?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
This study revealed melting behavior and thermal conductivity of four samples generated by sodium-concrete reaction (SCR). We prepared the samples using two methods such as firing mixtures of sodium (Na) and grinded concrete powder, and sampling depositions after the SCR experiments. In the former, the mixing ratios were determined from the past experiment. The latter simulated the more realistic conditions such as the temperature history and the distribution of Na and concrete. The thermogravimetry-differential thermal analyzer (TG-DTA) measurement showed the temperatures of the onset of the melting (solidus temperatures) were 865–942°C, but those of the samples containing metallic Na could not be clarified. In the two more realistic samples, the compression moldings in a furnace were observed. The observation revealed the softening temperature was 800–840°C and the solidus temperature was 840–850°C, which was 10–20°C lower than the TG-DTA results. The thermodynamics calculation of FactSage 7.2 revealed the solidus temperature was caused by melting of the some components such as Na2SiO3 and/or Na4SiO4 and NaAlO2. Moreover, the thermal conductivity was λ ~ 1–3 W/m-K, which was comparable to xNa2O - (1 - x)SiO2 (x = 0.5, 0.33, and 0.25), and that at 700°C was explained by NBO/T of EquationEquation (1)(1)
(1) .
1. Introduction
The interaction between sodium (Na) and concrete in the coexistence of high-temperature core debris during a postulated reactor vessel melt-through accident in sodium-cooled fast reactors (SFRs) is very important from the viewpoint of the containment integrity [Citation1] because the interaction generates a large amount of hydrogen gas from the Na pool and the core debris [Citation2,Citation3]. Up to date, the phenomenon has never been researched sufficiently due to the severe experimental conditions such as high-temperature, high-reactivity, and high-corrosive environment. However, the extensive researches on the interaction should be carried out for key issues of the safety assessment.
Japan Atomic Energy Agency (JAEA) has been developing the safety assessment method of the severe accidents such as the CONTAIN-LMR code [Citation4] and carried out the various experiments to understand the characteristics of the accidents. The authors’ experiments revealed the self-terminating behavior of the Na-concrete reaction (SCR), which had the great impacts on the consequences of the safety assessment [Citation5,Citation6]. The authors suggested that the reaction terminating was caused by deposition of the reaction products in the reaction zone. The deposition becomes a barrier for the chemical reaction. Furthermore, the persistent termination of the reaction needs that the deposited reaction products exist stable under a high-temperature condition.
The decay heat removal from the core debris is often used as the one of the major mitigation methods for the melt-through accident despite of the reactor types. However, in the light water reactors, crusts would generate on the surface of the fuel debris and suppress the heat transfer. Masaya Kondo et al. proposed the effective heat transfer coefficient was proportional to the porosity of the crust [Citation7]. In the SFRs, the SCR products could accumulate on the surroundings of the core debris and the concrete base mat if the leaked hot Na has contacted with the concrete directly during the reactor vessel melt-through accidents [Citation8]. Then, the products might disturb the heat removal from the fuel debris for the low thermal conductivity [Citation9]. The thermal conductivity is very important property for the heat transfer from the core debris when the SCR products cover the surface of core debris during the severe accidents. Furthermore, melting and relocation behavior of the deposited SCR products under high-temperature condition are a key issue to determine the material contacting with the core debris. When the products melt and relocate from the top of the core debris, the heat of the core debris can transfer directly the leaked coolant and the cover gas.
This research revealed the temperature of the onset of the melting (solidus temperature) of the reaction products for the relocation and the thermal conductivity of the SCR products as the important physical property for the heat transfer.
2. Experiments
2.1. Preparation of simulated SCR products
and show the preparing conditions for the SCR products and the compositions of the concrete, respectively. The mixing ratios were determined from the past SCR experiments, which showed 32: 68 wt.% and 60: 40 wt.% in the mass ratios of Na: concrete at the bottom and at the middle of the reaction zone, respectively [Citation5,Citation6]. The former ratio (S2LL1) is almost equivalent to the concentration of Na2SiO3 and the latter (S2HL1) means metallic Na remains in the products. shows the outlines of two preparing methods of the simulated SCR products. S2LL1 and S2HL1 were prepared by firing the mixtures of the above-mentioned concentrations at 630°C for 1 h in a muffle furnace in an argon atmosphere () [Citation9]. S3NH1 and S3NH2 were prepared by sampling deposits of reaction products after middle-scale SCR experiments to simulate the more realistic conditions such as the temperature history and the distribution of Na and concrete (). In the SCR experiments, 7.6 kg of Na and 23 or 47 kg of concrete blocks were used. The cover gas was argon. The details of experimental condition were referred to our previous paper [Citation8]. Before measuring the physical properties, the four samples were crushed in a crucible and fired at 1500°C in an inert gas to simulate the metallic Na vaporization due to the low saturated vapor pressure under the severe accident condition. The appearance of the samples was uniformly crystallized. However, S2HL1 hardly remained by the firing so that the physical properties were measured at only room temperature before the firing.
