?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
A novel chelator diethylenetriaminepentaacetic phenylenediamine (DTPAA) was successfully synthesized using p-phenylenediamine and diethylenetriaminepentaacetic acid. The as-synthesized DTPAA was covalently bonded to a supporting matrix of graphene oxide (GO), and a composite material (GO-DTPAA) was obtained. The structure of GO-DTPAA was characterized using Fourier-transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD). The morphology was observed using scanning electron microscope (SEM) and Raman spectroscopy. The adsorption of uranium(VI) from aqueous solution was investigated using GO-DTPAA via batch experiments. Results indicated that GO-DTPAA was a highly efficient absorbent for the removal of uranium (VI) from aqueous solution at pH 6.5. Kinetics and adsorption isotherm investigations revealed that the adsorption process was well-fitted by pseudo-second-order kinetics and by the Langmuir isotherm. The adsorption capacity of GO-DTPAA was as high as 485.0 mg·g−1 at 298.15 K, which was far greater than that of pristine GO (97.3 mg·g−1) at the same temperature.
1. Introduction
Uranium is a radioactive metal that occurs in nature and plays an important role in the nuclear industry. It is usually a product of uranium mining and hydrometallurgy. Unfortunately, a large amount of low concentration uranium-containing radioactive wastewater is produced at the same time. Uranium contamination, even at trace levels, is a threat to human health and the environment because of its chemical toxicity and radioactivity [Citation1]. To meet the requirements of nuclear energy for the future and to address environmental remediation, the removal, enrichment, purification, and recovery of uranium from waste streams are increasingly important. Thus far, various technologies have been proposed for treating uranium in radioactive wastewaters, and the most commonly used methods are ion exchange [Citation2], chemical precipitation [Citation3], solvent extraction [Citation4], microbiological repair [Citation5], and adsorption [Citation6]. However, most of these methods have some drawbacks. For instance, the chemical precipitation method requires efficient modulation of pH, coagulation produces toxic residues, and biological methods need strict controls. The cost of treating uranium ions by osmosis is high [Citation7,Citation8]. Of these methods, adsorption is considered an effective method because of its simplicity, feasibility, and easy operation. Currently, various materials, such as zeolite [Citation9], activated carbon [Citation10], mesoporous silica [Citation11], montmorillonite [Citation12], biomass, and polymeric materials [Citation13,Citation14], have been used as adsorbents for removing uranium(VI) (U(VI)). However, to increase absorption capacity and to enhance removal efficiency, it is still necessary to explore the design and functionalization of novel adsorbents.
Graphene oxide (GO) has become a hot topic for the uptake of inorganic and organic pollutants because of its ultrahigh surface area and large mechanical strength. In particular, abundant functional groups such as hydroxyl, carboxyl, and epoxy groups are present on the surface, and these provide a larger space for further modification [Citation15]. Consequently, GO is a promising adsorbent for removing U(VI) from aqueous solution. However, the maximum adsorption capacity of GO for removing U(VI) is lower than that of some other adsorbents because it lacks targeted functional groups. Additionally, GO nanosheets are prone to form aggregates in aqueous solution. In our previous work [Citation16], a three-dimensional graphene oxide sponge was assembled at 180 °C to reduce aggregate and improve adsorption capability of GO. Compared to pristine GO, the as-prepared GO sponge exhibited higher adsorption amount for uranium removal. Although a lot of progress has been made for enhancing sorption performance, further work still needs to be explored in depth to improve uranium adsorption capability.
It has been proposed that introducing suitable functional groups on the surface of GO can greatly increase its adsorption capability and improve its water-dispersion property, and these are important for practical applications of GO as an adsorbent. To date, researchers have prepared GO composite materials with organic functional groups, such as amidoxime, amino, imide, sulfonate, or phosphonate groups, to enhance the adsorption of U(VI) ions [Citation17–21].
Diethylenetriaminepentaacetic acid (DTPA) is widely used in a variety of fields, including agriculture, detergents, foods, polymers, and pharmaceuticals [Citation22]. In particular, DTPA can form a stable chelate with heavy metal ions because of its characteristic molecular structure. The structure of DTPA is similar to that of ethylenediaminetetraacetic acid (EDTA), but it has more carboxyl groups than EDTA, which can enhance its coordination ability with heavy metal ions. Furthermore, it can be produced on a large scale and at low cost. Hence, the immobilization of DTPA on the surface of various matrix materials for heavy metal uptake has attracted much attention. Additionally, amino groups also easily form stable chelates with heavy metal ions because of their high nucleophilicity and reactivity, and thus, many inorganic materials have been functionalized with amino groups [Citation23–26]. Compared to pristine inorganic materials, materials modified with amino groups have improved the adsorption capacity of U(VI) [Citation27]. If amino groups are grafted to the terminal of DTPA, it is predicted that the compounds have unexpected synergistic effects for removing U(VI) ions in an aqueous environment.
In this work, for the goal of improving the adsorption capacity of U(VI) ions, a new ligand DTPAA was synthesized by condensation reactions between amino groups of PPD and carboxyl groups of DTPA. As a surface modifier, DTPAA offers more nitrogen/oxygen-containing functional groups, which are conducive for forming uranium complexes and improving uranium removal from aqueous solution. The as-synthesized DTPAA was grafted onto the surface of GO under a mild experimental condition, and a novel absorbent (GO-DTPAA) with targeted functional groups was obtained. The chemical structure and composition of the as-prepared absorbent were confirmed using characterization methods. Applying the GO-DTPAA hybrid in the removal of U(VI) from aqueous solution was evaluated using batch experiments. The effects of various factors, such as pH, contact time, and initial concentration of the U(VI) ions, on the uptake behavior were investigated. The adsorption kinetic, equilibrium, and thermodynamic parameters were calculated. The adsorption mechanism is also discussed.
