duc et al. [Citation5] investigated the sorption of Se(IV) onto goethite and hematite by the CCM. It was reported that goethite and hematite, which have different acid–base properties, exhibit very similar sorption properties for Se(IV). The monodentate complexes ≡FeOSe(O)OH and ≡FeOSe(O)O− were assumed in the CCM analysis. Duc et al. [Citation6] assumed a bidentate complex (≡FeO)2SeO for the CCM calculation for the sorption of Se(IV) onto hematite. Fukushi and Sverjensky [Citation50] demonstrated the applicability of an extended TLM, which took into account the effect the electrostatics of water dipole desorption had on the measurements reported for the sorption of Se(VI) onto goethite by Rietra et al. [Citation16,17] and Hayes et al. [Citation12]. The sorption behaviors of Se(VI) were explained by a mixture of outer- and inner-sphere surface complexes [≡FeOH2+−SeO42−, (≡FeOH2+)2−SeO42−, and ≡FeOSeO3−]. Shibutani et al. [Citation3] obtained sorption data of Se(IV) onto goethite and biotite and analyzed them using the DLM. By assuming the surface site of biotite to be ≡FeOH, the model calculations showed good agreement with experimental results. Martinez et al. [Citation22] modeled the variation of the sorption of Se(IV) and Se(VI) onto magnetite as a function of pH by the TLM. The best fitting was obtained for Se(IV) by assuming two inner-sphere complexes (≡FeOHSeO32− and ≡FeHSeO30), while assuming an outer-sphere complex (≡FeOH2+−SeO42−) for Se(VI). Missana et al. [Citation25], Jordan et al. [Citation26], and Kim et al. [Citation27] investigated the sorption of Se(IV) onto magnetite by the diffuse double-layer model (DDLM). They supposed that Se(IV) sorbed onto magnetite by forming an inner-sphere monodentate species such as ≡FeOSeO2−. Goldberg [Citation51] investigated the adsorption behavior of Se(IV) on amorphous Fe oxides using the TLM. The dominant surface species predicted by the TLM were an inner-sphere complex (≡FeHSeO30) at pH < 9 and an outer-sphere complex (≡FeOH2+−SeO32−) at pH > 10.
In contrast to Se(IV) and Se(VI), the data of Se(−II) are scarce [Citation9,Citation13,Citation52] and the sorption mechanisms of Se(−II) are poorly understood. Although the exact speciation of selenium in the vitrified HLW waste is unclear, it is estimated as Se(IV) or Se(VI) because selenium is exposed to oxidizing conditions during the reprocessing of spent fuel, and SeO32− and SeO42− can substitute trigonal borate or tetrahedral silicate entities, respectively, in the glass network [Citation53–56]. Selenium had been considered to migrate as soluble SeO32−/SeO42− because of its slow reduction kinetics [Citation53–56], in spite of thermodynamic stability of Se(−II) under reducing disposal conditions. Recent works have demonstrated the abiotic reduction of Se(IV) and Se(VI) to Se(0) by several Fe(II)-bearing minerals [Citation24,Citation55–62] and to Se(−II) by Fe(0) nanoparticles [Citation63]. Understanding the sorption behavior of Se(−II) species is important; however, the sorption data of Se(−II) are scarce because of the experimental difficulties of maintaining the reducing state of Se. In the long term, the sorption behavior of Se(−II) would change because the composition of groundwater is likely to be changed by intrusion of saline groundwater for coastal repositories and alkaline groundwater induced by cementitious materials. For a long-term evaluation of the sorption of Se(−II), it is necessary to understand the sorption mechanisms, such as inner-sphere surface complexation and outer-sphere surface complexation.
In the present study, sorption data of Se(−II) onto iron-containing minerals, magnetite, goethite, ferrous oxide, and biotite were obtained by batch sorption experiments under reducing conditions. The sorption behaviors of Se(−II) were analyzed by the TLM with the Visual Minteq computer program. Sorption experiments for Se(IV) were also carried out under anaerobic conditions and the sorption behaviors were reproduced by the TLM to confirm the validity of this experimental method and surface parameters of the employed iron-containing minerals.
2. Materials
2.1. Iron-containing minerals
Goethite (α–Fe(III)OOH) and magnetite (Fe(II, III)3O4) samples were purchased from Rare Metallic Co. Ltd. Ferrous oxide (Fe(II)O) and biotite [K(Mg, Fe(II))3(Al, Fe(III))Si3O10(OH, F)2] were purchased from Nichika Inc. and Soekawa Chemical Co. Ltd., respectively. All of the mineral samples were in granulated form and used without pretreatment. For a determination of specific surface area of granulated samples, the air-permeability method and the gas adsorption method are generally used [Citation64]. The gas adsorption method is used more widely and can determine the total surface area including cracks and micro pores [Citation65]. By the reasons, the specific surface areas of employed minerals were measured by the Brunauer–Emmett–Teller gas adsorption method [Citation66] which is widely used and useful [Citation34]. The measured specific surface areas were 17.6 m2 g−1 (goethite), 1.6 m2 g−1 (magnetite), 0.2 m2 g−1 (ferrous oxide), and 4.6 m2 g−1 (biotite).
2.2. Selenium stock solution
A Se(−II) stock solution was prepared in a controlled-atmosphere glove box under Ar (O2 < 1 ppm) by the following procedure. A solution of 75Se (4 MBq cm−3, carrier: 100 μg cm−3 Na2SeO3) was purchased from GE Healthcare Limited. A 0.5 cm3 volume of a 98% aqueous solution of hydrazine monohydrate (N2H4·H2O) was mixed with a 0.5 cm3 volume of the 75Se solution in a polypropylene test tube and the mixture was stored for seven days to reduce Se(IV) to Se(−II) [Citation9]. The mixture was the diluted with distilled deionized water to 10 cm3. The solution was filtered through a 10,000 nominal molecular weight limit (NMWL) ultrafilter (USY–1, Toyo Roshi Kaisha Ltd.) to remove the precipitated fraction of Se. The filtrate was sampled to analyze the aqueous Se species by UV-vis spectrometry, which confirmed that Se was dissolved as a stable species (HSe− and Se42−) under reducing conditions [Citation9].
