Abstract
In the present experimental study, the prediction of water droplet evaporation on a zircaloy surface was investigated using various initial droplet sizes. To the best of our knowledge, this may be the first valuable effort for understanding the details of water droplet evaporation on a zircaloy surface. The initial contact diameters of the water droplets tested ranged from 1.76 to 3.41 mm. The behavior (i.e., time-dependent droplet volume, contact angle, droplet height, and contact diameter) and mode-transition time of the water droplet evaporation were strongly influenced by the initial droplet size. Using the normalized contact angle (θ*) and contact diameter (d*), the transitions between evaporation modes were successfully expressed by a single curve, and their criteria were proposed. To predict the temporal droplet volume change and evaporation rate, the range of θ* > 0.25 and d* > 0.9, which mostly covered the whole evaporation period and the initial contact diameter remained almost constant during evaporation, was targeted. In this range, the previous contact angle functions for the evaporation model underpredicted the experimental data. A new contact angle function of a zircaloy surface was empirically proposed, which represented the present experimental data within a reasonable degree of accuracy.
1. Introduction
Zircaloy has been widely used as a fuel cladding material for pressurized water reactor for more than three decades owing to its low absorption cross-section of thermal neutrons, high hardness, ductility, and corrosion resistance. In the postulated accidents (e.g., loss of coolant accident) of a nuclear reactor core or spent fuel pool, the evaporation phenomenon will be encountered in the nuclear fuel cladding, which is an important research topic. Since the Fukushima Daiichi nuclear accident in 2011, an accident-tolerant cladding has been under development to prevent a hydrogen explosion by lowering the oxidation rate during a severe accident. The surface modification of zircaloy cladding is being pursued to increase the boiling and quenching heat transfers and to minimize the oxidized layer. For this work, an evaporation experiment using water droplets of various sizes and a zircaloy surface is performed, which may be the first valuable effort for understanding the details of water droplet evaporation on a zircaloy surface.
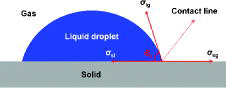
When a droplet is placed on a surface, the contact angle is determined by the force balance acting on the contact line formed between the liquid, gas, and solid, as shown in . This is generally described by Young's equation as a function of the interfacial tension,
(1)
where θ and σ are the contact angle and interfacial tension, respectively. The subscripts e, sg, sl, and lg are the equilibrium, solid–gas, solid–liquid, and liquid–gas, respectively.
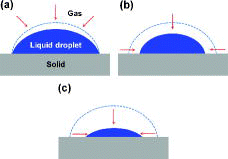
Once a liquid droplet begins to evaporate into the environment, the droplet shape changes. Picknett and Bexon [Citation1] reported that three distinct modes can be observed during the evaporation of a droplet, as shown in : (a) constant contact radius mode (CCRM), (b) constant contact angle mode (CCAM), and (c) shrinkage mode (SM). In the CCRM, the contact line, which is a perimeter between a liquid, gas, and solid, is pinned. The contact angle of the evaporating droplet decreases, while the contact area radius remains constant. In the CCAM, which is known as moving contact line mode, the contact area radius decreases and the contact angle remains constant. In the SM, both the contact angle and the contact area radius decrease simultaneously.