2. Experimental
2.1. Materials
Natural large-flake graphite (300 mesh, 99.8%) was obtained from Qingdao Jinrilai Graphite Co., Ltd. (China). PPD, 1-(3-dimethylaminopropyl)−3-ethylcarbodiimide hydrochloride (EDC), and N-hydroxysuccinimide (NHS) were purchased from J&K Scientific Co., Ltd. (China). DTPA was procured from Sigma-Aldrich Chemical Co., Ltd. (USA). A stock solution of U(VI) (1000 mg·L−1) was prepared by weighing 1.1792 g analytical grade U3O8 into a small beaker, 10 mL concentrated HCl and 2 mL 30% H2O2 were added and mixed well. The mixture was then heated to digest and steamed nearly dry. The residue was dissolved with 20 mL 1 mol·L−1 dilute nitric solution and transferred into a 1000 mL volumetric flask. The solution was diluted to the mark with water and shook well. Uranium working solution was prepared freshly by diluting the stock solution with water. U3O8was provided by the Faculty of Chemistry, Biology, and Material Science, East China University of Technology. All other chemicals and reagents used in this study were of analytical grade.
2.2. Synthesis of DTPAA
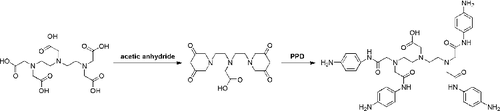
DTPA (12.5 mmol, 4.467 g) and acetic anhydride (8 mL) were added to a mixture of 12.5 mL of pyridine and 12.5 mL of acetonitrile at 50 °C. After stirring for 24 h, the mixture was filtered and washed with each acetic anhydride and ether, sequentially. The residue was dried at 50 °C under vacuum, and cyclic-diethylenetriaminepenta-acetic acid anhydride (denoted as cyclic-DTPA) was obtained. Cyclic-DTPA (357.4 mg, 1 mmol), PPD (4.5 mmol, 486.63 mg), and K2CO3 (10 g, 60 mmol) were added to a solution of 100 mL of dimethyl sulfoxide (DMSO), and stirred for 12 h at 65 °C. The mixture was filtered, and the filtrate was concentrated under reduced pressure. Finally, DTPAA was obtained as a brown oil without further purification. The synthesis route of DTPAA is illustrated in .
2.3. Preparation of GO-DTPAA
GO was synthesized according to a modified Hummers method as described in our precious work [Citation16]. The as-synthesized GO (150 mg) and EDC (384 mg) were dissolved in 50 mL of deionized water, the mixture was sonicated for 30 min, and then a phosphate buffer solution was added to maintain the pH between 7–7.5. Finally, NHS (231 mg, 2 mmol) and DTPAA (753 mg, 1 mmol) were added and stirred at room temperature for 4 h. The mixture was then filtered and washed with ethanol and deionized water, sequentially. Finally, the residue was dried at 50 °C for 24 h under vacuum to yield GO-DTPAA.
2.4. Characterization
The surface morphlogy of the prepared materials was characterized by using field emission scanning electron microscope (SEM NNS450) coupled with energy dispersive X-ray spectrometry (EDX) spectrometer (FEI, Czech) at 15 kv. The infrared spectra of the materials were recorded from KBr pallets on a Fourier Transform IR spectrophotometer Magna-IR 380 (Nicolet, USA) in order to analyze the structure groups of the materials. The XRD patterns of the materials were mounted by Cu Kα radiation generated in the Bruker D8 X-ray diffractometer at step scan increment of 0.02° and a dwell time of 2 s, the diffraction angle (2θ) from 5° to 50° was scanned. Raman measurements were carried out using an inVia + Reflex Raman spectrometer (Renishaw, UK). X-ray photoelectron spectroscopy was conducted on a Thermo Scientific Escalab 250Xi (USA).
2.5. Batch adsorption experiments
Batch adsorption experiments were carried out using a thermostatically controlled shaker under different conditions. In a typical experiment, 10 mg of pristine GO and GO-DTPAA adsorbent was equilibrated with 100 mL of uranium solution at a given concentration in an Erlenmeyer flask with stirring at room temperature. The pH of the solution was adjusted using negligible volumes of dilute solutions of nitric acid or sodium hydroxide. After reaching adsorption equilibrium, the solution was filtered using a 0.45 µm cellulose acetate membrane. The concentration of U(VI) in the supernatant was determined by the Arsenazo III photometric method. Each experiment was repeated three times, and the results given are the average values. The amount of adsorbed U(VI) was calculated from the difference of the uranium concentration in the initial solution and the equilibrated concentration in the aqueous solution using the following expression
(1)
(1)
where qe (mg·g−1) represents the amount of U(VI) ions adsorbed per unit mass of adsorbent at equilibrium, V (L) is the solution volume, and C0 (mg·L−1) and Ce (mg·L−1) are the initial and equilibrium concentrations of U(VI) ions, respectively. m (g) is the mass of the adsorbent.
