ABSTRACT
Lecanoraceae is one of the largest families of the Lecanoromycetes, with about 30 accepted genera, many of which, however, have uncertain status and/or circumscriptions. We assess the phylogenetic position of the genus Bryonora and its segregate Bryodina for the first time, using a six-locus phylogeny comprising the Lecanoraceae as well as closely related families. We find strong support for the placement of Bryonora in the Lecanoraceae, whereas there is no support for treating Bryodina as a genus separate from Bryonora. Hence, we reduce Bryodina to synonymy with Bryonora. Further, we describe Bryonora microlepis as new to science and transfer Lecanora castaneoides to Bryonora and L. vicaria to Miriquidica. A world key to Bryonora is included.
INTRODUCTION
Lecanoroid lichens form a vast, heterogeneous assemblage including crustose lichens with apothecial margins containing algal cells and, in most species, unicellular ascospores. For a long time, the type genus Lecanora Ach. represented the large majority of the described species of this group, especially after the introduction of Zahlbruckner’s highly influential but largely artificial taxonomic system (Zahlbruckner Citation1928). Subsequent work, from 1960 and onward, has sought to delimit more natural groups of lecanoroid lichens. Several segregates have been found to belong to other families than the Lecanoraceae, for example, Aspicilia A. Massal. (Megasporaceae), Squamarina Poelt (Squamarinaceae), and Trapelia M. Choisy (Trapeliaceae) (Wijayawardene et al. Citation2018). Others have been split from the genus Lecanora but were retained in the Lecanoraceae, for example, Myriolecis Clem., Palicella Rodr. Flakus & Printzen, and Protoparmeliopsis M. Choisy (Rodriguez Flakus and Printzen Citation2014; Wijayawardene et al. Citation2018; Zhao et al. Citation2016). Another such segregate is the genus Bryonora Poelt, which comprises 13 crustose, lichenized species mainly overgrowing dead bryophytes in arctic or alpine environments.
Bryonora was segregated from Lecanora by Poelt (Citation1983), mainly on the basis of the well-delimited apothecial cortex composed of strongly conglutinated hyphae. The species included in Bryonora also seemed to share ecological preferences, occurring mainly above the tree limit and usually growing on soil, plant debris, or bryophytes on acidic ground. Morphologically, they are also rather similar, i.e., in their brownish color, rather large and more or less stipitate apothecia, and usually poorly developed thallus. In addition, ascospores of Bryonora generally become septate, in contrast to the species remaining in Lecanora. Poelt included six species in Bryonora, three of which were newly described by him: B. castanea (Hepp) Poelt, B. corallina Poelt, B. curvescens (Mudd) Poelt, B. rhypariza (Nyl.) Poelt, B. stipitata Poelt, and B. yeti Poelt. Poelt also suggested that the diversification center of the genus was located in the high mountains of southern Asia.
Poelt and Obermayer (Citation1991) divided Bryonora into three infrageneric groups: section Bryonora, which included the types species of the genus (B. castanea) as well as four other species (B. curvescens, B. pruinosa, B. septentrionalis, and B. reducta) with poorly developed thallus and a cortex that does not refract polarized light (POL−); section Stipitantes, including three species (B. pulvinar, B. stipitata, and B. yeti) with subfruticose to fruticose thalli and a cortex that refracts polarized light(POL+); and section Rhyparizae, including the two species B. rhypariza and B. selenospora. This latter section was later segregated from Bryonora and placed in the new genus Bryodina Hafellner (Hafellner and Türk Citation2001). The thallus in B. rhypariza, the type species of Bryodina, is comparatively well developed, crust-forming, or squamulose. A further difference from species left in Bryonora is a paler apothecial margin, consisting of hyphae that are separated at the ends, not conglutinated all the way as in Bryonora. Finally, the ascospore wall is slightly thinner and the ecology seemingly differs, as B. rhypariza sometimes grows directly on rock (Hafellner and Türk Citation2001; Poelt and Obermayer Citation1991).
Since the first treatment of Bryonora, the number of species in the genus has more than doubled. In a paper addressing cyanotrophy in lichens, Poelt and Mayrhofer (Citation1988) described two new species of Bryonora and one variety: B. reducta Poelt & Mayrh., B. selenospora Poelt. & Mayrh., and B. rhypariza var. cyanotropha Poelt & Mayrh., all from Nepal. Holtan-Hartwig (Citation1991) showed that Lecanora castanea f. pruinosa (Th. Fr.) Th. Fr. is a distinct species and transferred it to Bryonora as B. pruinosa (Th. Fr.) Holt.-Hartw. In the same publication, he also described B. septentrionalis Holt.-Hartw from Fennoscandia and Russia. Poelt and Obermayer (Citation1991) provided an overview of the genus and described one new species from Nepal, B. pulvinar Poelt & Oberm., with two varieties. All known species of Bryonora up to that point were from the Northern Hemisphere, but Øvstedal (Øvstedal and Lewis Smith Citation2001) described B. peltata Øvstedal, from the Antarctic Peninsula and Fryday and Øvstedal (Citation2012) added a second species from the Southern Hemisphere, B. granulata Fryday, from the Falkland Islands.
In his original treatment of Bryonora, Poelt (Citation1983) also discussed some species that he did not include in the new genus, although they showed affinities to Bryonora. One of these was Lecanora castaneoides H. Magn., which he excluded from Bryonora on account of the thin apothecium cortex, a zeorine apothecial margin, and unicellular ascospores. It was noted that a zeorine margin was unusual within Bryonora and present only in B. rhypariza. Lecanora castaneoides also seemed to have a different ecology, occurring on weathered rocks rather than on soil or plant debris on the ground. Further, Poelt (Citation1983) excluded L. vicaria (Th. Fr.) Vain. (originally described as L. rhypariza ssp. vicaria Th. Fr.), as he found this species to deviate from Bryonora in the poorly delimited apothecium cortex and the zeorine apothecial margin.