The droplet evaporation is influenced by the liquid (e.g., surface tension, viscosity, and volatility), gas (e.g., relative humidity, temperature, and pressure), and solid (e.g., surface material, wettability, and roughness) properties [Citation2]. Among them, as interest in the surface effect on evaporation has heightened, researches into droplet evaporation have been carried out extensively using various surfaces [Citation3–7]. Birdi and Vu [Citation3] and Birdi et al. [Citation4] studied the behavior of evaporating water and n-octane droplets on smooth glass and Teflon surfaces. Both studies reported that the droplet evaporations of water on a glass surface and n-octane on a Teflon surface were stationary processes with a constant contact radius and linear evaporation rate. However, when the contact angle was over 90°, a constant contact angle with decreasing contact radius and a non-linear evaporation rate were observed. Shin et al. [Citation5] investigated the evaporation characteristics of a water droplet on hydrophilic (glass, θ = 58.64°), hydrophobic (octadecyltrichlorosilane; OTS, θ = 122.52°), and superhydrophobic (alkyl ketene dimer; AKD, θ = 160.59°) surfaces. On the glass surface, the contact angle, center height, and volume of the droplet decreased linearly over the entire evaporation time. On the OTS surface, three distinct stages, shown in , were observed. However, on the AKD surface, there was no contact line pinning period. Shin et al. [Citation6] examined the evaporation of a water droplet using non-patterned polydimethylsiloxane (PDMS) and patterned (i.e., submicron-sized post array) silicon surfaces. Both were hydrophobic surfaces with the similar initial contact angles. On the non-patterned PDMS surface, three distinct stages occurred during evaporation. On the other hand, on the patterned silicon surface, the contact angle decreased linearly, while the contact area remained constant until the droplet evaporated. Lee et al. [Citation7] investigated the behavior of an evaporating water droplet on three different hydrophobic surfaces. They used plain copper (θ = 115°), micro-structured copper (θ = 126°), and nano-structured copper oxide (θ = 159°) with coating of the same self-assembled monolayer material, as the test surfaces. In their work, the surface morphology had a great influence on the behavior of droplet evaporation. The surface structure determined the droplet state (i.e., Wenzel and Cassie) on the surface, which affected the motion of the contact line. The behavior of the contact line resulted in a change of the details in droplet evaporation (e.g., contact angle, contact area radius, droplet height, shrinking velocities of droplet radius, and height). However, to the best of our knowledge, none of the investigations using a zircaloy surface, currently used in the fuel cladding, have been performed.
The droplet evaporates slowly into the environment, which implies a quasi-equilibrium process. In general, the evaporation rate of a water droplet into the ambient air is modeled by Fick's law [Citation8,9],
(2) where Q, D, A, c, and r are the evaporation rate, vapor diffusivity in air, the surface area of the droplet undergoing diffusion, the vapor concentration, and the radial distance, respectively. From Equation (2), the droplet volume change with time can be obtained as below [Citation8,9]:
(3) where ρ, V, t, and R are the liquid density, droplet volume, time, and spherical radius, respectively. The subscripts s and ∞ are the saturated and ambient vapor concentrations, respectively. f(θ) is the contact angle function for a droplet evaporation, which represents the dependency of the evaporation rate on the contact angle.
The contact angle function, f(θ), is an important parameter to predict the temporal change of an evaporating droplet volume and evaporation rate, which has been modeled by some researchers. Picknett and Bexon [Citation1] proposed the empirical correlation of the contact angle function, using the problem of evaluating the capacitance of an isolated conducting body of the same size and shape as the droplet,
(4)
(5)
(6) where C is the capacitance of an equiconvex lens. Rowan et al. [Citation10] assumed that the vapor concentration gradient (dc/dr) is in an outward radial direction, and the same as (c∞−cs)/R. They mentioned that their model will be in the correct form for a uniform evaporation of a completely unsupported and spherical drop. In other words, it neglects the effect of evaporation occurring at the edge of a spherical cap-shaped droplet, and the following contact angle function was found:
(7) Bourges-Monnier and Shanahan [Citation11] assumed that the diffusion of liquid vapor from the droplet into the surrounding atmosphere during evaporation is purely radial and developed a model for a spherical cap shape, as follows:
(8) Song et al. [Citation8] performed experiments on droplet evaporation using 10 kinds of surfaces with a wide range of contact angles. They reported that the evaporation rate of a droplet is influenced by the surface condition and material as well as the contact angle. Even though the surfaces appeared to have the same contact angle with the same droplet shape, the evaporation behaved differently depending on the surface material and condition. Using their experimental data, they proposed simple contact angle functions for all surfaces to predict the volume change of an evaporating droplet.
Based on the works mentioned above, the foregoing approaches (Equations (4), (7) and (8)) considering only the geometry of an evaporating droplet on a surface still have a limitation in explaining the differences in the evaporation behavior on various surfaces [Citation8]. In addition, the surface materials and conditions having the same initial contact angle may have different contact angle functions from each other. Therefore, it is meaningful to perform the droplet evaporation experiments with the specific surface material used in the industrial field, and for nuclear reactor core application, zircaloy is a good candidate for droplet evaporation testing. Moreover, considering a wide range of industrial applications, the influence of water droplets of various sizes on the evaporation should be examined, because the initial droplet size may have a significant influence on the evaporation behavior.