A Se(IV) stock solution was prepared by diluting 0.5 cm3 volume of the 75Se solution to 10 cm3 with distilled deionized water.
3. Sorption experiments
The experimental runs were made following the procedure of the “Measurement Method of the Distribution Coefficient on the Sorption Process” compiled by the Atomic Energy Society of Japan [Citation67]. Batch sorption experiments for Se(−II) were performed in a controlled-atmosphere glove box under Ar at 25°C ± 1°C. A 0.5 cm3 volume of 98% N2H4·H2O solution was added to a 1000 cm3 volume of 0.01, 0.1, and 1 mol dm−3 sodium chloride (NaCl) solution. The concentration of N2H4·H2O was 0.01 mol dm−3. A 0.2 cm3 volume of Se(−II) stock solution was spiked into the NaCl solution. By filtering this solution through a 0.45 μm filter, experimental Se(−II) solutions (Se: 1.3 × 10−8 mol dm−3) were prepared. A 1 cm3 aliquot was sampled and the radioactivity of the solution was measured by γ-spectrometry (LOAX-51370/20-P, SEIKO EG&G) with a peak at 0.136 MeV to determine the initial concentration of Se.
Prior to the sorption runs, blank tests were carried out to check for precipitation of Se(−II). A 10 cm3 volume of the Se(−II) solution was poured into a polypropylene test tube. A 2 cm3 aliquot was sampled and filtered through a 10,000 NMWL ultrafilter preconditioned with a small amount of the sample solution. The filtrate was sampled (1 cm3) and its radioactivity was measured by γ-spectrometry. The Se concentration decreased from the initial concentration at pH < 7 but it did not decrease at pH > 7.
A mineral sample (1 g) was immersed in a 10 cm3 volume of the Se(−II) solution in a polypropylene test tube. For goethite, which has a large surface area, sorption runs using 0.1 g of the solid phase were added. The pH of the sample suspension was adjusted to 7–13 with sodium hydroxide (NaOH) solution or hydrochloric acid (HCl). The sample suspensions were agitated once a day. After two weeks, the pH, redox potential (Eh), and radioactivity of the solutions were measured. The pH was measured with a Sure-Flow combination glass electrode (ROSS 8172BNWP, Thermo Fisher Scientific Inc.), which is suitable for the measurement of high-ionic-strength and high-pH samples. The electrode was calibrated with standard pH buffer solutions of 7.00, 10.01, and 12.46. Because of the difference in activity coefficients between the standard buffers and the high-ionic-strength solutions, the observed pH value (pHobs) can shift from actual pH (−log aH+). The difference between pHobs and −log aH+ was determined by measuring the pHobs of NaCl/HCl and NaCl/NaOH solutions of known H+ and OH− concentrations [Citation68]. The Eh was determined against NHE using a platinum electrode combined with an Ag/AgCl reference electrode (ROSS 9180BNMD, Thermo Fisher Scientific Inc.) after checking its accuracy with saturated quinhydrone solutions. A 2 cm3 aliquot was sampled and filtered through a 10,000 NMWL ultrafilter preconditioned with a small amount of the sample solution. The filtrate was sampled (1 cm3) and the radioactivity of the solution was measured by γ-spectrometry to determine the equilibrated concentration of Se.
Batch sorption experiments for Se(IV) were also performed in the controlled-atmosphere glove box under Ar. The starting solution was prepared by adding a 0.2 cm3 volume of the Se(IV) stock solution to a 1000 cm3 volume of the NaCl solution, so the concentration of Se(IV) was 1.3 × 10−8 mol dm−3. Batch sorption runs for Se(IV) were performed in the same manner as those for Se(−II). The Eh of the Se(IV) solution without a redox buffer was not measured.
4. Results and discussion
4.1. Experimental results
4.1.1. Sorption behavior of Se(IV)
The sorption ratio, Rs (%), and the distribution coefficient, Kd (m3 kg−1), were calculated using the following equations, respectively:
(1)
(2) where cini is the initial concentration of Se (mol dm−3), ceq the equilibrated concentration of Se (mol dm−3), Vini the initial volume of the solution (m3), and M the weight of the solid phase (kg). The obtained pH, Rs, and Kd values are listed in –. The dominant Se(IV) species under the experimental conditions are HSeO3− or SeO32− () dependent on pH:
(3)
Table 1. Experimental variables and data for Se(IV) sorption on goethite.
Table 2. Experimental variables and data for Se(IV) sorption on ferrous oxide.
Table 3. Experimental variables and data for Se(IV) sorption on magnetite.
Table 4. Experimental variables and data for Se(IV) sorption on biotite.
Figure 1. Comparison of Kd values for Se(IV) obtained in this study (open marks) and previously reported ones (closed marks) for (a) goethite [Citation3,Citation12,Citation13,Citation15], (b) ferrous oxide, (c) magnetite [Citation21,Citation27], and (d) biotite [Citation3]. “0.01 M” represents the 0.01 mol dm−3 NaCl concentration. The previous data for ferrous oxide are not available.
![Figure 1. Comparison of Kd values for Se(IV) obtained in this study (open marks) and previously reported ones (closed marks) for (a) goethite [Citation3,Citation12,Citation13,Citation15], (b) ferrous oxide, (c) magnetite [Citation21,Citation27], and (d) biotite [Citation3]. “0.01 M” represents the 0.01 mol dm−3 NaCl concentration. The previous data for ferrous oxide are not available.](/cms/asset/35cb5fc7-31d5-41ee-a8c3-e07fec840572/tnst_a_864457_f0001_b.gif)
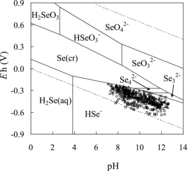
Figure 2. Experimental conditions of equilibrated solutions in a pH–Eh diagram for the H–O–Se system under standard conditions. The total concentration of Se is 10−8 mol dm−3. The circles, squares, triangles, and asterisks represent the conditions for the sorption experiments using goethite, ferrous oxide, magnetite, and biotite, respectively.