Both Bryonora and Bryodina are generally viewed as a members of the Lecanoraceae (e.g., Wijayawardene et al. Citation2018; Zhao et al. Citation2016), but it should be noted that Poelt and Obermayer (Citation1991) suggested, on anatomical grounds, that Bryonora (including the two species later transferred to Bryodina) was close to Protoparmelia M. Choisy, a genus that is now included in the Parmeliaceae (Wijayawardene et al. Citation2018). The genus Bryonora has so far received scant attention in molecular studies. An internal transcribed spacer (ITS) sequence from B. castanea was published by Grube et al. (Citation2004) and appeared in an unresolved position in an unrooted maximum likelihood distance tree including taxa from several families. The same sequence was utilized by Zhao et al. (Citation2016), and the branch containing B. castanea appeared as a polytomy in an unsupported clade with the genera Adelolecia Hertel & Hafellner, Frutidella Kalb, and Pyrrhospora Körb. and the Lecanora subfusca and L. subcarnea groups. Thus, the delimitation of Bryonora and Bryodina, as well as their phylogenetic positions, remains untested using comprehensive taxon sampling and multiple loci.
Our aims were to assess the phylogenetic position of Bryonora and to evaluate the delimitation of the genus, both in relation to its segregate Bryodina and with regard to species previously excluded from the genus by Poelt (Citation1983), i.e., Lecanora castaneoides and L. vicaria. In addition, we investigated material of a previously unknown species of the genus discovered in Norway.
MATERIALS AND METHODS
Morphology and anatomy.—
Measurements were made under a light microscope of material mounted in water, using an oil-immersion lens with a precision of 0.5 µm for measurements of finer anatomical structures (e.g., ascospores and paraphyses). Only well-developed ascospores lying outside the asci were measured. To examine color reactions of pigments and solubility of crystals, we used a 10% solution of KOH (abbreviated K), and a 10% solution of HNO3 (abbreviated N). Apical structures of asci were examined in an iodine solution (0.3–0.4% I, abbreviated I) after pretreatment with K (abbreviated KI). Algae in the upper cortex of Bryonora microlepis were observed in chlorine-zinc-iodine (ClZnI). We checked thallus reactions with K and C, and, in addition, with an ethanolic solution of para-phenylenediamine (abbreviated Pd). We performed high-performance thin-layer chromatography (HPTLC) (following the method described by Arup et al. Citation1993) and TLC (Orange et al. Citation2010), in both cases using solvent systems B and C. Material from the following herbaria was included in the study: LD, O, S, TRH, and UPS.
DNA extraction, amplification, and sequencing.—
We extracted total DNA using a Qiagen DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) or a E.Z.N.A. Plant DNA Kit (Omega Bio-tek, Norcross, Georgia) following the manufacturers’ instructions or, alternatively, with Chelex 100 (Bio-Rad, Hercules, California) following the procedure described by Ferencova et al. (Citation2017). For amplification and sequencing, we used the primers mrSSU1, mrSSU2, and mrSSU3R for the mitochondrial ribosomal small subunit 12S, hereafter mrSSU (Zoller et al. Citation1999); LRlecF and LrlecR for nuclear ribosomal large subunit 28S, hereafter LSU (Schneider et al. Citation2015); ITS1F, ITS5, and ITS4 for nuc rDNA ITS1-5.8-ITS2 (Gardes and Bruns Citation1993; White et al. Citation1990); MCM7-709for and MCM7-1348rev for the mini-chromosome maintenance complex 7, hereafter MCM7 (Schmitt et al. Citation2009); and fRPB2-5F and fRPB2-7cR with the nested primers RPB2-980F and RPB2-1554R for the RNA polymerase II complex subunit, hereafter RPB2 (Liu et al. Citation1999; Reeb et al. Citation2004). For the RNA polymerase II largest subunit, hereafter RPB1, we used the newly designed primers RPB1-Lec540for (5′-GAR ACN ATG GAY GAG CA-3´) and RPB1-Lec1950rev (5′-ACV CGA TGK CCC ATC AT-3′) with the nested primers RPB1-Lec800for (5′-ACV GTH TGY CAC AAC TG-3′) and RPB1-Lec1600rev (5′-RTC MAC WCK YTT MCC CAT-3´) or RPB1-Lec1400rev (5′-ACA TTS CCG YTY GCA CG-3′). For polymerase chain reactions (PCRs) for mrSSU, ITS, and RPB2, we followed the protocols of Svensson and Westberg (Citation2021). For LSU, MCM7, and RPB1, we used VWR Red Taq Polymerase Master Mix (VWR International, Belgium) following the manufacturer’s protocols. For LSU, we used the following PCR thermal profile: an initial hold at 94 C for 3 min; followed by 35 cycles of denaturization at 94 C for 30 s, annealing at 57 C for 45 s, and polymerization at 72 C for 1 min 45 s; and finally a hold at 72 C for 10 min. For MCM7, we used the following PCR thermal profile: an initial hold at 94 C for 3 min; followed by 3 cycles of denaturization at 94 C for 45 s, annealing at 57 C for 45 s, and polymerization at 72 C for 1 min 45 s; then 4 cycles of 94 C for 40 s, 54 C for 40 s, and 72 C for 45 s; 4 cycles of 94 C for 40 s, 51 C for 40 s, and 72 C for 45 s; 30 cycles of 94 C for 30 s, 48 C for 30 s, and 72 C for 45 s; and finally a hold at 72 C for 10 min. For RPB1, we used the following PCR thermal profile for the first PCR: an initial hold at 94 C for 3 min; followed by 15 cycles of denaturization at 94 C for 30 s, annealing at 65 C for 45 s (decreasing 1 C each cycle), and polymerization at 72 C for 1 min 20 s; then 28 cycles of 94 C for 30 s, 50 C for 45 s, and 72 C for 1 min 20 s; and finally a hold at 72 C for 10 min. For the second PCR, using nested primers, we used the same PCR cycling thermal profile as for the first PCR. PCR products were subsequently purified with ExoCleanUp FAST (VWR International, Belgium). In addition, three ITS sequences (MW927468–MW927470) were provided by the Canadian Center for DNA Barcoding (CCDB; http://www.ccdb.ca), using the primer pairs ITS1F/ITS4 or ITS5/ITS4.