The objectives of this work are to investigate the behavior of water droplet evaporation on a non-heated zircaloy surface using various initial droplet sizes, and propose the transition criteria between evaporation modes and contact angle function to predict the temporal volume change and evaporation rate of water droplets.
2. Experimental details
2.1. Experimental set-up and method
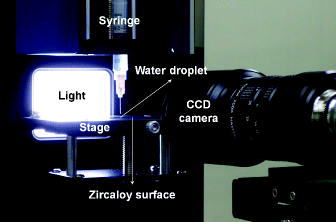
shows the experimental set-up for an evaporation test of a water droplet, which consists of a charge-coupled device (CCD) camera, syringe, light, stage, and computer system for image processing. As a test specimen, a zircaloy surface widely used in nuclear fuel cladding was prepared. In , the properties of zircaloy are shown and compared with other materials. The test specimen, 90 mm (length) × 13 mm (width) × 0.5 mm (thickness) in size, was mechanically polished using ultra fine sandpaper (2000-grit). It was cleaned with methanol and acetone in an ultrasonic bath. Next, it was rinsed with deionized water several times, and then, completely dried. Using a syringe, a deionized water droplet was carefully placed on a non-heated zircaloy surface. The initial contact diameter, defined as the diameter of the area formed between a water droplet and zircaloy surface, was in the range of 1.76–3.41 mm, and the initial contact angle was about 58°. During evaporation, the images of the droplet were recorded every 30 sec by a CCD camera and computer system. The total evaporation time was measured by a stopwatch. The room temperature and relative humidity were maintained using a constant temperature and humidity control system (ZERO Eng., SS-2000) within 23–24 °C and 33%–34%, respectively, and monitored by a thermo-humidity meter (SATO, PC-5000TRH-II).
Table 1. Properties of zircaloy and other materials (at room temperature).
2.2. Characterization of sessile water droplet
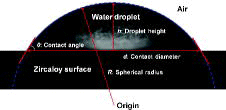
In , the captured image of a sessile water droplet on a zircaloy surface is shown with the definitions of the parameters used in this work. To determine whether the shape of a droplet on a test surface is assumed to be a spherical cap, the Bond number, defined as the ratio of gravitational force to surface tension force, is calculated using Equation (9),
(9)
A spherical cap-shaped water droplet can be characterized by the contact diameter, droplet height, contact angle, and spherical radius, and can be described if two of these parameters are known [Citation9]. In this work, the contact diameter and droplet height were measured using an image processing technique. The contact angle, spherical radius, and droplet volume at any instant in time were then determined through the following equations:
(10)
(11)
(12) where h and d are the droplet height and contact diameter, respectively.
3. Results and discussion
3.1. Behavior of evaporating water droplets on zircaloy surface
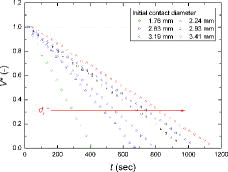
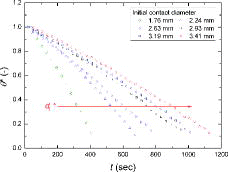
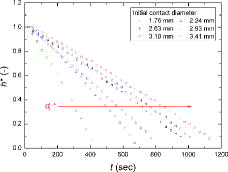
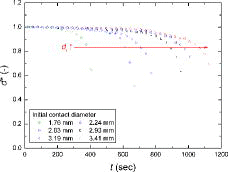
In –, the normalized droplet volume, contact angle, droplet height, and contact diameter, defined as Equation (13), over time are shown for the exemplified water droplet sizes, respectively.