3. Results and discussions
3.1. Characterization of GO-DTPAA composites
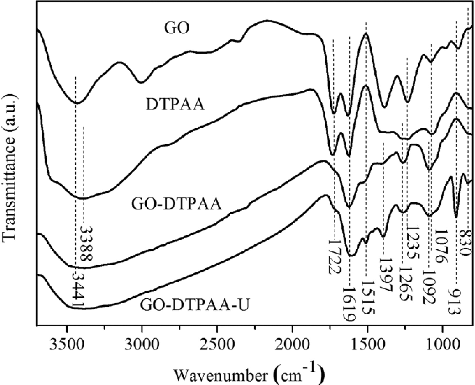
FTIR spectra of pristine GO, DTPAA, and GO-DTPAA before and after adsorption (denoted as GO-DTPAA-U) are shown in . The main characteristic bands of pristine GO were the peaks at 3441, 1722, 1619, 1397, 1235, and 1076 cm−1 [Citation28]. The band at 3415 cm−1 resulted from the stretching vibrations of −OH bonds, and the band at 1722 cm−1 was assigned to the stretching vibrations of C=O in carboxylic acid. The peaks at 1619, 1397, 1235, and 1076 cm−1 corresponded to the stretching vibrations of aromatic C=C, carboxyl C–O–H, epoxy C−O–C, and alkoxy C−O bonds [Citation29,Citation30], respectively. The characteristic bands of DTPAA at 3388, 1722, 1619, and 1265 cm−1 were ascribed to the stretching vibration of N–H in amine groups, C=O in carbonyl groups, aromatic C=C bonds, and C−N groups, respectively [Citation31]. As for GO-DTPAA, the new adsorption peak at 1515 cm−1 was attributed to the N–H bending vibration of the amide group. Compared to pristine GO, the peak at 1722 cm−1 was not present in the spectrum of GO-DTPAA, and this proved that DTPAA was successfully grafted onto the surface of GO. After adsorption, new adsorption peaks appeared in the spectrum of GO-DTPAA-U at 913 and 830 cm−1. These were attributed to the vibration of the O=U=O bond [Citation32] and the out-of-plane bending vibration of the N–H bond, suggesting that the U(VI) ions were bonded to the DTPAA ligands on the surface of GO-DTPAA.
To further clarify the uranium adsorption mechanism, pristine GO and the GO-DTPAA hybrid before and after U(VI) adsorption were determined using XPS, and the results are shown in . As can be seen from (a), the spectra consist of carbon, oxygen, nitrogen, and uranium. The appearance of the N 1 s binding energy peak of GO-DTPAA directly confirms that the amino group of DTPAA was grafted onto the surface of GO via the carbodiimide reaction. The peaks of the C 1 s, O 1 s, and N 1 s of GO-DTPAA were simulated using two-component Gaussian–Lorentzian sum functions. Deconvolution of the C 1 s peak showed the presence of sp2-hybridized carbon, as seen in (b), the sp2-hybridized carbons were: C−C/C= C (∼284.6 eV), C−O/C−N (∼286.6 eV), and C=O (∼288.7 eV). The peak fitting of the O 1 s spectrum in (c) revealed the presence of C=O (∼531.5 eV), C−O−C (∼532.5 eV), and C−OH (∼533.5 eV) species. From (d), the deconvoluted N 1 s spectra indicated the presence of –NH−C=O (∼399.6 eV) and a single–NH2 (∼400.5 eV) group, confirming the functionalized GO-DTPAA. After U(VI) adsorption, the XPS spectrum of GO-DTPAA-U ((e)) indicated that two strong binding energy peaks corresponding to U 4f7/2 and U 4f5/2 appeared at ∼381.9 and ∼392.6 eV, respectively, and were assigned to UO22+ [Citation33], suggesting that U(VI) was effectively adsorbed by the GO-DTPAA composite. To understand the element contents of the samples, the results of elemental analysis of pristine GO, GO-DTPAA, and GO-DTPAA-U are listed in . Compared with GO, the ratio of O/C for GO-DTPAA decreased and the ratio of N/C for GO-DTPAA increased, demonstrating that nitrogen-containing groups of DTPAA were grafted to GO via reaction between the amino groups of DTPAA and the carboxyl groups of pristine GO. The results also indicate that the atomic percentage of the C 1 s and N 1 s spectra of GO-DTPAA-U decreased, whereas that of O 1 s increased. These results reveal that nitrogen atoms of amino groups in GO-DTPAA chelated with uranyl ions via a coordination reaction.
Figure 3. XPS spectra of (a) total survey scan, (b) C 1 s of GO-DTPAA, (c) O1s of GO-DTPAA, (d) N 1 s of GO-DTPAA, and (e) U 4f of GO-DTPAA-U.
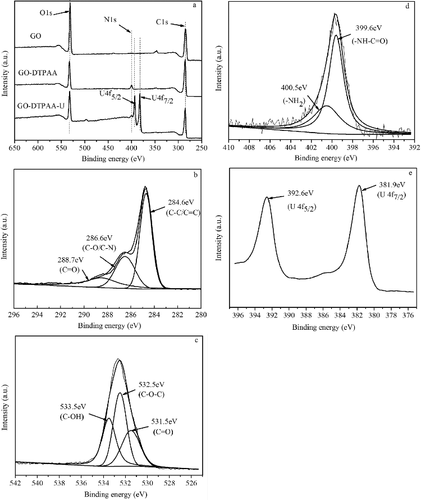
Table 1. Composition of GO, GO-DTPAA, and GO-DTPAA-U
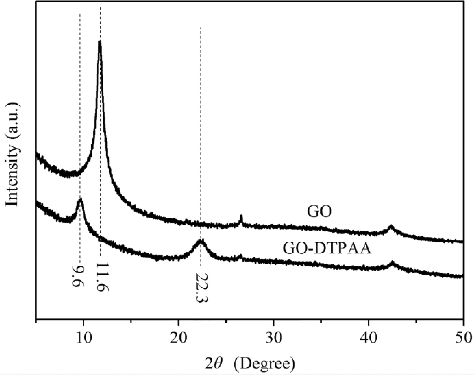
The XRD patterns of GO and GO-DTPAA are shown in . It is clear that an intense diffraction peak from (002) is at 2θ = 11.6° and corresponds to the typical peak associated with GO nanosheets and a d-spacing of 0.76 nm, which might depend on the method of preparation and on the number of layers of water in the gallery space of the material [Citation34]. After being treated with DTPAA, the peaks of the resulting functionalized GO nanosheets located at 2θ = 9.6° and 22.3° were much weaker and broader. Correspondingly, the interlayer spacing of GO nanosheets expanded to 0.92 nm because of the wrinkling or presence of functional groups between the interlayer structures caused by the intercalation of DTPAA [Citation35]. The interlayer spacing of functionalized GO was reduced to 0.39 nm because of the stitching of the exfoliated graphene nanosheets [Citation36], suggesting that DTPAA was adequately grafted onto the graphene layers and intercalated in the interlayer space of GO. It is clear that the amine groups of DTPAA successfully bonded with the carboxyl groups on the surface of the GO nanosheets.