Taxon sampling.—
To determine the phylogenetic position of Bryonora, we assembled a sequence data set with a selection of species and genera from the Lecanoraceae, a couple of closely related families, and a more distant outgroup. We included sequences from five currently accepted species of Bryonora, as well as two species (Lecanora castaneoides and L. vicaria) previously considered but never included in the genus. Sequences from a potentially undescribed species of Bryonora were also included. The included specimens of B. castanea and B. rhypariza belonged to these species in the strict sense; that is, not to any of the varieties of these species that have been described from the Himalayas (Poelt Citation1983; Poelt and Obermayer Citation1991). We included various species of the genus Lecanora s.l., including species from the Lecanora subfusca group, L. polytropa group, L. subcarnea group, and L. symmicta group and L. formosa. We also included species of the genera Miriquidica Hertel & Rambold, Myriolecis, Rhizoplaca Zopf, Palicella, Protoparmeliopsis, and Lecidella Körb., which belong to the Lecanoraceae as well (Wijayawardene et al. Citation2018; Zhao et al. Citation2016). To test whether Bryonora has affinities to Protoparmelia, we included two species of this genus in addition to two other genera in the Parmeliaceae (Bryoria Brodo & D. Hawksw. and Evernia Ach.). We further included representatives from the Cladoniaceae (the genus Cladonia P. Browne) and the Stereocaulaceae (the genera Hertelidea Printzen & Kantvilas and Stereocaulon (Schreb.) Schrad.), as these families are sisters to the Lecanoraceae (Miadlikowska et al. Citation2014; Schmull et al. Citation2011). As an outgroup, we selected the Rhizocarpaceae (the genus Rhizocarpon Ramond ex DC.), as this family appears as basal to the Lecanoromycetidae (Miadlikowska et al. Citation2014), which includes all other families included in the analysis. For the selected genera and species, we downloaded sequences of mrSSU, LSU, ITS, MCM7, RPB1, and RPB2 from GenBank ().
Table 1. Sequence data used for the phylogenetic analysis, with GenBank accession numbers and voucher information
Sequence alignment and partitioning scheme.—
For the non-protein-coding markers (mrSSU, ITS, and LSU), we estimated separate alignments using PASTA (Mirarab et al. Citation2015), with the mask option activated, MAFFT (algorithm L-INS-i) as the aligner, OPAL for the pairwise merging, and FastTree as the tree estimator, with GTR+Γ as the model for molecular evolution. PASTA is an iterative method that optimizes the alignment using maximum likelihood; hence, we did not conduct any subsequent filtering of ambiguous regions of the resulting alignments. However, inspection of the alignment of LSU revealed problems due to the presence of long introns in several species. We therefore estimated the LSU data set using MAFFT with the algorithm E-INS-i, which is designed to handle sequence data sets with multiple conserved domains divided by long gaps (Katoh et al. Citation2019). From the MAFFT alignment of LSU, we removed one intron from Evernia prunastri (226 bp), three from Lecanora polytropa (50, 203, and 236 bp), one from Miriquidica complanata (60 bp), and one from M. gyrizans (79 bp). We then estimated the LSU alignment using PASTA, as above. To avoid potential problems with missing data, the ends of all the PASTA alignments were trimmed.
For the protein-coding genes (MCM7, RPB1, and RPB2), we estimated alignments with MAFFT using the algorithm E-INS-i (Katoh et al. Citation2019). After aligning the sequences, we identified several noncoding introns in the RPB1 and RPB2 alignments, which we removed before any further analysis was performed. No such introns were detected in the alignment of MCM7. The first 65 bp of our newly generated RPB1 sequences for Bryonora were, although apparently protein-coding, not easily alignable with the rest of the RPB1 alignment and ended with a stop codon. We therefore removed the first 65 bp of the RPB1 for Bryonora before analysis.
We checked for incongruence among markers by performing a separate maximum likelihood analysis of each alignment using IQ-TREE with 500 nonparametric bootstrap replicates (Nguyen et al. Citation2015). The six single-marker trees from these analyses were then compared to identify any conflicting results, defined as conflicting clades with >75% bootstrap support. We found no such conflicts and therefore decided to concatenate the six alignments into one. The final alignment is included in SUPPLEMENTARY MATERIAL 1.
We assessed the division of the concatenated alignment into partitions using PartitionFinder 2.1.1. (Lanfear et al. Citation2017), which also allows for simultaneous estimation of models of molecular evolution for the partitions. We restricted the estimation to models implemented in MrBayes 3.2.6., used AICc (Akaike Information Criterion with small sample correction) for model selection, assumed linked branched lengths (= edge-proportional model), and used the “greedy” algorithm (Lanfear et al. Citation2012). We assessed the division of the concatenated alignment into 14 partitions: mrSSU, LSU, ITS1, 5.8S, ITS2, and independent 1st, 2nd, and 3rd codon positions for each of the three protein-coding genes MCM7, RPB1, and RPB2.