Table 1. Preparing conditions: concentrations of Na, Si, Al, and Ca, heat-treatment (temperature and reaction time), cover gas condition and XRD results
Table 2. Compositions of the concrete
Figure 1. Outlines of preparation of the SCR products: (a) Firing the mixtures in a muffle furnace (uniformed concentration) [Citation9], (b) SCR tests (ununiformed concentration) [Citation8].
![Figure 1. Outlines of preparation of the SCR products: (a) Firing the mixtures in a muffle furnace (uniformed concentration) [Citation9], (b) SCR tests (ununiformed concentration) [Citation8].](/cms/asset/9eb1cc6c-7cad-401b-bfa3-73ad90a421c3/tnst_a_1599744_f0001_b.gif)
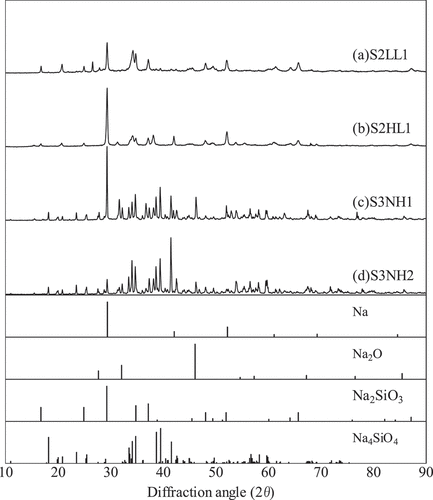
X-ray diffraction (XRD) measurement identified the chemical formula of the main components in the samples before firing at 1500°C. shows the XRD spectra of the four samples and the reference peaks of Na, Na2O, Na2SiO3, and Na4SiO4 according to the International Centre for Diffraction Data (ICDD) card. shows the dispensed concentrations of S2LL1 and S2HL1 and the analyzed ones of S3NH1 and S3NH2, together. The concentrations of Na, Si, Al, and Ca in S2LL1 and S2HL1 were calculated from the mixing ratios of the Na and the concrete according to the specified concentration of . On the other hand, the concentrations of Na in S3NH1 and S3NH2 were analyzed by Atomic Absorption Spectrometry (AAS) and those of Si, Al, and Ca were analyzed by Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) before firing at 1500°C.
2.2. Melting behavior
The solidus temperatures of the SCR products were analyzed by a thermogravimetry-differential thermal analyzer (TG-DTA) of NETZSCH 2000SE. The heating rate and the temperature range were set to 10 K/min and from room temperature to 1500°C. Furthermore, the melting behavior of the compression moldings (10 mmɸ×3.5 mmH) for S3NH1 and S3NH2 was observed directly in a furnace replaced by argon gas. Then, the characteristic temperatures of rounding the shape and spreading the skirt were identified as a softening temperature and a solidus temperature, respectively.
2.3. Thermal conductivity
The density (ρ), the specific heat (Cp) and the thermal diffusivity (α) were measured to find the thermal conductivity (λ) using λ = ρCpα.ρ at room temperature was measured with the Archimedes’ principle using an argon gas and the temperature dependency was measured by a thermomechanical analyzer of Rigaku TMA8140. Cp was measured by a differential scanning calorimetry of NETZSCH STA449C Jupiter as reference of standard Al2O3. α was measured by a laser flash method of ULVAC TC-9000.