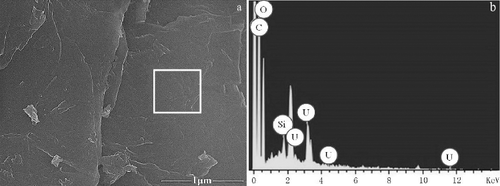
The surface topologies of the GO-DTPAA composite adsorbed uranium was observed using SEM. As seen in (a), the nanosheets had rough and wrinkled surface structures and a layered structure. Moreover, energy dispersive spectroscopy (EDS) ((b)) of a selected area on the surface of GO-DTPAA was performed to determine its chemical composition after adsorption. Results show that a higher uranium peak occurred at 3.2 keV, demonstrating that U(VI) was adsorbed on GO-DTPAA.
Figure 5. SEM images of (a) GO-DTPAA adsorbed uranium and (b) the EDS image of GO-DTPAA adsorbed with U(VI).
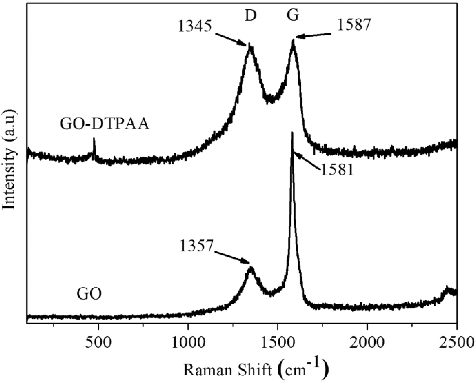
Raman spectroscopy is considered as an effective technique for characterizing composite materials. To investigate the effect of GO modified with DTPAA, the Raman spectra of pristine GO and the GO-DTPAA hybrid were measured and are shown in . The D and G bands of pristine GO appear at 1357 and 1581 cm−1, while the D and G bands of GO-DTPAA appear at 1345 and 1587 cm−1, respectively. The D band originates from the stretching vibration of sp3 carbon atoms, which induces defects and disorders, whereas the G band originates from the stretching vibration of sp2 carbon atoms, corresponding to the first-order scattering of the E2g mode [Citation37–39]. Generally, the intensity ratio of the D and G bands is used to evaluate the degree of disorder in the carbon structure. It is clear that the ratio ID/IG (0.19) for GO-DTPAA is larger than that of pristine GO (0.93), confirming that DTPAA was successfully immobilized on the surface of GO.
3.2. Effects of pH on U(VI) adsorption
The effects of pH on uranium uptake on pristine GO and the GO-DTPAA composite were investigated in the pH range of 2.0–8.0. As seen in , the uptake of uranium on GO-DTPAA composite indicates a faster increasing at pH < 6.0, while the sorption on pristine shows a slight increasing at pH < 5.5. The pristine GO nanosheets and the GO-DTPAA composite achieved their maximum amount adsorbed at pH values of 5.5 and 6.5, respectively. The adsorption of uranium onto the adsorbent was affected by the presence of various U(VI) hydrolysis products in aqueous solution [Citation40]. In the pH range of 3.0–7.5, uranium exists in the forms of (UO2)(OH)+, (UO2)2(OH)22+, and (UO2)3(OH)5+. At lower pH (pH < 4.0), the dominant uranium ion species in aqueous solution is UO22+, and higher concentrations of H+ compete with UO22+ for the binding sites on the surface of the adsorbent, resulting in a lower sorption of U(VI) on the composites. With an increase in pH, the adsorbent surface becomes more negatively charged, and the uptake of positively charged species is more feasible. In this work, pH 5.5 and 6.5 were optimal for the adsorption of uranium by the pristine GO nanosheets and GO-DTPAA composite, respectively. From the results, it is observed that the adsorption capacity of GO-DTPAA was much higher than that of GO. It is attributed to the amine and carbonxyl groups on the surface of the GO-DTPAA composite, which show a great affinity for binding with metal ions. The positively charged species of U(VI) can be coordinated with these functional groups of GO-DTPAA via ligand–metal interactions.
3.3. Effects of contact time
The effects of contact time on the removal of U(VI) using pristine GO and the GO-DTPAA composite are illustrated in . It can be seen that U(VI) uptake increases rapidly at initial stage for both sorbents, and then gradually reaches to equilibrium state. The fast uptake at initial stage is attributed to a large number of available sorption sites in the sorbents. For pristine GO, the uptake of U(VI) reached equilibrium state fastly in 30 min. But to GO-DTPAA, three hours were required to come into equilibrium. The maximum amounts of U(VI) adsorption for GO and the GO-DTPAA composite are observed to be about 97.31 and 439.42 mg·g−1 at equilibrium state, respectively. The required longer equilibrium time and higher sorption amount for GO-DTPAA composite is mainly attributed to the structural properties of a great number of functional groups on the surface of the adsorbent.