The uncertainty of Kd was often larger than the most probable value under the conditions that the Rs was higher than 99% or lower than about 10%. For such data, the maximum or minimum values were shown in the tables.
The Rs values for goethite were almost 100% at pH < 10 and decreased to a few percent at around pH 12 (). In , the obtained and previously reported [Citation3,Citation12,Citation13,Citation15] Kd values for goethite are plotted versus pH; the obtained Kd values agree with the previous ones. The Kd values show negative dependences on pH and are independent of the NaCl concentration. The sorption tendency was also in agreement with the previous one [Citation12]. The dominant sorption mechanism of Se(IV) onto goethite has been reported to be inner-sphere surface complexation [Citation12,Citation52,Citation69]. The results of the current study can also be explained by inner-sphere surface complexation.
The Rs values for ferrous oxide were almost 100% at pH < 10 and decreased to a few percent at around pH 11 (). The obtained Kd values for ferrous oxide are plotted in . It was found that the Kd values were independent of the ionic strength. Although previous sorption data for ferrous oxide cannot be found, the pH dependences of Kd values for ferrous oxide show the same tendency as those for goethite. The dominant sorption mechanism of Se(IV) onto ferrous oxide can be estimated to be inner-sphere surface complexation from similarities of pH and ionic strength dependence of Kd to those for goethite. The difference in the sorption properties between Fe(II) and Fe(III) oxides was not significant in this experiment.
The Rs values for magnetite were almost 100% at pH < 9 and decreased to a few percent around pH 11(). The obtained and previously reported [Citation21,Citation27] Kd values for magnetite are plotted versus pH in . The Kd values were independent of the ionic strength. The obtained data were in agreement with those from Kim et al. [Citation27] obtained in Ar-filled glove box. On the other hand, the data from Fujikawa and Fukui [Citation21] are more than one order of magnitude lower than the data obtained in this study. The lower Kd values are due to the oxidation of Se(IV) to low adsorbable Se(VI) in their experiments conducted under the atmospheric conditions [Citation21]. The dominant sorption mechanism of Se(IV) onto magnetite has been reported to be inner-sphere surface complexation [Citation22,Citation27]. The results of the current study can also be explained by inner-sphere surface complexation.
The Rs values for biotite were almost 100% at pH 7 and decreased to a few percent at around pH 10 (). The obtained and previously reported [Citation3] Kd values for biotite are plotted versus pH in . The Kd values were independent of the ionic strength. The pH dependences of the Kd values for biotite are similar to those obtained by Shibutani et al. [Citation3]. Although the sorption mechanism of Se(IV) onto biotite has not been reported, it can be estimated to be inner-sphere surface complexation from the similarities of pH and the ionic strength dependence of Kd to those for goethite and magnetite.
4.1.2. Sorption behavior of Se (−II)
Experimental data from the sorption experiments are summarized in –. The experimental conditions for Se(−II) are plotted on a pH−Eh diagram for the system H−O−Se [Citation2] and shown in . Based on the pH and Eh of the equilibrated experimental solutions in all of the experimental runs, the dominant Se(−II) species in the experimental solution was estimated to be HSe−.
Table 5. Experimental variables and data for Se(−II) sorption on goethite (using 0.1 g of the solid phase).
Table 6. Experimental variables and data for Se(−II) sorption on goethite (using 1 g of the solid phase).
Table 7. Experimental variables and data for Se(−II) sorption on ferrous oxide.
Table 8. Experimental variables and data for Se(−II) sorption on magnetite.
Table 9. Experimental variables and data for Se(−II) sorption on biotite.
The Rs values for goethite were almost 100% at pH < 10 and decreased to 30% at pH 12.5 in the experimental runs using 0.1 g of the solid phase (). The Rs values were almost 100% at pH < 11 and decreased to 70% at pH 12.5 in the experimental runs using 1 g of the solid phase (). The obtained Kd values for goethite – from 2.0 × 10−2 to 38 m3 kg−1 – are plotted versus pH in . The Kd values obtained from the experiments using 1 g of the solid phase were consistent with those obtained using 0.1 g of the solid phase.
The Rs values for ferrous oxide were almost 100% at pH < 9.5 and decreased to a few percent at pH 12 (). The obtained Kd values for ferrous oxide, 5.4 × 10−4 to 1.1 m3 kg−1, are plotted versus pH in . The influence of the NaCl concentration on the sorption was not significant. The Kd values for ferrous oxide were approximately 2 orders of magnitude lower than those for goethite. The difference in Kd is likely to be due to the difference in the specific surface areas (17.6 m2 g−1 for goethite and 0.2 m2 g−1 for ferrous oxide). The pH dependences of Kd values for ferrous oxide show the same tendencies as those for goethite.
The Rs values for magnetite were almost 80% at pH 7 and gradually decreased to 10% at pH 12 (). The obtained Kd values for magnetite are plotted versus pH in . The variation in Kd was not systematically related to the ionic strength. The Kd values of Se(−II) for magnetite, 6.8 × 10−4 to 5.6 × 10−2 m3 kg−1, are 1–3 orders of magnitude lower than those for goethite, and the pH dependences of the Kd values for magnetite are also lower than those for goethite.
The Rs values for biotite were almost 90% at pH 8 and gradually decreased to 30% at pH 12.5 (). The obtained Kd values for biotite, 2.7 × 10−3 to 1.3 × 10−1 m3 kg−1, versus pH are plotted in . The pH dependences of the Kd values of Se(−II) for biotite show the same tendencies as those for magnetite.