3. Results
3.1. Melting behavior
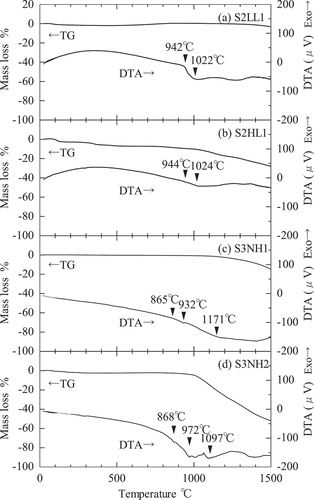
shows the TG-DTA curves for S2LL1, S2HL1, S3NH1, and S3NH2. The DTA curve of S2LL1 decreased rapidly around 942°C and the TG curve of S2LL1 did not show a drastic change. Because the main components were Na2SiO3 and SiO2 from XRD results and SiO2 was very stable below the melting point of 1710°C, the rapid decrease around 942°C was found to be the solidus temperature of S2LL1 by Na2SiO3 melting. In S3NH1 and S3NH2, the DTA curves decreased slightly around 865°C and 868°C, respectively and the TG curves did not change drastically below 1000°C. They might correspond to the melting of the some components in the products. The solidus temperature of S2HL1 could not be clarified because the TG decreased continuously over 100°C by evaporation of the metallic Na.
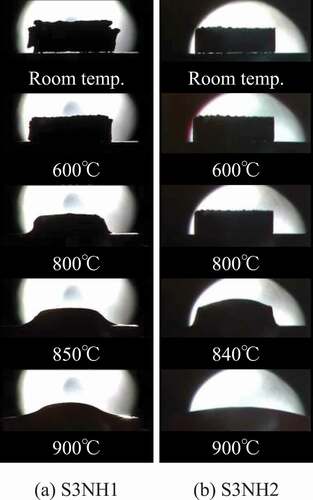
In addition, the melting behavior of S3NH1 and S3NH2 in a muffle furnace was observed directly to clarify the solidus temperatures. shows the shape change of the compression moldings of S3NH1 and S3NH2 at room temperature, 600°C, 800°C, the solidus temperature (840–850°C) and 900°C, respectively. The shapes did not change largely below 600°C. As heating, the corners of the compression moldings became rounded around 800–840°C, the skirts spread largely around 840–850°C and they spread sufficiently at 900°C. Therefore, the softening temperatures and the solidus temperatures were found to be 800–840°C and 840–850°C, respectively. The characteristic temperatures obtained by the direct observations were 10–20°C lower than the small decreases in the DTA curves.
3.2. Thermal conductivity
shows the measured values of the densities (ρ kg/m3), the specific heat (Cp
J/kg-K), and the thermal diffusivities (α
m2/s) in S3NH1 and S3NH2. The open and closed circles and squares in show the thermal diffusivities for S2LL1, S2HL1, S3NH1, and S3NH2 as a function of temperature measured by the laser flash method. The error bars show unavoidable errors from the experimental device. The bold solid, the bold dashed, and the thin solid lines show the thermal diffusivities of xNa2O - (1 - x)SiO2 (x = 0.5, 0.33–0.35, and 0.25) to compare with the main components in the products, respectively obtained by the calculation of α = ρCp/λ using the literatures [Citation10–Citation13]. The thermal diffusivity of S2HL1 is the highest among the four samples because it contains an amount of Na. The thermal diffusivities of S2LL1, S3NH1, and S3NH2 are ranged at 0.3–1.3 m2/s, which shows the almost same as the xNa2O - (1 - x)SiO2 (x = 0.5, 0.33–0.35, and 0.25). Furthermore, the thermal diffusivities obtained by the present experiment show insignificant temperature dependence or slightly decreasing.
Table 3. The densities (ρ kg/m3), the specific heat (Cp
J/kg-K), and the thermal diffusivities (α
m2/s) in S3NH1 and S3NH2 obtained by the experiments
Figure 5. Thermal diffusivities of S2LL1, S2HL1, S3NH1, and S3NH2 and thermal diffusivities of xNa2O - (1 - x)SiO2 (x = 0.5, 0.33–0.35, and 0.25) [Citation10–Citation13] together.
![Figure 5. Thermal diffusivities of S2LL1, S2HL1, S3NH1, and S3NH2 and thermal diffusivities of xNa2O - (1 - x)SiO2 (x = 0.5, 0.33–0.35, and 0.25) [Citation10–Citation13] together.](/cms/asset/77fbce6c-9ae2-460b-a945-44e618b4640b/tnst_a_1599744_f0005_b.gif)
shows the thermal conductivity for S2LL1 [Citation9], S2HL1 [Citation9], S3NH1, and S3NH2 by the calculation of λ = ρCpα as mentioned in Section 2.3. The thermal conductivity is ranged at 0.9–2.8 W/m-K, which is almost comparable to those of xNa2O - (1 - x)SiO2 (x = 0.5, 0.33, and 0.25). The thermal conductivity of S2LL1 increases slightly with temperature. However, that of S3NH1 and S3NH2 decreases slightly with temperature. From , the reason is the ρ and the α in S3NH1 and S3NH2 decrease monotonously at least below 600°C, and the Cp increases monotonously except for 500°C for S3NH1 and 200°C for S3NH2, which might be caused by transitions. Furthermore, the thermal conductivity shows the same temperature dependence as 0.5Na2O-0.5SiO2, which is the same dependency as the thermal diffusivities. The thermal conductivity of the samples is ranged from that of 0.5Na2O-0.5SiO2 to that of 0.25Na2O-0.75SiO2.