The best model fit was achieved when the 1st codon positions of MCM7 and RPB1 were merged into one partition and when the 2nd and 3rd codon positions of RPB1 and RPB2 were likewise merged into a single partition each. For the resulting 11 partitions, GTR+Γ was selected as the best model for ITS1 and RPB2 1st; GTR+Γ+I for mrSSU, LSU, RPB1 1st + MCM7 1st, RPB1 2nd + RPB2 2nd, and MCM7 3rd; SYM+Γ for ITS2; SYM+Γ+I for RPB1 3rd + RPB2 3rd; HKY+Γ+I for MCM7 2nd; and K80+Γ+I for 5.8S.
Phylogenetic analysis.—
We performed phylogenetic analyses on the concatenated, partitioned alignments, implementing the models of molecular evolution from PartitionFinder using MrBayes 3.2.6. (Ronquist et al. Citation2012). We used flat Dirichlet priors for the substitution rates and state frequencies, an exponential (1) distribution for the gamma shape parameter, a compound Dirichlet prior (α = 1, β = 0.1) for branch lengths, and uniform distributions for invariant sites and topology. We performed two runs of four Markov chain Monte Carlo (MCMC) chains each, three heated and one cold. For initial test runs, we set the temperature of the heated chains to 0.20 but reduced this to 0.10 to improve swapping rates. The sample frequency was set to every 100th generation. The analysis was halted when convergence was reached, which was defined as an average standard deviation of split frequencies below 0.01. The fraction of trees discarded as burn-in was set to 25%. We considered a posterior probability of 0.95 or higher as high support.
In addition to the Bayesian analysis, we also performed maximum likelihood analyses of the concatenated alignment with IQ-TREE, using the same partitioning scheme and models of molecular evolution as for the Bayesian analysis. As in the Bayesian analysis, we used edge-proportional partition models. We assessed branch support by running 500 nonparametric bootstrap replicates. We considered a bootstrap value above 85% as high support.
RESULTS
We generated 50 new sequences (). The final alignment included 68 terminals, 215 sequences, and had 4784 characters, 1763 of which were parsimony-informative. The analysis halted after 11.4 million generations, resulting in a posterior of 85 681 samples. The majority-rule consensus tree is shown in , with bootstrap values from the maximum likelihood analysis above 70% added.
Figure 1. Majority-rule consensus tree based on a Bayesian MCMC analysis of mrSSU, LSU, ITS, MCM7, RPB1, and RPB2, showing the phylogenetic position of Bryonora in the Lecanoraceae. Branch support is given as posterior probability (PP)/bootstrap support (BS). Bootstrap support values are from a corresponding maximum likelihood analysis. Only BS values >70% are shown. GenBank accession numbers and voucher information are given in .
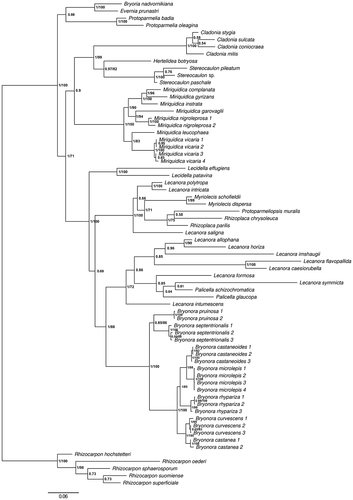
We found high support (posterior probability [PP] = 1, bootstrap [BS] = 100) for a monophyletic Bryonora, including two main clades: one clade (PP = 1, BS = 100) with five of the seven species, including the type species of both Bryonora (B. castanea) and Bryodina (B. rhypariza), and a second clade (PP = .85, BS = 86) with Bryonora pruinosa and B. septentrionalis (). The phylogenetic position of Bryonora is firmly within the Lecanoraceae, in a highly supported (PP = 1, BS = 88) clade together with, for example, the Lecanora subfusca group and the genus Palicella.
The two candidates for inclusion in Bryonora, Lecanora castaneoides and L. vicaria, were recovered in different parts of the tree. L. castaneoides was found nested within Bryonora, whereas L. vicaria was sister to Miriquidica leucophaea (PP = 1, BS = 83) in a monophyletic Miriquidica (PP = 1, BS = 100). The two species are revised below. Finally, the suspected undescribed species was recovered in Bryonora as well and is newly described as Bryonora microlepis below.
TAXONOMY
Bryonora castaneoides (H. Magn.) Arup, M. Svensson & M. Westb., comb. nov.
Figure 2. A–B. Bryonora castaneoides. A. Habit of form strongly associated with cyanobacteria and a thallus of Vestergrenopsis showing scattered thallus areoles “floating” on a carpet of Stigonema. Note apothecia in upper left hand corner that grow directly on the thallus of Vestergrenopsis (Du Rietz 889c, UPS L-115750). B. Habit of cracked areolate thallus and apothecia not strongly associated with cyanobacteria and cyanolichens (Westberg ULR223, UPS L-880215). Bars = 1 mm.

MycoBank MB842776
≡ Lecanora castaneoides H. Magn., Ark Bot 33A:107. 1946.
Typification: SWEDEN. LYCKSELE LAPPMARK: Tärna par., Björkfors, by a brook at 600 m, 12 Jul 1924, A.H. Magnusson 8143 (UPS L-189715!, lectotype, designated here, MBT10005453; S L2044!, isolectotype).
= ?Lecanora erythrophaea H. Magn., Ark Bot 33A:110. 1946.
Typification: [SWEDEN. LYCKSELE LAPPMARK:] “Tärna: Umfors, rocks on shore of Överuman, mixed with Ionaspis odora and Pyrenopsis sp., [no date] hb. Stm [= Stenholm]” (cited from protolog, material not located).