3.4. Effects of initial uranium concentration
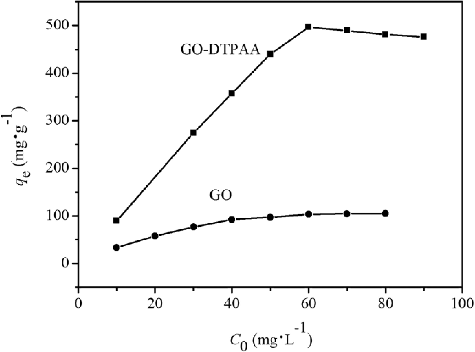
The influence of the initial concentration on U(VI) removal by pristine GO and GO-DTPAA was tested using U(VI) ion concentrations in the range of 10–85 mg·L−1. As shown in , U(VI) uptake by the two adsorbents increased with an increase in the initial U(VI) ion concentration. The adsorption of U(VI) on the GO-DTPAA adsorbent was much higher than that of pristine GO. The increase of U(VI) uptake with an increase in the initial U(VI) ion concentration was due to an increase in the concentration gradient and an increase in the frequency of adsorbent–adsorbate interactions. With an increase in the U(VI) concentration, the active sites of the adsorbent were occupied by more uranium ions, and the process of adsorption was more effective, resulting in an increased value of qe. For the case of GO-DTPAA, the adsorption amount decreases a little when uranium concentration exceeds 60 mg·L−1. It is because that the species of U(VI) in aqueous solution partly depend on total U(VI) concentrations. Uranyl ions are slowly hydrolyzed to uranyl hydroxides with increasing concentration, which may cause a slight decrease in uranium uptake.
3.5. Adsorption kinetics
Two typical adsorption kinetics models, pseudo-first-order and pseudo-second-order equations were used to interpret the adsorption mechanism of pristine GO and GO-DTPAA composite. The linear forms are expressed in EquationEquations (2)(2)
(2) and (Equation3
(3)
(3) ) [Citation41]. The parameters of the adsorption kinetics models were calculated. The pseudo-first-order kinetic model is given as
(2)
(2)
The pseudo-second-order kinetic model can be represented by the general expression
(3)
(3)
where qe (mg·g−1) is the amount of adsorbed metal ions at equilibrium, and qt (mg·g−1) is the amount of adsorbed metal ions at a given time t. k1 (min−1) and k2 (g·mg−1·min−1) are the corresponding adsorption rate constants of the first-and second-order adsorptions, respectively.
The dynamic parameters qe, k1, and k2 and the correlation coefficient R2 of the pseudo-first-order and pseudo-second-order kinetics models were calculated and are presented in . Results show that the R2 values of the pseudo-second order kinetics model for the two adsorbents are better than those of the pseudo-first order kinetics model. For each of the two adsorbents, the calculated equilibrium adsorption capacity, q2, cal (100.5 mg·g−1 for GO and 476.19 mg·g−1 for GO-DTPAA) is close to the experimental adsorption capacity, qe, exp (97.31 mg·g−1 for GO and 439.42 mg·g−1 for GO-DTPAA). It was concluded that the uranium adsorption behaviors of pristine GO and the GO-DTPAA composite both follow a pseudo-second-order kinetics model. The good fit to the pseudo-second-order kinetic model suggests that U(VI) adsorption on the pristine GO and GO-DTPAA occurred by chemical adsorption, and the valency forces are involved in adsorption process through sharing or exchanging of electrons between adsorbent and adsorbate [Citation42].
Table 2. Adsorption kinetic parameters of uranium by GO and GO-DTPAA
3.6. Adsorption isotherms
To investigate the interaction mechanism of the solid–liquid interface between U(VI) ions and the two adsorbents, three isotherm models were used to simulate the sorption data. The three models were the Freundlich, Langmuir, and Dubinin–Radushkevich (D–R) isotherm models, which are commonly used models for describing adsorption equilibrium in wastewater treatment applications.
The Langmuir isotherm model assumes that monolayer uptake occurs at the surface of an adsorbent with homogeneous binding sites [Citation43]. Mathematically, the model is generally given by EquationEquation (4)(4)
(4)
(4)
(4)
where Ce (mg·L−1) is the concentration of the metal ions at equilibrium, qe (mg·g−1) is the amount of adsorbed metal ions at equilibrium, qm (mg·g−1) is the maximum adsorption capacity, and KL (L·mg−1) is the adsorption equilibrium constant.
The Freundlich expression is an exponential equation based on the assumption that sorption behavior occurs on the sites of a heterogeneous surface with different binding energies [Citation44]. The equation is represented as the following expression
(5)
(5)
where Ce (mg·L−1) is the concentration of metal ions at equilibrium, and qe (mg·g−1) is the amount of adsorbed metal ions at equilibrium. Kf (mg·g−1) represents the Freundlich constant, which is related to the adsorption capacity, and n indicates the adsorption intensity of the adsorbent.
The D–R isotherm model can be used to describe uptake on both homogeneous and heterogeneous surfaces. This is much more general than the Langmuir model because it does not have the restrictions of surface properties. The D–R isotherm model is represented by the general expression [Citation45]
(6)
(6)
where qe (mg·g−1) is the amount of adsorbed metal ions at equilibrium, qm (mg·g−1) is the maximum adsorption capacity, β (mol2· kJ−2) is the activity coefficient related to the mean uptake energy, and ϵ is the Polanyi adsorption potential. The Polanyi adsorption potential is expressed as
(7)
(7)
where R is the ideal gas constant (8.314 J·mol−1·K−1), T is the absolute temperature in Kelvin (K), and Ce (mg·L−1) is the concentration of metal ions at equilibrium.