Figure 6. Thermal conductivity of S2LL1 [Citation9], S2HL1 [Citation9], S3NH1, and S3NH2 and the thermal conductivity of xNa2O - (1 - x)SiO2 (x = 0.5 [Citation10], 0.33 [Citation11], and 0.25 [Citation10]) together.
![Figure 6. Thermal conductivity of S2LL1 [Citation9], S2HL1 [Citation9], S3NH1, and S3NH2 and the thermal conductivity of xNa2O - (1 - x)SiO2 (x = 0.5 [Citation10], 0.33 [Citation11], and 0.25 [Citation10]) together.](/cms/asset/5938be63-a02d-4aa4-958f-8431ff1ea4b4/tnst_a_1599744_f0006_b.gif)
4. Discussion
The present experiment shows the melting behavior and the thermal conductivity of the SCR products are comparable to those of the Na silicates which is a major component in the products. However, because the concrete contains the other components such as Al2O3 and CaO, we will discuss their effects by the thermodynamics calculation.
shows the estimated molar ratios of Na2O, SiO2, Al2O3, and CaO in S2LL1, S2HL1, S3NH1, and S3NH2 after vaporizing the residual metallic Na. In the estimation, the amount of Na silicate was calculated from those of Na or Si in . The amounts of Na2O and SiO2 were estimated from that of the Na silicate. The excessive Si in this Na silicate calculation added to the estimated amount of SiO2 because SiO2 was obtained by the XRD (S2LL1 in ). Similarly, the excessive Na added to the estimated amount of Na2O in the case of S3NH1, and was assumed as metallic Na in the case of S2HL1 and S3NH2 as shown in . The amounts of the Al2O3 and CaO were calculated from those of Al and Ca in . The XRD could not identify the chemical formulas of the aluminum and the calcium compounds because the strong signals of the main components such as the Na silicates hid their weak signals. Therefore, Al2O3 and CaO which were the same chemical formulas as the original concrete were assumed. Then, the total molar ratios were set to 100 mol.%.
Table 4. Estimated molar ratios of Na2O, SiO2, Al2O3, and CaO after Na evaporation
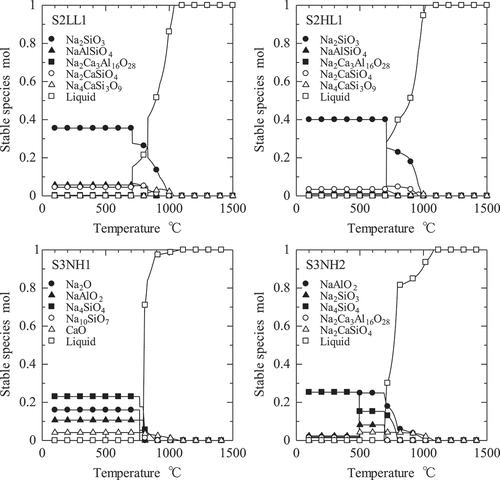
shows the stable species of S2LL1, S2HL1, S3NH1, and S3NH2 obtained by the thermodynamics calculation of FactSage 7.2 under the assumption of . The open square in shows the liquid slag phase and the others show the solid species. The lowest temperatures of the liquid phase which mean the temperature of the onset of the melting (solidus temperature) are 708–710°C and 800°C in S2LL1, S2HL1, S3NH2, and S3NH1, respectively. In S2LL1 and S2HL1, the solidus temperatures of 708–710°C are caused by Na2SiO3 which is the main component. In S3NH1 and S3NH2, they are caused by Na4SiO4 and/or Na2SiO3, and NaAlO2. The unreacted Na2O in S3NH1 reduces to Na10SiO7 at 768°C which is lower than the solidus temperature. Namely, the solidus temperature depends on the melting behavior of the some components of the Na silicates such as Na2SiO3 and/or Na4SiO4, and the Na aluminates such as NaAlO2. On the other hand, because the calcium components are the most stable under the high temperature, they start melting above 900°C. The thermodynamics calculation predicted the all species melted perfectly below 1100°C at least. Therefore, the liquidus temperature strongly depended on the calcium components, which melted perfectly below 1100°C at least.