(13)
The initial water droplet size strongly affected the behavior (i.e., normalized parameters of Equation (13)) and mode-transition time of the evaporating droplet. A larger initial contact diameter (i.e., larger initial droplet volume) needed a longer time to be completely evaporated. The general trends in the normalized droplet volume, contact angle, and droplet height during the evaporation were similar, as shown in , in that they gradually decreased over time. However, the contact diameter showed a different behavior from the other parameters. As shown in , for all cases, the contact diameters during evaporation remained the same as the initial values (i.e., d* ≈ 1) for a certain period (i.e., the contact line is pinned) and then sharply decreased with time (i.e., the contact line is depinned). Based on , for the water droplets of all sizes evaporating on a zircaloy surface, CCRM and SM occurred, without CCAM. In the early stage of evaporation, the contact angle decreased with a constant contact diameter (i.e., CCRM) for a certain period, and then both the contact angle and contact diameter decreased over time (i.e., SM). As the initial contact diameter of water droplet increased, the period required to maintain a constant contact diameter became longer.
The depinning of the contact line is determined based on the competition between the unbalanced Young's force and frictional force [Citation14]. In , a frictional force between the droplet and the surface leads to the pinning of the contact line. As the water droplet evaporates, the unbalanced Young's force increases owing to a decrease in the droplet height and contact angle. When the unbalanced Young's force dominates over the frictional force, the contact line is depinned.
Figure 9. Force balance acting on contact line during evaporation [Citation14].
![Figure 9. Force balance acting on contact line during evaporation [Citation14].](/cms/asset/1dfd05b7-2b26-4ffa-824f-94cca052a821/tnst_a_873359_f0009_oc.jpg)
In , using the captured images, the change in droplet shape for di = 1.76 mm during the evaporation was exemplified and shown as the contours. CCRM and SM were clearly observed.
3.2. Transition criteria of evaporation modes
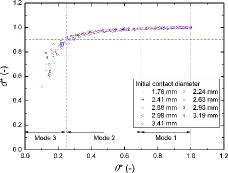
The evaporation modes, as shown in , are determined by changes in the contact angle and contact diameter. To generalize the transition criteria between the evaporation modes of a water droplet on a zircaloy surface, the experimental data were replotted in , using the normalized contact angle and contact diameter. Interestingly, experimental data for the droplets of all sizes were almost placed on a single curve, which implies that the normalized contact angle and contact diameter are good parameters to describe and generalize the transitions between evaporation modes.
In this study, the evaporation modes were divided into three groups, as shown in and elaborated: In Mode 1, the normalized contact diameter was the same as the initial value, while the normalized contact angle was decreased by about 30%. In Mode 2, the change of the normalized contact diameter appeared much smaller than that of the normalized contact angle. In this mode, the reductions in the normalized contact angle and contact diameter were 45% and 10%, respectively. Finally, in Mode 3, both the normalized contact angle and contact diameter decreased drastically. Based on these observations, the transition criteria for evaporation modes of a water droplet on a zircaloy surface were proposed based on the normalized contact angle and contact diameter, considering the initial water droplet size, as shown below.
(14)
3.3. Proposed contact angle function
In this section, based on the experimental data, the previous contact angle functions are compared and assessed. The contact angle function of a zircaloy surface is then explored to predict the temporal volume change and evaporation rate of a sessile water droplet.
The volume of evaporating water droplet at any instant in time can be estimated by the following relationship:
(15) where Δt is the time step, and the subscript k is the value of the parameter at each time step. From the initially given water droplet volume (V1) and contact angle (θ1), the droplet volume (V2) after a certain time (Δt) can be simply obtained using Equation (15). The evaporation rate of the initial droplet volume (Q1) is calculated by Equation (3). In such a case, the contact angle function, f(θ), is the key parameter to predict the temporal water droplet volume change and evaporation rate. In addition, to estimate the subsequent volume of an evaporating droplet using Equations (10)–(12), another factor, among the contact diameter, droplet height, contact angle, and spherical radius, should be known with the droplet volume (V2) obtained by Equation (15). In this work, Modes 1 and 2, which mostly covered the whole evaporation period, are targeted. In these modes, since the contact diameter during the evaporation decreases by only 10%, the contact diameters of the droplet can be assumed to remain the same as the initial value (i.e., d* ≈ 1). Then, using Equations (3), (10)–(12), and (15) with the assumption of d* ≈ 1 in Modes 1 and 2 (i.e., θ* > 0.25 and d* > 0.9), the temporal volume change of an evaporating water droplet can be predicted, if the contact angle function is given.