The value of β is a constant in the D–R model and is related to the free energy change (E, KJ·mol−1). The free energy change is the energy associated with the transfer of one mole of metal ions to the surface of the solid from infinity in the solution and can be calculated using the following equation
(8)
(8)
The thermal parameters are listed in . According to the values of the correlation coefficient, R2, the behaviors of U(VI) adsorption onto pristine GO (R2 = 0.9966) and GO-DTPAA (R2 = 0.9950) are Langmuir type isotherms. The maximum adsorption capacities, qm, calculated from Langmuir model were 122.4 and 485.0 mg·g−1, respectively, at 298.15 K. The results indicate that the GO-DTPAA composite shows a better adsorption capacity than pristine GO. The higher value of Langmuir isotherm parameter KL for GO-DTPAA also indicates that the GO-DTPAA composite is more favorable than GO. For Freundlich model, if the numerical value of n is equal to one, the partition between the two phases is independent of the concentration. If n > 1, it implies a normal adsorption. If n < 1, it indicates a cooperative adsorption [Citation46]. In present work, the value of n is larger than 1, which suggests that the uranium adsorption is a normal adsorption process. Kf represents the adsorption capacity when metal ion equilibrium concentration equals to 1. Usually, the higher the Kf value the greater the adsorption intensity [Citation47]. The value of Kf for GO-DTPAA is much higher than that of GO, indicating excellent adsorption performance of GO-DTPAA composite. The numerical value of E is used to evaluate the reaction mechanism between the adsorbent and adsorbate. Usually, the adsorption is governed by chemical forces if E is in the range of 8–16 kJ·mol−1, whereas physical forces affect the adsorption if E is less than 8 kJ·mol−1 [Citation48]. The E values of GO and GO-DTPAA were calculated to be 12.11 and 12.87 kJ·mol−1. Both of these values suggest that the adsorption mechanism is a chemical process.
Table 3. Langmuir, Freundlich, and D–R isotherm parameters of pristine GO and GO-DTPAA
3.7. Thermodynamic studies
Thermodynamic parameters, such as the Gibbs free energy change (ΔG0), enthalpy change (ΔH0), and entropy change (ΔS0), can be calculated at different temperatures using EquationEquations (9)(9)
(9) and (Equation10
(10)
(10) ) [Citation49].
(9)
(9)
where R (8.314 J·mol−1·K−1) is the ideal gas constant and T (K) is the absolute temperature in Kelvin. The lnKd term was obtained at different temperatures with a fixed initial concentration of 50 mg·L−1. The values of ΔH0 and ΔS° can be calculated from the slope and intercept of the plot of lnKd vs. 1/T.
(10)
(10)
where Kd is the value of the distribution coefficient.
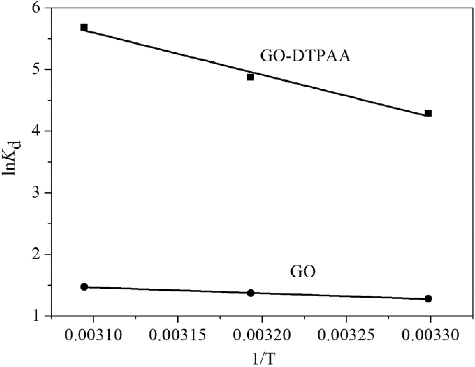
lnKd vs. 1/T for pristine GO and GO-DTPAA were plotted and are shown in . The calculated values of ΔH0, ΔS0, and ΔG0 at different temperatures (303, 313, and 323 K) are given in . The positive values of ΔH0 and ΔS0 demonstrate that the uptake was an endothermic process and that the randomness of the system increased at the solid–liquid interface during the uptake process using both pristine GO and the GO-DTPAA. The values of ΔG0 decreased with an increase in temperature, suggesting that the adsorption of U(VI) on pristine GO and the GO-DTPAA composite are spontaneous processes in nature and that they favor uptake at high temperature. Moreover, in comparison with the values of ΔG0 at different temperatures, it is clear that the adsorption of uranium by GO-DTPAA was more favorable than that by GO. The results may suggest some structural changes in GO-DTPAA and indicate that GO-DTPAA is a potentially useful adsorbent for the removal of uranium ions from aqueous solution.
Table 4. Thermodynamic parameters for U(VI) adsorption onto GO and GO-DTPAA
3.8. Comparison with other GO-based adsorbents
For the purpose of evaluating the prospects of potential applications, the efficiency of uranium removal using GO-DTPAA was compared with that of other adsorbents. The comparisons are summarized in . According to the results, the maximum adsorption capacity for U(VI) in this work was 485.0 mg·g−1, which is larger than that reported in the literature for other adsorbents [Citation16,Citation18,Citation50–53], illustrating that the GO-DTPAA composite material may have great prospects for applications in removing U(VI) from aqueous solution.
Table 5. Comparison of U(VI) adsorption capacity by different sorbents
4. Conclusions
In this work, functionalized GO (GO-DTPAA) was synthesized by introducing the chelating reagent DTPAA onto GO with the goal of using this synthesized material for the removal of uranium ions from aqueous solution. Pristine GO and the as-synthesized GO-DTPAA before and after adsorption were characterized using various analytical techniques: FTIR, XRD, SEM, Raman, and XPS. Results demonstrated that amine-groups were grafted successfully onto the surface of GO. The uptake properties of the GO-DTPAA composite were compared to the sorption by pristine GO using the batch method. It was found that the adsorption processes of pristine GO and GO-DTPAA were well fitted to the Langmuir isotherm model and to the pseudo-second order kinetics model. The D–R isotherm model demonstrated that chemisorption plays a dominant role in the adsorption process. Results indicated that the uranium adsorption capacity of the GO-DTPAA hybrid materials was larger than that of pristine GO. The thermodynamic parameters revealed that adsorption of uranium ions by pristine GO and by GO-DTPAA are feasible, spontaneous, and endothermic.