Figure 7. Temperature dependency of the stable species of S2LL1, S2HL1, S3NH1, and S3NH2 obtained by the thermodynamics calculation of the FactSage 7.2.
A lattice vibration plays an important role in a thermal conductivity of crystals at high temperature because a phonon conducts majority of heat. However, thermal conductivity of glass or glassy slag governed by a short-range order depends on the structures of SiO44- and the cations such as Na+, Ca2+, or Al3+. Nonbridging oxygen ions per tetrahedrally coordinated cation (NBO/T) provides the proportion of tetrahedrally coordinated cations. Here, SiO2 and Al2O3 work as a network-former, but Na2O and CaO work as a network-breaker [Citation14]. Furthermore, the Na silicates such as xNa2O - (1 - x)SiO2 (x ≤ 0.5) are known to occur a glass transition below 520°C [Citation12], and the SCR samples could underlie a glass transition around the specific temperatures of the specific heat. Therefore, we could discuss the measured thermal conductivity around 700°C as well as the glass-like materials.
Mills and Susa [Citation15] and Hasegawa et al. [Citation16] suggested the relation between thermal conductivity and the NBO/T for various slags under high-temperature condition. The NBO/T is following:
Where is the mass ratio of the i component. The thermal conductivity in molten silicate of Al2O3-CaO-Na2O-SiO2 decreases with NBO/T increasing, and reaches to the constant value of about 1.5. shows the thermal conductivity of the literature data for the molten Al2O3-CaO-Na2O-SiO2 [Citation16] and the measured data of S3NH1 and S3NH2 at 700°C. The NBO/T of S3NH1 and S3NH2 are about 3.9 and 1.8 because they have many amounts of Na2O. The measured thermal conductivity shows 1.29 and 1.63, which are explained by EquationEquation (1)
(1)
(1) .
Figure 8. Relation between the thermal conductivity of S3NH1 and S3NH2 at 700°C and the molten Al2O3-CaO-Na2O-SiO2 [Citation16] and NBO/T.
![Figure 8. Relation between the thermal conductivity of S3NH1 and S3NH2 at 700°C and the molten Al2O3-CaO-Na2O-SiO2 [Citation16] and NBO/T.](/cms/asset/eefd9f31-b60e-4a8f-b891-ecbc65831662/tnst_a_1599744_f0008_b.gif)
5. Conclusion
This study revealed the melting behavior and the thermal conductivity of the four solid SCR products. We prepared the samples in the two methods. One of the methods is firing the mixtures of Na and grinded concrete powder in the muffle furnace replaced by the argon gas. The mixing ratios were determined from the past SCR experimental data [Citation5]. The other is sampling the depositions of the SCR products after the middle-scale SCR experiments to simulate more realistic conditions [Citation8]. The XRD measurements identified Na2SiO3 and/or Na4SiO4 as the main components in the products.
The TG-DTA measurements showed that the solidus temperatures were about 865–942°C, but those of the samples containing residual metallic Na could not be clarified because of the Na evaporation. Furthermore, for S3NH1 and S3NH2, the observation in the furnace revealed the softening temperature was about 800–840°C and the solidus temperature was 840–850°C, which was 10–20°C lower than the TG-DTA results. The thermodynamics calculation using FactSage 7.2 revealed that the temperature of the onset of melting, which was nearly to the softening temperature, was caused by melting of the some components such as Na2SiO3 and/or Na4SiO4, and NaAlO2. The liquidus temperature strongly depended on the calcium components, which melted perfectly below 1100°C at least.
The thermal conductivity was λ ~ 1–3 W/m-K, which were comparable to xNa2O - (1 - x)SiO2 (x = 0.5, 0.33, and 0.25). According to the literatures [Citation11,Citation16], the thermal conductivity of the molten slags depends to the NBO/T. The thermal conductivity of S3NH1 and S3NH2 at 700°C shows 1.29 and 1.63 W/m-K which are explained by EquationEquation (1)(1)
(1) .