4.2. Analysis of the sorption behavior by the TLM
4.2.1. Sorption behavior of Se (IV)
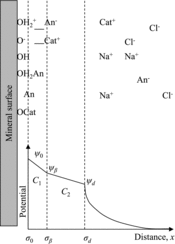
The sorption behaviors of Se(IV) were analyzed by the TLM. shows a schematic representation of the TLM. The protonation and deprotonation reactions of ≡FeOH can be expressed by
(4)
(5)
Figure 3. The Kd values for Se(−II) obtained in this study: (a) goethite, (b) ferrous oxide, (c) magnetite, and (d) biotite. Open marks and closed marks represent the data obtained using 1 g and 0.1 g of the solid phase, respectively.
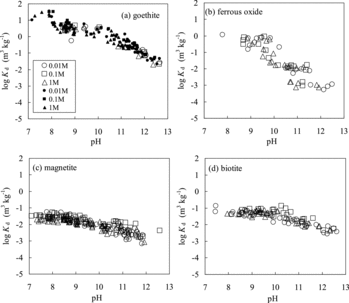
Figure 5. Comparison of Rs values of Se(IV) predicted using the TLM (lines) with experimentally measured ones (marks): (a) goethite, (b) ferrous oxide, (c) magnetite, (d) biotite (calculated with KintSeO3 = 1015.1), and (e) biotite (calculated with KintSeO3 = 1016.1).
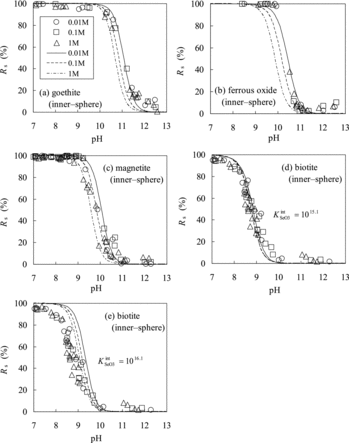
Figure 6. Comparison of Rs values of Se(−II) predicted using the TLM (lines) with experimentally measured ones (marks): (a) goethite (using 0.1 g of the solid phase; assuming inner-sphere complexation), (b) goethite (0.1 g of the solid phase; outer-sphere complexation), (c) goethite (1 g of the solid phase; inner-sphere complexation), (d) goethite (1 g of the solid phase; outer-sphere complexation), (e) ferrous oxide (inner-sphere complexation), and (f) ferrous oxide (outer-sphere complexation).
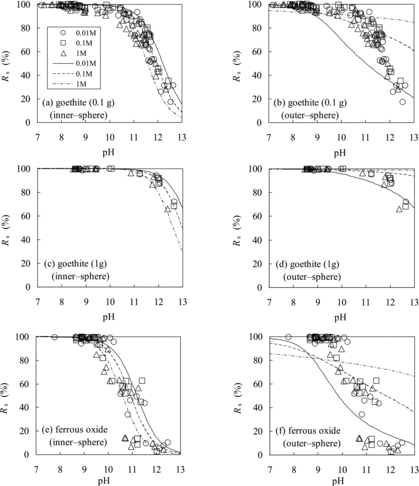
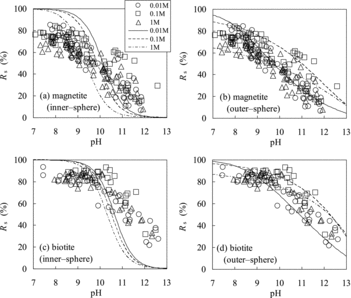
Figure 7. Comparison of Rs values of Se(−II) predicted using the TLM (lines) with experimentally measured ones (marks): (a) magnetite (assuming inner-sphere complexation), (b) magnetite (outer-sphere complexation), (c) biotite (inner-sphere complexation), and (d) biotite (outer-sphere complexation).
In this model, the activity of protons at any location {H+}i is related to the bulk activity {H+}b by the Boltzmann distribution:
(6) where F is the Faraday constant (96,485 C mol−1), ψi the electric potential (V) at any given location, R the gas constant (8.3145 J K−1 mol−1), and T the temperature (298.15 K). The intrinsic surface acidity constants of ≡FeOH are given by using the surface potential ψ0 as an electrostatic correction factor:
(7)
(8) where the square brackets represent concentrations (mol dm−3) and the curly brackets represent activities (the activity coefficients for the surface species are assumed to be equal [Citation34]). The sorption of the electrolyte ions, Na+ and Cl−, can be described as outer-sphere surface complexation [Citation12,Citation70]:
(9)
(10)
(11)
(12) where ψβ is the surface potential at the β plane. The sorption of Se(IV) onto ≡FeOH sites can be described as inner-sphere surface complexation [Citation12,Citation22,Citation27,Citation52,Citation69]. Biotite includes aluminol (≡AlOH) and silanol (≡SiOH) sites on the edge surfaces, in addition to ≡FeOH sites [Citation3]. In this study, the sorption of Se(IV) onto biotite was analyzed by assuming inner-sphere surface complexation with ≡FeOH sites, as reported by Shibutani et al. [Citation3]. The inner-sphere surface complexation of Se(IV) with ≡FeOH sites can be described by assuming a simple monodentate surface complex (≡FeSeO3−) [Citation12,Citation27] as
(13)
(14)
Considering all the species in equilibrium, the surface site density Ns (C m−2) and the surface charge density σ (C m−2) at the 0, β, and d planes are written as [Citation32,Citation71]
(15)
(16)
(17)
(18) where S is the specific surface area (m2 g−1), V the volume of the solution (dm3), W the weight of the solid sample (g), Ic the ionic strength (mol dm−3), ψ the electric potential (V), ϵ the relative dielectric constant of water (78.5 at 25°C [Citation32]), ϵ0 the permittivity of free space (8.854 × 10−12 C V−1 m−1), and z the ionic charge (valid for a symmetrical electrolyte). Considering the electrostatics of the layers, the following equations are obtained:
(19)
(20)
(21) where C1 is the inner-layer capacitance (F m−2) and C2 is the outer-layer capacitance (F m−2). The values of Rs and Kd were calculated as
(22)
(23)
The TLM was calculated by the Visual Minteq computer program with the Davies equation for activity correction [Citation32].