Thallus variable, from more or less (±) invisible or discernible only around the apothecia to thin, ± confluent to cracked areolate, without a well-defined cortex, K−, C−, Pd−. When associated with cyanophilous lichens or free-living Stigonema, the thallus may be better developed, consisting of scattered areoles, sometimes appearing on a carpet of cyanobacteria and then often with well-defined cortex, 12–20 µm thick, of ± isodiametric cells. Areoles 0.1–0.9 mm diam, usually 0.05–0.2(–0.4) mm thick, even to finely scurfy or minutely granular, beige, brownish to grayish beige, dark gray to brown; medulla with crystals not refracting polarized light, not dissolving in K; photobiont trebouxioid with cells 7–14 µm diam, but sometimes colonies of Nostoc or Stigonema occur within or are clearly associated with the thallus.
Apothecia common, zeorine, scattered or often aggregated, round to angular from compression or irregular, slightly immersed but more commonly adnate to sessile, 0.3–0.6(–1.0) mm diam; disc ± flat to convex, sometimes ± flexuous, dark reddish brown to medium brown, slightly glossy, rarely thinly white pruinose; thalline margin usually deeply suppressed below the proper margin and not possible to see from above, but occasionally conspicuous, even, to 50 µm thick, at base of apothecium, cortex 12–20 µm, well defined, of ± isodiametric cells; proper margin up to 75 µm thick but suppressed and invisible in convex apothecia, concolorous with disc or slightly darker, somewhat glossy, level with disc or somewhat prominent; hymenium (55–)60–70 µm thick, hyaline with only upper part brown; epihymenium brown, without any crystals; paraphyses simple to slightly branched, 2.0–2.5 µm wide with upper cell slightly wider, 3.0–4.0(–4.5) µm; hypothecium hyaline, 70–150 µm thick, consisting of short and irregular to almost isodiametric, thick-walled hyphae; excipulum pale with brown outer part, consisting of radiating, branching hyphae with narrow cell lumina, often with crystals refracting polarized light (POL+), dissolving in K but not in N; amphithecium with crystals not refracting polarized light (POL−), not or partly dissolving in K, but not in N; asci 8-spored, 46–58 × 13–16 µm, of the Lecanora type; ascospores 0–1(–3)-septate, hyaline, narrowly ellipsoid to ± cylindrical, (11–)13–15.4–18(–21) × 4–4.8–5.5(–6) µm, length/width ratio 2.2–3.2–5.0 (n = 91, 9 specimens). Pycnidia not seen.
Chemistry: No lichen substances detected by HPTLC.
Ecology and distribution: Bryonora castaneoides grows directly on siliceous to calciferous rocks, often on various types of slate. It usually grows intermixed with other crustose lichens, often with species of Placynthium and Vestergrenopsis, or seemingly associated with free-living cyanobacteria, mainly of the genera Nostoc and Stigonema. Most of the known localities are near fresh water of creeks and lakes that become inundated or sprayed from time to time. The association with cyanobacteria or lichens containing cyanobacteria seems to stimulate thallus growth and development as well as apothecial growth: a well-defined thallus cortex seems to develop only when abundant cyanobacteria are associated with the lichen thallus. Cells of Nostoc are sometimes even found inside the thallus of B. castaneoides together with the normal trebouxioid algae.
So far, B. castaneoides is known only from Norway (including Svalbard) and Sweden, but it is likely that the species has been overlooked and that the distribution is wider. All localities are within the Scandinavian mountain range or in arctic areas.
Notes: We located two syntypes of Lecanora castaneoides in UPS and S, of which we designate the largest collection (UPS L-189175) as the lectotype. Poelt (Citation1983) cited an “isotype” in M without giving further collection data, but no material matching the protologue can presently be found there (A. Beck, pers. comm., 2021).
Magnusson described Lecanora erythrophaea in the same work as L. castaneoides (Magnusson Citation1946), but he did not compare the two species. According to Magnusson (Citation1946), the holotype of L. erythrophaea was 1.2 × 0.6 cm and grew intermixed with other lichens in a collection made by Carl Stenholm and kept in his personal herbarium. However, the holotype of L. erythrophaea could not be located in GB, where Stenholm’s herbarium is now lodged (E. Larsson, pers. comm., 2020). Lecanora erythrophaea has only been reported once after its description (Magnusson Citation1952). The specimen that this report was based on (UPS L-789780!) is Bryonora castaneoides, which is probably why Santesson included L. erythrophaea in the synonymy of L. castaneoides in the Fennoscandian checklist (Santesson Citation1993; Santesson et al. Citation2004). As the description of L. erythrophaea is similar to that of L. castaneoides, we also tentatively include L. erythrophaea in the synonymy of L. castaneoides.
Lecidea hilarescens Nyl., described based on material from Greenland (Nylander Citation1862), was suggested to be related to B. castaneoides by Printzen (Citation1995). The holotype of L. hilarescens (H-Nyl 21207!) has discrete, ± white thallus areoles, rather light, flesh-colored apothecia, ascospores 9 × 3–4 µm, and an epihymenium with brown crystals that dissolve in K. In our opinion, L. hilarescens is not likely to belong in Bryonora and may be a member of the Lecanora polytropa group.
Within the genus Bryonora, B. castaneoides is well characterized by the combination of saxicolous habit, ellipsoid, 0–1-septate ascospores, and a lack of lichen substances. The most closely related species in our phylogeny were B. rhypariza and B. microlepis, both species that, apart from several other differences in anatomy and ecology, have secondary compounds (norstictic acid in the former and stictic acid in the latter). The most similar species in the genus appears to be B. reducta, which was described from a single collection from a high-elevation (3200 m above sea level [a.s.l.]) locality in Nepal by Poelt and Mayrhofer (Citation1988). Like B. castaneoides, it has uniformly brown apothecia, no secondary chemistry, and grows in a mat of Stigonema (Poelt and Mayrhofer Citation1988; Poelt and Obermayer Citation1991). The ascospores in B. reducta differ from those of B. castaneoides by being somewhat thicker and dumbbell-shaped (Poelt and Mayrhofer Citation1988; Poelt and Obermayer Citation1991) and are unlike any ascospores we have seen in the examined material of B. castaneoides. It is possible that B. reducta represents a later synonym for B. castaneoides, but given the difference in ascospore shape and the large distance between the locality for B. reducta and the known distribution of B. castaneoides, more data are, in our opinion, needed to allow for firm conclusions.