To obtain the contact angle function for a zircaloy surface, based on the measurement data at each time step, the values of the contact angle function were calculated using Equation (16) derived from Equations (3) and (15) with the environment properties corresponding to the ambient temperature and relative humidity,
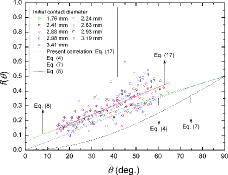
(16) In , the values of the contact angle function estimated in Modes 1 and 2 are shown and compared with the previous correlations of Equations (4), (7), and (8). Even though the test conditions (e.g., surface and environment conditions) were tried to be carefully and precisely controlled and maintained in this work, the experimental data were likely to be somewhat scattered because the droplet evaporation phenomena might be sensitively influenced by the surface and environment conditions. In , the measured values of contact angle function seemed to be independent of the initial water droplet size. In these modes, the correlation of Bourges-Monnier and Shanahan [Citation11] (i.e., Equation (8)) appeared to have a higher value than that of Rowan et al. [Citation10] (i.e., Equation (7)). This is because the correlation of Bourges-Monnier and Shanahan [Citation11] takes the effect of evaporation at the edge of the droplet into account. All previous correlations underpredicted the present experimental data, which implies that the previous droplet evaporation correlations may have a limitation in predicting the temporal volume change and evaporation rate for a water droplet on a zircaloy surface.
In this work, for the application of nuclear fuel cladding, the contact angle function of Equation (17) to predict the behavior of an evaporating water droplet on a zircaloy surface was proposed as a linear form through the data regression process in Modes 1 and 2 and then, plotted in ,
(17) where θ must be in radian. Equation (17) was developed using the experimental data in the range of θ* > 0.25 and d* > 0.9 for 1.76–3.41 mm in the initial contact diameter of a water droplet on a horizontal plate.
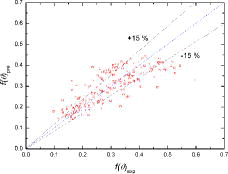
In , the measured values of the contact angle function for water droplet evaporation on a zircaloy surface are compared with the proposed correlation of Equation (17). The empirical correlation represented the present experimental data within an error of about 15%.
In the nuclear reactor core or spent fuel pool under severe accidents, the nuclear fuel cladding will be under a heated condition, and the oxide layer may be formed on its surface. Radiation-induced surface activation (RISA) phenomenon could be one of the important factors to be considered for an improved evaporation model. RISA is known to be caused by cathodic and anodic reactions due to irradiation of radioactive rays on the oxidized metal surface, which is able to change the surface wettability [Citation15]. Also, Equation (17) was simply and empirically developed for engineering use, based on the experimental data. For the better prediction and generalization of droplet evaporation, the physical properties and conditions of surface should be considered in the detailed model. To do this, the droplet evaporation tests using various surface conditions of different material, contact angle, morphology, and roughness are needed. Therefore, further experiments considering these parameters mentioned above should be carried out, which are left as future work.
4. Conclusions
In the present experimental study, the behavior of water droplet evaporation on a non-heated zircaloy surface was investigated using various initial droplet sizes. Then, the transition criteria between evaporation modes and new contact angle function for a zircaloy surface were proposed to predict the temporal volume change and evaporation rate of the water droplets. The following conclusions can be summarized.
The initial water droplet size had a significant influence on the behavior (i.e., droplet volume, contact angle, droplet height, and contact diameter) and mode-transition time of an evaporating droplet. By increasing the initial droplet size, the water droplet lasted for a longer time, and the pinning period of the contact line became longer. Among the evaporation modes, CCRM and SM occurred without CCAM.
Using the normalized contact angle and contact diameter, the transition between evaporation modes was successfully expressed by a single curve. The transition criteria were generalized and proposed considering the initial droplet size.
The previous contact angle functions underpredicted the present experimental data. A new contact angle function for a zircaloy surface was proposed empirically as a linear form, which represented the present experimental data within a reasonable degree of accuracy.