For the TLM parameters on the sorption of Se(IV) onto goethite, the values reported by Hayes et al. [Citation12] were used in this calculation. The KintClvalue, which was not reported by Hayes et al. [Citation12] is in the range of 6.4–9.0 in the literatures [Citation3,Citation52,Citation70,Citation72–74]; the intermediate value of 8.0 reported by Fukushi and Sverjensky [Citation50] was adopted. The model parameters are summarized in . shows the comparison of the Rs values of Se(IV) for goethite calculated using TLM with the experimentally measured values and there is good agreement between the two.
Table 10. Model parameters and equilibrium constants for the TLM calculation.
The TLM parameters for ferrous oxide are not available. Instead, the parameters for goethite were used to analyze the sorption onto ferrous oxide based on the similarities of pH dependence of Kd for ferrous oxide to that for goethite (). shows the comparison of the predicted Rs values for ferrous oxide with the experimentally measured values and there is good agreement between the two.
The sorption behavior of Se(IV) onto magnetite has been analyzed using DDLM by Kim et al. [Citation27]. The surface site density (ns = 2.5 sites nm−2), surface acidity constants (log = 5.1 and log
= −9.1), and the surface complexation constant
(24)
(25) reported by Kim et al. [Citation27] were adopted in this calculation. The
value for magnetite was calculated to be 1016.1 from Equations (3) and (25), which was 1 order of magnitude higher than that for goethite (1015.1). For the values of capacitances and the surface complexation constants for Na+ and Cl−, which were not reported by Kim et al., the values C1 = 1.2 F m−2 [Citation72], C2 = 0.2 F m−2 [Citation72], logKintNa = −6.8 [Citation75], and logKintCl = −8.0 (the same as for goethite) were adopted. shows the comparison of Rs values of Se(IV) for magnetite calculated, using TLM with the experimentally measured values and there is good agreement between the two.
The surface acidity constants of biotite were reported by Chakraborty et al. [Citation76] (log = 4.6 and log
= −6.4); however, other parameters are not available. The TLM parameters for magnetite were used for the analysis of sorption behavior onto biotite. For
, the value for goethite (1015.1) was more suitable than that for magnetite (1016.1) ( and ). The calculated values show good agreement with the experimentally measured values.
From the calculation results, the validity of surface parameters for employed iron-containing minerals was confirmed.
4.2.2. Sorption behavior of Se (−II)
The sorption behaviors of Se(−II) were also analyzed by the TLM. The chemical properties of selenium are known to be similar to those of sulfur which is chalcogen congener (the group 16 elements of the periodic table) [Citation54,Citation77]. The stokes radii, conductivity and softness of HSe− are 110 pm, 70.4 cm2 Ω−1 mol−1 and 0.44, respectively, and these values are similar to those of hydrosulfide ion (HS−), 110 pm, 65 cm2 Ω−1 mol−1 and 0.65, respectively [Citation78]. In addition, the incorporation of selenide into iron sulfides, such as pyrite (FeS2) and mackinawite (FeS), can be seen [Citation54–56,Citation77]. Therefore, the sorption behavior of HSe− onto iron-containing minerals is expected to be similar to that of HS−. Although the inner-sphere surface complexation reaction of HSe− with ≡FeOH sites has not been reported, it can be estimated from its chemical similarity to HS− as [Citation79–81]
(26)
(27)
The outer-sphere surface complexation of HSe− can be described as
(28)
(29)
The values of Rs and Kd were calculated as
(30)
(31)
The TLM parameters for the surface characteristics of the minerals were the same as those used for Se(IV) ().
The obtained Rs values for goethite using 0.1 g of the solid phase versus pH are plotted with the results of the TLM calculation by assuming inner-sphere surface complexation () and outer-sphere surface complexation (). The sorption tendency for goethite can be well explained by inner-sphere surface complexation but not by outer-sphere surface complexation (logK′intHSe = 12). The inner-sphere surface complexation constant for goethite,KintHSe, was determined by a least-squares fitting of the model calculations to the experimental Rs data using 0.1 g of the solid phase (). The obtained value, logKintHSe = 6.0, was slightly higher than the value for HS−,
(32) which is derived from the equilibrium constants of the reactions
(33) and
(34)
The experimental Rs data using 1 g of the solid phase were reproduced by assuming inner-sphere surface complexation with logKintHSe = 6.0 () but not by outer-sphere surface complexation (logK′intHSe = 12) (). In addition, the experimental Rs data for ferrous oxide were also successfully reproduced by the TLM calculation by assuming inner-sphere surface complexation with the KintHSevalue for goethite (logKintHSe = 6.0) () but not by outer-sphere surface complexation (logK′intHSe = 13) [].
The Kd values of Se(−II) for magnetite showed different tendencies from those of goethite and ferrous oxide (). The obtained Rs values for magnetite are plotted versus pH with the results of the TLM calculation by assuming inner-sphere surface complexation (logKintHSe = 6.0) in . The sorption tendency for magnetite cannot be explained by inner-sphere surface complexation. The data plot show vertical dispersion and the dependences of the data of Se(−II) on pH and ionic strength are unclear as compared to those of Se(IV). The large variation in the sorption data of Se(−II) may have been caused by polymerization of the HSe− species. The dominant species of Se(−II) were estimated to be HSe− under the experimental pH–Eh conditions, but HSe− can partially polymerize to a lower adsorbable divalent anion species, such as Se32− and Se42−, depending on the redox potentials ():
(35)
(36)
The variation of several tens of percent in the Rs data could be induced by the uncertainty of the Eh measurements at the boundary area (). shows the results of the model calculation by assuming an outer-sphere surface complexation with logK′intHSe = 11.7 as compared with the experimentally obtained Rs data. The model calculation predicted the Rs values of Se(−II) for magnetite reasonably well. Although the variation in data is too large to obtain the exact value of logK′intHSe, it can be estimated to be around 12. The value of logK′intHSe = 12 is comparable with the previously reported values for the outer-sphere complexation of Se(IV) and Se(VI) [Citation12]: log for goethite (13.4) and HFO (12.8) and log
for goethite (14.0) and HFO (12.8), described as
(37)
(38)
The obtained Rs values of Se(−II) for biotite are plotted as a function of pH in and . The pH dependences of the Rs values for biotite show the same tendencies as those for magnetite and cannot be explained by inner-sphere surface complexation (logKintHSe = 8) (). shows the results of the model calculation by assuming outer-sphere surface complexation with logK′intHSe = 11.8 as compared with the experimental Rs data. Although the variation in data is significant, logK′intHSecan be estimated to be around 12. The sorption behavior of Se(−II) onto biotite can thus be explained by assuming outer-sphere surface complexation.