Additional specimens examined: NORWAY. SØR-TRØNDELAG: Oppdal, Blesebekken, 1.4 km SE of Kongsvoll, on rocks at the brook, 1030 m, 5 Aug 1964, Tibell 2259 (UPS L-138332); SVALBARD: Hopen, Husdalen, 1982, Skye (UPS L-204360). SWEDEN. JÄMTLAND: Åre par., Handöl Rapids in river Handölan, W of Lake Ånnsjön, on exposed, flat rock on the shore, ca. 0.2 m above the water level, 29 Jul 1994, Owe-Larsson H94-89 (UPS L-588742); LULE LAPPMARK: Jokkmokk par., Padjelanta National Park, along the Nordkalotten trail ca. 3.6 km S of the Staloluokta cabins, at small lake in the river Viejevágge, on Placynthium on sloping rock surface of slate at the waterfall, alt. 665 m, 28 Jul 2004, Arup L04065 (LD); LYCKSELE LAPPMARK: Tärna par., Ume älv, Över-Umans sydvästligaste vik, “Elyna-ön,” alt. 530 m, svagt sluttande häll, övergeolitoral, [= Ume river, the southwestern most bay of Lake Över-Uman, the ”Elyna-island”, alt. 530 m, gently sloping rock surface on the shore] 17 Aug 1960, Du Rietz 889c (UPS L-115750); “ön 138,” SV-sidan, strandklippor av kalkfri glimmerskiffer, [= the island “138”, SW-side, mica-schistose rocks on the shore] 29 Aug 1960, Du Rietz 2169c (UPS L-119225) [HPTLC: nil]; Vilasund, shore of lake Över-Uman below Mårtensson’s boarding house, alt. 520 m, on medium-sized boulder in the middle geolitoral zone, 25 Aug 1963, Du Rietz 499d (LD, O); ibid., 28 Aug 1963, Du Rietz 588a (UPS L-128417) [HPTLC: nil]; TORNE LAPPMARK: Jukkasjärvi par., Björkliden, 675 m W of the railway station, on exposed, sloping slate rocks on E side the creek Rákkasjohka, elev. ca. 520 m, 6 Aug 2013, Arup L13343 (LD); Abisko National Park, sekt. 7, Abiskojaures östra strand, på strandblock, [= the eastern shore of Lake Abiskojaure, on boulder by the shore] 14 Aug 1943, Santesson (UPS L-123023); Vassitjåkko, on moist stone, ca. 700 m, 26 Jul 1921, Magnusson 5920 (LD, O, S); Karesuando par., S of Maunu, just E of the bridge over the stream Kiltijoki, on rock by the stream, 29 Aug 2019, Westberg & Odelvik KAR156 (UPS L-989349); Kopparåsen, regios subalpina, among boulders, 700 m, 1 Aug 1921, Magnusson 6133b (UPS L-789780); ÅSELE LAPPMARK: Vilhelmina par., 1.9 km NE of Fatmomakke Church, rapids in semiopen, old growth coniferous/deciduous forest, on schistose rock, 27 Jul 2017, Westberg ULR223 (UPS L-880215) [HPTLC: nil]; ibid., Svensson 3156 (UPS).
Bryonora microlepis Haugan & Timdal, sp. nov.
Figure 3. A–E. Bryonora microlepis. A–C. Habit of sterile thallus. Note the pale margin of the lobes and squamules. D–E. Section through squamule, stained in lactophenol cotton blue. A, C–E. Timdal 13869, O L-201412. B. Klepsland 625, holotype, O L-175933. Bars: A–C = 1 mm; D–E = 50 µm.

MycoBank MB842775
Typification: NORWAY. OPPLAND: Vågå, Jønndalen, S slope of Mt. Raudnebb, 61.9532°N, 9.0950°E (± 7 m), 890 m alt., eroded rock outcrop and stabilized sandy soil in S-facing scree, 7 Aug 2011, Jon T. Klepsland 625 (O L-175933!, holotype [TLC: stictic acid]).
Diagnosis: Thallus composed of scattered to conglomerate, up to 0.6 mm diam, rounded, plane to convex, shiny brown, gray-edged squamules containing stictic acid.
Etymology: The name refers to the characteristic, small thallus squamules.
Thallus up to ca. 3 cm diam or irregularly spreading, squamulose, attached by rhizohyphae; hypothallus not seen; squamules small, up to 0.6 mm diam, rounded, plane to convex, first dispersed, later forming conglomerate cushions, adnate to ascending, sometimes partly imbricate, brown, usually gray-edged, shiny, usually with a network of shallow fissures in the cortex, K+ yellow, Pd+ orange; thallus anatomy of the “Kegelrinden” type (Poelt Citation1958), i.e., with conical cortex sections extending into the center of the squamule flanked by pockets of algal layer and medulla (–E); cortex without remnants of algae (ClZnI!) or crystals (POL−), composed of strongly conglutinated, anticlinally oriented, thick-walled, up to 5 µm wide hyphae with thread-shaped lumina in inner part and shorter, angular to rounded lumina in upper part; algal layer and medulla composed of loosely interwoven hyphae, containing crystals dissolving in K, KI−; photobiont unicellular green algae, up to 10 µm diam. Apothecia and pycnidia not seen.
Chemistry: Stictic acid detected by TLC.