Acknowledgments
This work was supported by the National Natural Science Foundation of China [grant number 21667002], [grant number 21367001]; Key Research and Development Projects of Jiangxi Province [grant number 20161BBG70085]; Fundamental Science on Radioactive Geology and Exploration Technology Laboratory [grant number RGET1607]; National Scientific and Technological Cooperation Project of Jiangxi Province [grant number 20161BBH80046].
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mudd GM, Diesendorf M. Sustainability of uranium mining and milling: toward quantifying resources and eco-efficiency. Environ Sci Technol. 2008;42:2624–2630.
- Kitagaki T, Kaneshiki T, Nomura M, et al. Uranium separation from a simulant fuel debris solution using a benzimidazole-type anion exchange resin. J Nucl Sci Technol. 2016;53:1639–1646.
- Ohashi Y, Tanaka Y, Tsunashima Y, et al. Development of methods for recovering uranium from sludge-like uranium generated in decontamination of metal wastes. J Nucl Sci Technol. 2017;54:382–390.
- Turanov AN, Karandashev VK, Boltoeva M, et al. Synergistic extraction of uranium(VI) with TODGA and hydrophobic ionic liquid mixtures into molecular diluent. Sep Purif Technol. 2016;164:97–106.
- Tsurutat T. Removal and recovery of uranium using microorganisms isolated from Japanese uranium deposits. J Nucl Sci Technol. 2006;43:896–902.
- Pan DQ, Fan QH, Fan FY, et al. Removal of uranium contaminant from aqueous solution by chitosan@attapulgite composite. Sep Purif Technol. 2017;177:86–93.
- Mohan D, Pittman CU Jr. Arsenic removal from water/wastewater using adsorbents – a critical review. J Hazard Mater. 2007;142:1–53.
- Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. J Environ Manage. 2011;92:407–418.
- Bakatula EN, Molaudzi R, Nekhunguni P, et al. The removal of arsenic and uranium from aqueous solutions by sorption onto iron oxide-coated zeolite (IOCZ). Water Air Soil Pollut. 2017;228:5–18.
- Mahmoud ME, Khalifa MA, Wakeel YME, et al. Engineered nano-magnetic iron oxide-urea-activated carbon nanolayer sorbent for potential removal of uranium (VI) from aqueous solution. J Nucl Mater. 2017;487:3–22.
- Ji GJ, Zhu GR, Wang XH, et al. Preparation of amidoxime functionalized SBA-15 with platelet shape and adsorption property of U(VI). Sep Purif Technol. 2017;174:455–465.
- Liu XY, Wang LH, Zheng Z, et al. Molecular dynamics simulation of the diffusion of uranium species in clay pores. J Hazard Mater. 2013;244:21–28.
- Arica MY, Bayramoglu G. Polyaniline coated magnetic carboxymethylcellulose beads for selective removal of uranium ions from aqueous solution. J Radioanal Nucl Chem. 2016;310:711–724.
- Hu L, Yan XW, Yao CG, et al. Preparation of amidoximated coaxial electrospun nanofibers for uranyl uptake and their electrochemical properties. Sep Purif Technol. 2016;171:455–465.
- Wang Z, Zhu W, Qiu Y, et al. Biological and environmental interactions of emerging two-dimensional nanomaterials. Chem Soc Rev. 2016;45:1750–1780.
- Liu SJ, Li S, Zhang HX, et al. Removal of uranium(VI) from aqueous solution using graphene oxide and its amine-functionalized composite. J Radioanal Nucl Chem. 2016;309:607–614.
- Shao DD, Li JX, Wang XK. Poly(amidoxime)-reduced graphene oxide composites as adsorbents for the enrichment of uranium from seawater. Sci China Chem. 2014;57:1449–1458.
- Chen LL, Zhao DL, Chen SH, et al. One-step fabrication of amino functionalized magnetic graphene oxide composite for uranium(VI) removal. J Colloid Interface Sci. 2016;472:99–107.
- Song WC, Wang XX, Wang Q, et al. Plasma-induced grafting of polyacrylamide on graphene oxide nanosheets for simultaneous removal of radionuclides. Phys Chem Chem Phys. 2015;17:398–406.
- Zhang ZB, Qiu YF, Dai Y, et al. Synthesis and application of sulfonated graphene oxide for the adsorption of uranium(VI) from aqueous solutions. J Radioanal Nucl Chem. 2016;310:547–557.
- Liu X, Li JX, Wang XX, et al. High performance of phosphate-functionalized graphene oxide for the selective adsorption of U(VI) from acidic solution. J Nucl Mater. 2015;466:56–64.
- Haney CR, Buehler PW, Gulati A. Synthesis and characterization of a novel DTPA polymerized hemoglobin based oxygen carrier. Biochim Biophys Acta. 2005;1725:358–369.
- Fellenz N, Perez-Alonso FJ, Martin PP, et al. Chromium (VI) removal from water by means of adsorption-reduction at the surface of amino-functionalized MCM-41 sorbents. Micropor Mesopor Mater. 2017;239:138–146.
- Pana YF, Cai PX, Farmahini M, et al. Amino-functionalized alkaline clay with cationic star-shaped polymer as adsorbents for removal of Cr(VI) in aqueous solution. Appl Surf Sci. 2016;385:333–340.
- Peng W, Xie ZZ, Cheng G, et al. Amino-functionalized adsorbent prepared by means of Cu(II) imprinted method and its selective removal of copper from aqueous solutions. J Hazard Mater. 2015;294:9–16.
- Yin N, Wang K, Wang LZ, et al. Amino-functionalized MOFs combining ceramic membrane ultrafiltration for Pb (II) removal. Chem Eng J. 2016;306:619–628.