5. Conclusion
The sorption data of Se(−II) and Se(IV) onto the iron-containing minerals, goethite, ferrous oxide, magnetite, and biotite were obtained by batch sorption experiments. The obtained Kd values of Se(IV) agree with previously reported values and were successfully reproduced by the TLM calculations by assuming inner-sphere complexation, the validity of this experimental method and surface parameters used for the TLM calculations were confirmed.
The Kd values of Se(−II) were obtained in a range of 2.0 × 10−2 to 38 m3 kg−1 for goethite, 5.4 × 10−4 to 1.1 m3 kg−1 for ferrous oxide, 6.8 × 10−4 to 5.6 × 10−2 m3 kg−1 for magnetite, and 2.7 × 10−3 to 1.3 × 10−1 m3 kg−1 for biotite at pH 7–13. The sorption data and the TLM calculations suggest that the dominant sorption mechanisms of Se(−II) were inner-sphere complexation (≡FeSe−) for goethite and ferrous oxide but outer-sphere complexation (≡FeOH2+−HSe−) for magnetite and biotite. The equilibrium constant for inner-sphere complexation (logKintHSe) was determined to be 6.0 and that for outer-sphere complexation (logK′intHSe) was estimated to be approximately 12.
Acknowledgements
The authors acknowledge Mr. M. Kamoshida for experimental measurements.
Additional information
Funding
References
- Japan Nuclear Cycle Development Institute (JNC). H12: Project to establish the scientific and technical basis for HLW disposal in Japan – Second progress report on research and development for the geological disposal of HLW in Japan. Japan: JNC; 2000.
- Olin A, Nolang B, Osadchii EG, Ohman L-O, Rosen E. Chemical thermodynamics of selenium. Amsterdam: Elsevier; 2005.
- Shibutani T, Nishikawa T, Inui S, Uchidate N, Yui M. Study on sorption behavior of Se on rocks and minerals. Japan: Power Reactor and Nuclear Fuel Development Corporation; 1994 [ in Japanese].
- Peak D, Sparks DL. Mechanisms of selenate adsorption on iron oxides and hydroxides. Environ Sci Technol. 2002;36:1460–1466.
- Duc M, Lefevre G, Fedoroff M, Jeanjean J, Rocuchaud JC, Monteil-Rivera F, Dumonceau J, Milonjic S. Sorption of selenium anionic species on apatites and iron oxides from aqueous solutions. J Environ Radioact. 2003;70:61–72.
- Duc M, Lefe´vre G, Fedoroff M. Sorption of selenite ions on hematite. J Colloid Interface Sci. 2006;298:556–563.
- Xia X, Kameiv G, Iijima K, Shibata M, Ohnuki T, Kozai N. Selenium sorption in a sedimentary rock/saline groundwater system and spectroscopic evidence. Mater Res Soc Symp Proc. 2006;932:933–942.
- Jan YL, Wang TH, Li MH, Tsai SC, Wei YY, Teng SP. Adsorption of Se species on crushed granite: a direct linkage with its internal iron-related minerals. Appl Radiat Isot. 2008;66:14–23.
- Iida Y, Tanaka T, Yamaguchi T, Nakayama S. Sorption behavior of selenium(-II) on rocks under reducing conditions. J Nucl Sci Technol. 2011;48:279–291.
- Videnska K, Havlova V. Retention of anionic species on granite: influence of granite composition. Paper presented at: Proceedings WM2012 Conference; 2012 Feb 26–Mar 1; Phoenix, AZ.
- Balestrieri L, Chao T. Selenium adsorption by goethite. Soil Sci Soc Am J. 1987;51:1145–1151.
- Hayes KF, Papelis C, Leckie JO. Modeling ionic strength effects on anion adsorption at hydrous oxide/solution interfaces. J Colloid Interf Sci. 1988;125:717–726.
- Ticknor KV, Harris DR, Vandergraaf TT. Sorption/Desotption studies of selenium on fracture-filling minerals under aerobic and anaerobic conditions. Canada: Atomic Energy of Canada Limited;1988.
- Zhang P, Sparks DL. Kinetics of selenate and selenite adsorption/desorption at the goethite/water interface. Environ Sci Technol. 1990;24:1848–1856.
- Ticknor KV, McMurry J. A study of selenium and tin sorption on granite and goethite. Radiochim Acta. 1996;73:149–156.
- Rietra RPJJ, Hiemstra T, Van Riemsdijk WH. The relationship between molecular structure and ion adsorption on variable charge minerals. Geochim Cosmochim Acta. 1999;63:3009–3015.
- Rietra RPJJ, Hiemstra T, Van Riemsdijk WH. Comparison of selenate and sulfate adsorption on goethite.J Coll Interf Sci. 2001;240:384–390.
- Su C, Suarez DL. Selenate and selenite sorption on iron oxides: an infrared and electrophoretic study. Soil Sci Soc Am J. 2000;64:101–111.
- Rovira M, Giménez J, Martínez M, Martínez-Lladó X, de Pablo J, Martí V, Duro L. Sorption of selenium(IV) and selenium(VI) onto natural iron oxides: goethite and hematite. J Hazard Mater. 2008;150:279–284.