Ecology and distribution: Bryonora microlepis grows on calcareous mineral soil in small crevices of sloping rock faces at sun-exposed sites, on south- to west-facing slopes or boulderscrees in open north boreal forest to low alpine areas. Most of the known localities harbor a range of rare or red-listed lichens typical of xeric and calcareous habitats. The collection from Møre og Romsdal deviates by being from a lowland locality on a rocky lake shore, but also here the species grows on calciferous substrate, probably weakly influenced by trickling water. The species is known from six localities in the continental parts of South Norway; five are from the upper valleys east of the mountain chain and one from an inner fjord area of western Norway. All localities are in rain-shadow areas, and the annual precipitation is mostly below 500 mm.
Notes: The species differs from the other species of the genus in the thallus being composed of small, gray-edged squamules that are first dispersed and later form conglomerate cushions. The “Kegelrinden” thallus anatomy is unique within the genus, and so is the presence of stictic acid. It might be mistaken for a Miriquidica, since gray-edged squamules are common in that genus, as is the occurrence of stictic acid. No stictic acid–containing Miriquidica is known to grow on soil, however.
Additional specimens examined: NORWAY. HEDMARK: Folldal, Tverrgjelet, 62.1521°N, 9.7195°E (± 707 m), 1160–1200 m alt., on calcareous soil on S-facing slope, 18 Jul 1996, Haugan 4974 (O L-29315); OPPLAND: Dovre, Grimsdalen, Buåi, 62.0658°N, 9.5749°E (± 7 m), 1090 m alt., S-facing, steep slope, calcareous rock, 4 Aug 2005, Timdal 10536 (O L-148965); Nord-Fron, Kvikne, Halvhuda, 61.5741°N, 9.6074°E (± 7 m), 645 m alt., calciferous rock outcrop in grazed meadow, 28 May 2011, Haugan & Timdal 9931 (O L-173267) [TLC: stictic acid]; Vang, Smådalsfjellet, S of Sletteskaret, 61.063°N, 8.5595°E (± 10 m), 1270 m alt., rock outcrop in the alpine zone, 9 Sep 2015, Timdal 13869 (O L-201412); MØRE OG ROMSDAL: Molde, Hestneset, 62.455°N, 8.2434°E (± 7 m), 151 m alt., sun-exposed rock along lake shore, 25 Apr 2009, Haugan 8628 (O L-161432) [TLC: stictic acid].
Miriquidica vicaria (Th. Fr.) Arup, Haugan & Timdal, comb. nov.
Figure 4. A–D. Bryonora castanea, B. pruinosa, B. rhypariza, and Miriquidica vicaria. A. Habit of B. castanea with apothecium margin more or less concolorous with disc (Kristinsson 24861, UPS L-996123). B. B. pruinosa with its apothecial margin typically darker than the disc (Hellbom & Hellbom s.n., UPS L-7552246). C. B. rhypariza differs from most species in the genus by a well-developed thallus and conspicuous thalline margin (Du Rietz s.n., UPS L-118215). D. Habit of Miriquidica vicaria, in this case rather similar to a Bryonora, but differing in a well-developed thallus where the margin of the areoles is often paler than the center and in exclusively having simple ascospores (Nordin 7784, UPS L-721906). Bars = 1 mm.

MycoBank MB842777
≡ Lecanora rhypariza ssp. vicaria Th. Fr., Lichenogr Scand 1(1):271. Citation1871.
≡ Lecanora vicaria (Th. Fr.) Vain., Meddeland Soc Fauna Fl Fenn 10:208. Citation1883.
Typification: [SWEDEN. HÄRJEDALEN: Tännäs par.,] Funäsdalen, 1867, P.J. Hellbom s.n. (UPS L-023730!, lectotype, designated here, MBT10005454; isolectotypes G G00292207 [image!], UPS L-023729!).
Thallus up to 4 cm diam, squamulose, dispersed to continuous; hypothallus not seen; squamules roundish, up to 4 mm diam and 1.5 mm thick, weakly to strongly convex, beige to greenish brown, gray- or pale-edged, soon becoming cerebriform, i.e., consisting of a conglomeration of squamule sections, each with a paler edge, esorediate, K+ red, Pd+ orange; cortex up to 200 µm thick, composed of anticlinally arranged, branching and anastomosing, thick-walled hyphae with cylindrical lumina, containing crystals dissolving in K with yellow effusion and subsequent precipitation of red, needle-shaped crystals (POL+); algae green, coccoid, up to 13 μm diam; medulla lacking crystals, K−, Pd−, KI−. Apothecia indistinctly lecanorine when young, soon becoming biatorine with a narrow or more or less excluded thalline margin, round to irregular, plane to convex, pale brown when young, later dark brown to blackish, up to 1.5 mm diam, immersed as young but later adnate; proper exciple slightly raised or level with disc, 25–70(–90) µm thick, paler to darker than disc, brown to pale in the rim, pale brown in inner part; hymenium 50–70(–75) µm thick, hyaline; epihymenium yellowish green-brown, K+ olive-green, N−; hypothecium pale, –350 µm thick; paraphyses mainly simple, sometimes anastomosing, 1.0–1.5 wide with tip to 3 µm wide; asci 8-spored, of the Lecanora type, ascospores simple, narrowly ellipsoid, (8.5–)10–11.4–13.0–(15.0) × (4.0–)5–5.1–6.0–(6.5) μm (n = 80).
Chemistry: Norstictic acid (major) and connorstictic acid (minor) detected by TLC.
Ecology and distribution: The species is terricolous, muscicolous, or saxicolous on dry siliceous to somewhat calciferous rock. It is known from open subalpine to low alpine areas. Miriquidica vicaria is so far only known from Norway and Sweden.
Notes: We located three collections of Miriquidica vicaria from which a lectotype could be designated. The collection UPS L-023730 is originally from T. Fries’ herbarium and is hence the specimen on which the description of Lecanora rhypariza var. vicaria was based. It is also the largest and best developed, and we therefore designate this specimen the lectotype. A possible later synonym to M. vicaria is Lecanora rubida Wirth, which was described from France (Nordin Citation2015; Wirth Citation1981). An evaluation of the status of L. rubida is pending further studies of more material.