Nomenclature
| ρ | = | Density kg/m3 |
| Cp | = | Specific heat J/g-K |
| α | = | Thermal diffusivity m2/s |
| λ | = | Thermal conductivity W/m-K |
| = | Nonbridging oxygen ions per tetrahedrally coordinated cation | |
| xi | = | Mass ratio of i component |
Acknowledgments
The authors gratefully acknowledge the valuable team contributions made by a large number of the colleagues in Japan Atomic Energy Agency (JAEA). Especially, the authors would like to express their thanks to Mr. H. Seino for the experimental advice. The authors would like to express their thanks to Mr. T. Watanabe for operation of Sodium Handling Training Facility related to SCR experiments. Also, the authors would like to express their thanks to Dr. T. Kawai from Kobelco Research Institute for his valuable technical supports and advice.
This work was supported by Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT) and Japan Science and Technology Agency [Development of Estimation Technology for Availability of Measure for Failure of Containment Vessel in Sodium Cooled Fast Reactor]. The presented work is a part of the result of “Development of Estimation Technology for Availability of Measure for Failure of Containment Vessel in Sodium Cooled Fast Reactor” conducted by the charge from the University of Fukui as the MEXT fund research program.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Ludewig H, Powers DA, Hewson JC, et al. Sodium fast reactor safety and licensing research plan volume II. U. S.: Sandia National Laboratories; 2012. (Report no. SAND 2012-4259).
- Ohno S, Seino H, Miyahara S. Development of severe accident evaluation technology (Level 2 PSA) for sodium-cooled fast reactors. (5) Identification of dominant factors in ex-vessel accident sequences. Proc. 2009 International Congress on Advances in Nuclear Power Plants (ICAPP ‘09); 2009 May 10–14;Tokyo (Japan).
- Swanson DG, Castle JN. Safety evaluation report related to the construction of the clinch river breeder reactor plant. Tennessee (U.S.): U. S. nuclear commission; 1983. (Report no. NUREG-0968).
- Miyahara S, Seino H, Konishi K, et al. Development of fast reactor containment safety analysis code, CONTAIN-LMR (1) Outline of development project. Proc. 23rd international conference on nuclear engineering; 2015 May 17–21;Chiba (Japan).
- Kawaguchi M, Doi D, Seino H, et al. A study on self-terminating behavior of sodium-concrete reaction. J Nucl Sci Tehnol. 2016 Jul;53(12):2098–2107.
- Kawaguchi M, Miyahara S, Uno M. A study on self-terminating behavior of sodium-concrete reaction (2). J Nucl Sci Tehnol. 2018 Mar;55(8):874–884.
- Kondo M, Nishida A, Sugimoto J Study of overall heat transfer coefficient from upper crust to overlaying water during MCCI. Proc. 23rd international conference on nuclear engineering; 2015 May 17–21; Chiba (Japan).
- Kawaguchi M, Miyahara S, Uno M Discussion about sodium-concrete reaction in presence of internal heater. Proc. 26th Int. Conf. on Nucl. Eng. (ICONE26); 2018 Jul 22–26;London (England).
- Kawaguchi M, Miyahara S, Uno M. Thermophysical properties of sodium-concrete reaction products. Netsu Sokutei. 2018;45(1):2–8. [in Japanese].
- Nagata K, Susa M, Goto K. Thermal conductivities of slags for ironmaking and steelmaking. Tetsu-to-Hagane. 1983;69(11):1417–1424. [in Japanese].
- Hayashi M, Ishii H, Susa M, et al. Effect of ionicity of nonbridging oxygen ions on thermal conductivity of molten alkali silicates. Phys Chem Glasses. 2001 Feb;42(1):6–11.
- Knoche R, Dingwell DB, Seifert FA, et al. Non-linear properties of supercooled liquids in the system Na2O-SiO2. Chem Geol. 1994 Sep;116:1–16.
- Donato B, Carlo G, Giulio O, et al. Ab initio thermodynamic and thermophysical properties of sodium metasilicate, Na2SiO3, and their electron-density and electron-pair-density counterparts. J Phys Chem A. 2016 Oct;120(44):8881–8895.
- Mills KC. SLAG ATLAS 2nd Edition: 1 Structure of liquid slags. Dusseldorf (Germany): Verlag Stahleisen GmbH; 1995. p. 1–8.
- Mills KC, Susa M. Slag Atlas. 2nd ed. D-Dusseldorf (Germany): European communities; 1995. ( Report no. ECSC Research 7210-CF/107).
- Hasegawa H, Kowatari T, Shiroki Y, et al. Thermal conductivity of molten silicate of Al2O3-CaO-Na2O-SiO2 measured by means of a front heating-front detection laser flash method. Metall Mater Trans B. 2012 Dec;43(6):1413–1419.