- Das S, Hendry MJ, Essilfie-Dughan J. Adsorption of selenate onto ferrihydrite, goethite, and lepidocrocite under neutral pH conditions. Appl Geochem. 2013;28:185–193.
- Fujikawa Y, Fukui M. Radionuclide sorption to rocks and minerals: effect of pH and inorganic anion. Part. 2. Sorption and speciation of selenium. Radiochim Acta. 1997;76:163–172.
- Martinez M, Gimenez J, Pablo JD, Rovira M, Duro L. Sorption of selenium(IV) and selenium(VI) onto magnetite. Appl Surf Sci. 2006;252:3767–3773.
- Loyo R, Scheinost AC, Simonoff M, Nikitenko SI. Immobilization of selenite on Fe3O4 and Fe/Fe3C ultrasmall particles. Environ Sci Technol. 2008;42:2451–2456.
- Scheinost AC, Kirsch R, Banerjee D, Fernandez-Martinez A, Zaenker H, Funke H, Charlet L. X-ray absorption and photoelectron spectroscopy investigation of selenite reduction by FeII-bearing minerals. J Contam Hydrol. 2008;102:228–245.
- Missana T, Alonso U, Scheinost AC, Granizo N, García-Gutiérrez M. Selenite retention by nanocrystalline magnetite: role of adsorption, reduction and dissolution/co-precipitation processes. Geochim Cosmochim Acta. 2009;73:6205–6217.
- Jordan N, Lomenech C, Marmier N, Giffaut E, Ehrhardt JJ. Sorption of selenium(IV) onto magnetite in the presence of silicic acid. J Colloid Interface Sci. 2009;329:17–23.
- Kim SS, Min JH, Lee JK, Baik MH, Choi J-W, Shin HS. Effects of pH and anions on the sorption of selenium ions onto magnetite. J Environ Rad. 2012;104:1–6.
- Davis JA, Leckie JO. Surface ionization and complexation at the oxide/water interface 3. Adsorption of anions.J Coll Interf Sci. 1980;74:32–43.
- Parida KM, Gorai B, Das NN, Rao SB. Studies on ferric oxide hydroxides: III. Adsorption of selenite on different form of iron oxyhydroxides. J Colloid Interface Sci. 1997;185:355–362.
- Hayes KF, Leckie JO. Modeling ionic-strength effects on cation adsorption at hydrous oxide-solution interfaces. J Colloid Interface Sci. 1987;115:564–572.
- Manceau A, Charlet L. The mechanism of selenate adsorption on goethite and hydrous ferric oxide. J Colloid Interface Sci. 1994;168:87–93.
- Stumm W, Morgan JJ. Aquatic chemistry. 3rd ed. New York: John Wiley & Sons; 1996.
- Hayes KF, Redden G, Ela W, Leckie JO. Surface complexation models - an evaluation of model parameter-estimation using FITEQL and oxide mineral titration data. J Colloid Interface Sci. 1991;142:448–469.
- Dzombak DA, Morel FMM. Surface complexation modeling: hydrous ferric oxide. New York: Wiley-Interscience; 1990.
- Stumm W, Huang CP, Jenkins SR. Specific chemical interactions affecting the stability of dispersed systems. Croatica Chem Acta. 1970;42:223–245.
- Huang C, Stumm W. Specific adsorption of cations of hydrous γ-Al2O3. J Colloid Interface Sci. 1973;43:409–420.
- Hohl H, Stumm W. Interaction of Pb2+ with hydrous γ-Al2O3. J Colloid Interface Sci. 1976;55:281–288.
- Schindler PW, Gamsjager H. Acid-base reactions of the TiO2 (Anatase) water interface and the point of zero charge of TiO2 suspensions. Kolloid-Z.u.Z. Polymere. 1972;250:759–763.
- Stumm W, Hohl H, Dalang F. Interaction of metal ions with hydrous oxide surfaces. Croat Chim Acta. 1976;48:491–504.
- Stumm W, Kummert R, Sigg L. A ligand-exchange model for the adsorption of inorganic and organic-ligands at hydrous oxide interfaces. Croat Chem Acta. 1980;53:291–312.
- Westall J, Hohl H. A comparison of electrostatic models for the oxide/solution interface. Adv Colloid Interface Sci. 1980;12:265–294.
- Turner DR. A uniform approach to surface complexation modeling of radionuclide sorption. USA: Center for Nuclear Waste Regulatory Analyses; 1995.
- Yates DE, Levine S, Healy TW. Site-binding model of the electrical double layer at the oxide/water interface. J Chem Soc, Faraday Trans 1. 1974;70:1807–1818.
- Davis JA, James RO, Leckie JO. Surface ionization and complexation at oxide-water interface .1. Computation of electrical double- layer properties in simple electrolytes. J Colloid Interface Sci. 1978;63:480–499.
- Davis JA, Leckie JO. Surface ionization and complexation at the oxide/water interface II. Surface properties of amorphous iron oxyhydroxide and adsorption of metal ions. J Colloid Interface Sci. 1978;67:90–107.
- Blesa MA, Maroto AJG, Regazzoni AE. Boric-acid adsorption on magnetite and zirconium dioxide. J Colloid Interface Sci. 1984;99:32–40.
- Hayes KF, Leckie JO. Mechanism of lead-ion adsorption at the goethite-water interface. ACS Sym Ser. 1986;323:114–141.
- Goldberg S. Use of surface complexation models in soil chemical-systems. Adv Agron. 1992;47:233–329.
- Goldberg S. Sensitivity of surface complexation modeling to the surface site density parameter. J Colloid Interface Sci. 1991;145:1–9.
- Fukushi K, Sverjensky DA. A surface complexation model for sulphate and selenate on iron oxides consistent with spectroscopic and theoretical molecular evidence. Geochim Cosmochim Acta. 2007;71:1–24.