Nomenclature
| A | = | diffusion area (mm2) |
| Bo | = | Bond number (-) |
| c | = | vapor concentration (g/mm3) |
| cp | = | specific heat at constant pressure (J/kg·K) |
| D | = | vapor diffusivity in air (mm2/s) |
| d | = | contact diameter (mm) |
| f(θ) | = | contact angle function (-) |
| g | = | gravitational acceleration constant ( = 9.81 m/s2) |
| h | = | droplet height (mm) |
| k | = | thermal conductivity (W/m·K) |
| Q | = | evaporation rate (g/s) |
| R | = | spherical radius (mm) |
| r | = | radial distance (mm) |
| t | = | time (sec) |
| Δt | = | time step (sec) |
| V | = | droplet volume (mm3) |
Greek letters
| θ | = | contact angle (° or radian) |
| ρ | = | density (g/mm3) |
| σ | = | interfacial tension (mN/mm) |
Subscripts
| 1 | = | the first droplet |
| 2 | = | the second droplet |
| BM&S | = | Bourges-Monnier and Shanahan's model [Citation11] |
| d | = | contact angle changed by evaporation |
| e | = | equilibrium |
| exp | = | experimental data |
| i | = | initial |
| k | = | value at each step |
| lg | = | liquid–gas |
| P&B | = | Picknett and Bexon's model [Citation1] |
| pre | = | predicted value |
| R | = | Rowan et al.'s model [Citation10] |
| s | = | saturated vapor concentration |
| sg | = | solid–gas |
| sl | = | solid–liquid |
| ∞ | = | ambient vapor concentration |
Superscript
| * | = | normalized |
Additional information
Funding
References
- Picknett RG, Bexon R. The evaporation of sessile or pendant drops in still air. J Colloid Interface Sci. 1977;61:336–350.
- Dugas V, Broutin J, Souteyrand E. Droplet evaporation study applied to DNA chip manufacturing. Langmuir. 2005;21:9130–9136.
- Birdi KS, Vu DT. Wettability and the evaporation rates of fluids from solid surface. J Adhes Sci Technol. 1993;7:485–493.
- Birdi KS, Vu DT, Winter A. A study of the evaporation rates of small water drops placed on a solid surface. J Phys Chem. 1989;93:3702–3703.
- Shin DH, Lee SH, Jung JY, Yoo JY. Evaporating characteristics of sessile droplet on hydrophobic and hydrophilic surfaces. Microelectron Eng. 2009;86:1350–1353.
- Shin DH, Lee SH, Choi CK, Retterer S. Evaporation and wetting dynamics of sessile water droplets on submicron-scale patterned silicon hydrophobic surfaces. J Micromech Microeng. 2010;20:055021.
- Lee CY, Zhang BJ, Park J, Kim KJ. Water droplet evaporation on Cu-based hydrophobic surfaces with nano- and micro-structures. Int J Heat Mass Transfer. 2012;55:2151–2159.
- Song H, Lee Y, Jin S, Kim HY, Yoo JY. Prediction of sessile drop evaporation considering surface wettability. Microelectron Eng. 2011;88:3249–3255.
- Yildirim Erbil H, McHale G, Newton MI. Drop evaporation on solid surfaces: constant contact angle mode. Langmuir. 2002;18:2636–2641.
- Rowan SM, Newton MI, McHale G. Evaporation of microdroplets and the wetting of solid surfaces. J Phys Chem. 1995;99:13268–13271.
- Bourges-Monnier C, Shanahan MER. Influence of evaporation on contact angle, Langmuir. 1995;11:2820–2829.
- Siefken LJ, Coryell EW, Harvego EA, Hohorst JK. SCDAP/RELAP5/MOD 3.3 code manual: MATPRO – a library of materials properties for light-water-reactor accident analysis. Washington (DC): Nuclear Regulatory Commission; 2001.
- Incropera FP, DeWitt DP. Fundamentals of heat and mass transfer. New York (NY): Wiley; 2002; p. 905–908.
- Shi L, Shen P, Zhang D, Lin Q, Jiang Q. Wetting and evaporation behaviors of water–ethanol sessile drops on PTFE surfaces. Surface Interface Anal. 2009;41:951–955.
- Takamasa T, Hazuku T, Okamoto K, Mishima K, Furuya M. Radiation induced surface activation on Leidenfrost and quenching phenomena. Exp Thermal Fluid Sci. 2005;29:267–274.