- Morsy AMA, Ali AH. Sorption of uranium from waste effluent solutions by mesoporous carbon impregnated with trioctylamine. Radiochemistry. 2017;59:152–159.
- Sutar DS, Narayanam PK, Singh G, et al. Spectroscopic studies of large sheets of graphene oxide and reduced graphene oxide monolayers prepared by Langmuir−Blodgett technique. Thin Solid Films. 2012;520:5991–5996.
- Pei Z, Li L, Sun L, et al. Adsorption characteristics of 1,2,4-trichlorobenzene, 2,4,6-trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon. 2013;51:156–163.
- Xu J, Wang L, Zhu Y. Decontamination of bisphenol A from aqueous solution by graphene adsorption. Langmuir. 2012;28:8418–8425.
- Gao BJ, Gao YC, Li YB. Preparation and chelation adsorption property of composite chelating material poly(amidoxime)/SiO2 towards heavy metal ions. Chem Eng J. 2010;158:542–549.
- Barkleit A, Foerstendorf H, Li B, et al. Coordination of uranium(VI) with functional groups of bacterial lipopolysaccharide studied by EXAFS and FT-IR spectroscopy. Dalton Trans. 2011;40:9868–9876.
- Pan N, Li L, Ding J, et al. Preparation of graphene oxide-manganese dioxide for highly efficient adsorption and separation of Th(IV)/U(VI). J Hazard Mater. 2016;309:107–115.
- Stobinski L, Lesiak B, Malolepszy A, et al. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J Electron Spectrosc Relat Phenom. 2014;195:145–154.
- Kim NH, Kuila T, Lee JH. Simultaneous reduction, functionalization and stitching of graphene oxide with ethylenediamine for composites application. J Mater Chem A. 2013;1:1349–1358.
- Hu L, Jiang PP, Zhang PB, et al. Amine-graphene oxide/waterborne polyurethane nanocomposites: effects of different amine modifiers on physical properties. J Mater Sci. 2016;51:8296–8309.
- Chen J, Yao B, Li C, et al. An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon. 2013;64:225–229.
- Yang G, Cao J, Li L, et al. Carboxymethyl chitosan-functionalized graphene for label free electrochemical cytosensing. Carbon. 2013;51:124–133.
- Fang M, Long J, Zhao WF, et al. pH-responsive chitosan-mediated graphene dispersions. Langmuir. 2010;26:16771–16774.
- Camacho LM, Deng SG, Parra RR. Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater. 2010;175:393–398.
- Wang GH, Wang XG, Chai XJ, et al. Adsorption of uranium (VI) from aqueous solution on calcined and acid-activated kaolin. Appl Clay Sci. 2010;47:448–451.
- Wang Y, Gu ZX, Yang JJ, et al. Amidoxime-grafted multiwalled carbon nanotubes by plasma techniques for efficient removal of uranium(VI). Appl Surf Sci. 2014;320:10–20.
- Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40:1361–1403.
- Freundlich H. Concerning adsorption in solutions. Z Physik Chem A. 1906;57:385–470.
- Dubinin MM, Radushkevich LV. The equation of the characteristic curve of activated charcoal. Proc Acad Sci USSR Phys Chem Sect. 1947;55:331–337.
- Qin J, Qiu FX, Rong XS, et al. Removal of basic fuchsin dye from aqueous solutions using graphite oxide modified aromatic polyurethane foam material. Toxicol Environ Chem. 2014;96:849–860.
- Liu SJ, Luo MB, Li JQ, et al. Adsorption equilibrium and kinetics of uranium onto porous azo-metal organic frameworks. J Radioanal Nucl Chem. 2016;310:353–362.
- Senol S, Meral E. Uranium adsorption studies on aminopropyl modified mesoporous sorbent (NH2-MCM-41) using statistical design method. J Nucl Mater. 2010;406:285–292.
- Guo XY, Du B, Wei Q, et al. Synthesis of amino functionalized magnetic graphenes composite material and its application to remove Cr(VI), Pb(II), Hg(II), Cd(II) and Ni(II) from contaminated water. J Hazard Mater. 2014;278:211–220.
- Yu SJ, Wang J, Song S, et al. One-pot synthesis of graphene oxide and Ni-Al layered double hydroxides nanocomposites for the efficient removal of U(VI) from wastewater. Sci China Chem. 2017;60:415–422.
- Lingamdinne LP, Choi YL, Kim IS, et al. Preparation and characterization of porous reduced graphene oxide based inverse spinel nickel ferrite nanocomposite for adsorption removal of radionuclides. J Hazard Mater. 2017;326:145–156.
- Yang PP, Liu Q, Liu JY, et al. Bovine serum albumin-coated graphene oxide for effective adsorption of uranium(VI) from aqueous solutions. Ind Eng Chem Res. 2017;56:3588–3598.
- Shao L, Zhong JR, Ren YM, et al. Perhydroxy-CB[6]decorated graphene oxide composite for uranium (VI) removal. J Radioanal Nucl Chem. 2017;311:627–635.

![Figure 7. Effects of pH on U(VI) adsorption onto GO and GO-DTPAA. (C[U(VI)] = 50 mg·L−1 and T = 25 °C).](/cms/asset/035ed8d0-bb6a-4e67-bfd2-74812fc30ec4/tnst_a_1439415_f0007_b.gif)
![Figure 8. Effects of time on U(VI) adsorption onto GO and GO-DTPAA. (C[U(VI)] = 50 mg·L−1, pH = 6.5 and T = 25 °C).](/cms/asset/58b2256b-b99e-4dfc-97ec-42c10f71f2a9/tnst_a_1439415_f0008_b.gif)