- Goldberg S. Modeling selenite adsorption envelopes on oxides, clay minerals, and soils using the triple layer model. Soil Sci Soc Am J. 2013;77:64–71.
- Kim SS, Min JH, Baik, MH, Kim GN, Choi JW. Estimation of the behaviors of selenium in the near field of repository. Nucl Eng Tech. 2012;44:945–952.
- Iseghem PV, Lemmens K, Pirlet V. The leaching of Se, Sn, Zr, Pd from vitrified high-level waste in clay slurries. Proceedings of Mobile Fission and Activation Products in Nuclear Waste Disposal; 2007 Jan 16–19; La Baule, France.
- Cannière PD, Maes A, Williams S, Bruggeman C, Beauwens T, Maes N, Cowper M. Behaviour of selenium in Boom Clay. Belgium: SCKžCEN; 2010.
- Curti E, Aimoz L, Kitamura A. Selenium uptake onto natural pyrite. J Radioanal Nucl Chem. 2013;295:1655–1665.
- Diener A, Neumann T, Kramar U, Schild D. Structure of selenium incorporated in pyrite and mackinawite as determined by XAFS analyses. J Contam Hydrol. 2012;133:30–39.
- Myneni SCB, Tokunaga TK, Brown GE. Abiotic selenium redox transformations in the presence of Fe(II, III) oxides. Science. 1997;278:1106–1109.
- Scheinost AC, Charlet L. Selenite reduction by mackinawite, magnetite and siderite: XAS characterization of nanosized redox products. Environ Sci Technol. 2008;42:1984–1989.
- Breynaert E, Bruggeman C, Maes A. XANES-EXAFS analysis of Se solid-phase reaction products formed upon contacting Se(IV) with FeS2 and FeS. Environ Sci Technol. 2008;42:3595–3601.
- Breynaert E, Scheinost AC, Dom D, Rossberg A, Vancluysen J, Gobechiya E, Kirschhock CEA, Maes A. Reduction of Se(IV) in boom clay: XAS solid phase speciation. Environ Sci Technol. 2010;44:6649–6655.
- Kang ML, Chen FR, Wu SJ, Yang YQ, Bruggeman C, Charlet L. Effect of pH on aqueous Se(IV) reduction by pyrite. Environ Sci Technol. 2011;45:2704–2710.
- Charlet L, Kang ML, Bardelli F, Kirsch R, Géhin A, Grenèche JM, Chen, F. Nanocomposite pyrite-greigite reactivity toward Se(IV)/Se(VI). Environ Sci Technol. 2012;46:4869–4876.
- Olegario JT, Yee N, Miller M, Sczepaniak J, Manning B. Reduction of Se(VI) to Se(-II) by zerovalent iron nanoparticle suspensions. J Nanopart Res. 2010;12:2057–2068.
- Tubaki J, Suzuki M, Kanda Y. Nyuumonn ryuushi funntai kougaku. Tokyo: Nikkan kogyo shimbun Ltd.; 2009 [ in Japanese].
- Johnson MFL. Estimation of the zeolite content of a catalyst from nitrogen adsorption isotherms. J Catalysis. 1978;52:425–431.
- Brunauer S, Emmett PH, Teller E. Adsorption of gasses in multimolecular layers.J Am Chem Soc. 1938;60:309–319.
- Atomic Energy Society of Japan (AESJ). Measurement method of the distribution coefficient on the sorption process. Japan: AESJ; 2006 [ in Japanese].
- Iida Y, Yamaguchi T, Tanaka T, Nakayama S. Solubility of selenium at high ionic strength under anoxic conditions. J Nucl Sci Technol. 2010;47:431–438.
- Boult KA, Cowper MM, Heath TG, Sato H, Shibutani T, Yui M. Towards an understanding of the sorption of U(VI) and Se(IV) on sodium bentonite. J Contam Hydrol. 1998;35:141–150.
- Carroll SA, Roberts S, Criscenti LJ, O’Day PA. Suraface complexation model for strontium sorption to amorphous silica and goethite. Geochem Trans. 2008;9:2.
- Sasaki T, Terakado Y, Kobayashi T, Takagi I, Moriyama H. Analysis of sorption behavior of cesium ion on mineral components of granite. J Nucl Sci Technol. 2007;44:641–648.
- Sahai N, Sverjensky DA. Evaluation of internally-consistent parameters for the triple- layer model by the systematic analysis of oxide surface titration data. Geochim Cosmochim Acta. 1997;61:2801–2826.
- Sverjensky DA. Prediction of the speciation of alkaline earths adsorbed on mineral surfaces in salt solutions. Geochim Cosmochim Acta. 2006;70:2427–2453.
- Balistrieri LS, Murray JW. The surface chemistry of goethite (α-FeOOH) in major ion seawater. Am J Sci. 1981;281:788–806.
- Criscenti LJ, Sverjensky DA. The role of electrolyte anions ClO4−, NO3−, and Cl− in divalent metal (M2+) adsorption on oxide and hydroxide surfaces in salt solutions. Am J Sci. 1999;299:828–899.
- Chakraborty S, Wolthers M, Chatterjee D, Charlet L. Adsorption of arsenite and arsenate onto muscovite and biotite mica. J Colloid Interface Sci. 2007;309:392–401.
- Bouroushian M. Electrochemistry of metal chalcogenides. Berlin, Heidelberg: Springer-Verlag. 2010.
- Marcus Y. Ion properties. New York: Marcus Dekker, Inc.; 1997.
- Afonso MD, Stumm W. Reductive dissolution of iron(iii) (hydr)oxides by hydrogen sulfide. Langmuir. 1992;8:1671–1675.
- Peiffer S, Dos SA, Wehrli B, Gaechter R. Kinetics and mechanism of the reaction of hydrogen sulfide with lepidocrocite. Environ Sci Technol. 1992;26:2408–2413.
- Poulton SW. Sulfide oxidation and iron dissolution kinetics during the reaction of dissolved sulfide with ferrihydrite. Chem Geol. 2003;202:79–94.