In our phylogenetic reconstruction (), the species is resolved well inside the Miriquidica clade, as the sister of M. leucophaea (Flörke ex Rabenh.) Hertel & Rambold. Miriquidica leucophaea is somewhat similar to M. vicaria but differs in forming a strictly saxicolous, areolate, usually gray thallus on a black hypothallus, having lecideine apothecia, and in containing miriquidic acid instead of norstictic acid. The muscicolous M. squamulosa Fryday, known from the subantarctic Campbell Island, is the only other muscicolous Miriquidica species containing norstictic acid. This species is characterized by being cephalodiate, having convex squamules with lobulate margins, and having immersed, lecideine, black apothecia (Fryday Citation2008). Miriquidica ventosa (Vain.) Timdal, known from Finland and Russia, is also muscicolous or saxicolous and has gray-edged squamules, but the squamules are concave and sorediate and the species contains miriquidic acid (Andreev Citation2004; Owe-Larsson and Rambold Citation2001; Timdal Citation1993). Other Miriquidica species containing norstictic acid are strictly saxicolous, forming a typical areolate thallus (not with gray-edged squamules), and have black, lecideine apothecia. M. vicaria may also be confused with Psorinia conglomerata (Ach.) Gotth. Schneid., but that species has biatorine, black apothecia even when young, a more greenish epihymenium, and, at least partly, 1-septate ascospores. The asci of P. conglomerata have a well-developed ocular chamber, i.e., they are more similar to the Bacidia type. There are two chemotypes of P. conglomerata: (i) atranorin and stictic acid (major) and (ii) atranorin and norstictic acid (major) (Timdal Citation1992); atranorin does not occur in M. vicaria.
Additional specimens examined: NORWAY. SOGN OG FJORDANE: Årdal, Sletterust, along Mannsbergelvi, 61.2753°N, 8.0230°E, 1060 m alt., muscicolous on calciferous rock, 13 Jun 2011, Haugan 10185 (O L-173700) [TLC: norstictic acid, connorstictic acid]; TROMS: Målselv, Mt. Rostafjell, Rostaaksla, 69.0470°N, 19.5341°E, 700 m alt., 26 May 1984, Timdal 4134 (O L-17360) [TLC: norstictic acid]. SWEDEN. JÄMTLAND: Undersåker par., Gåsåliden, close to path from Edsåsen, on pebbles on gravelly ground, alt. 850 m, 63.27833°N, 13.12662°E, 24 Aug 2015, Nordin 7884 (UPS L-723005); Mt. Middagsvalen, SW slope, ca. 1.5 km NW of Vallbo, on soil and mosses, alt. 845 m, 63.13912°N, 13.04358°E, 21 Aug 2015, Nordin 7784 (UPS L-721906), on bryophytes, alt. 855 m, 63.13983°N, 13.04343°E, 21 Aug 2015, Nordin 7784 (UPS L-721936); LYCKSELE LAPPMARK: Tärna par., Ume älv, Över-Umans sydvästligaste vik, [= Ume river, the southwesternmost bay of Lake Över-Uman] 66.06–66.07°N, 14.32–14.33°E, alt., 530 m, 29 Aug 1960, Du Rietz 2194b (UPS: L-119263); TORNE LAPPMARK: Jukkasjärvi par., Björkliden, N side of the river Rakkasjohka, just N of the trail from Björkliden Fjällby to Njula, alt. 495 m, 68.40528°N, 18.66981°E, 6 Aug 2013, Westberg (UPS L-794945).
A WORLD KEY TO THE GENUS BRYONORA
In previous works (Poelt Citation1983; Poelt and Obermayer Citation1991), three Bryonora species (B. castanea, B. rhypariza, and B. pulvinar) were divided into varieties. We have kept these varieties in the key, as they may, in some cases, represent distinct species and at any rate deserve further study. Species that do not occur in the Nordic countries have not been studied by us, and data for the key are thus partly compiled from the literature (Fryday and Øvstedal Citation2012; Holtan-Hartwig Citation1991; Øvstedal and Lewis Smith Citation2001; Poelt Citation1983; Poelt and Mayrhofer Citation1988; Poelt and Obermayer Citation1991).
1. On rock or on other lichens on rock 2
1′. On soil, mosses, or plant debris 4
2. Apothecium sections K+ red (norstictic acid); ascospores subglobose to kidney-shaped or spirally twisted, 15–19 × 7–9 µm; in Himalaya B. selenospora
2′. Apothecium sections K− (without norstictic acid); ascospores ellipsoid or dumbbell-shaped 3
3. Ascospores often dumbbell-shaped, (12–)14–16(–19) × 5–7 µm; Himalaya B. reducta
3′. Ascospores ellipsoid, (11–)13–18(–21) × 4–5.5(–6) µm; Norway, Sweden, Svalbard B. castaneoides
4. Thallus composed of scattered to conglomerate, shiny brown, gray-edged squamules; containing stictic acid; apothecia unknown B. microlepis
4′. Thallus various, not of gray-edged squamules; not containing stictic acid; usually apotheciate 5
5. Apothecium sections K+ red (norstictic acid) 6
5′. Apothecium sections K− (without norstictic acid) 11
6. Thallus well-developed and apothecia with a distinct thalline margin 7
6′. Thallus usually poorly developed and apothecia without a distinct thalline margin, appearing biatorine 9
7. Thallus on cyanobacteria; apothecium margin usually suppressed B. rhypariza var. cyanotropha
7′. Thallus not on cyanobacteria; apothecium margin usually thick and conspicuous 8
8. Ascospores 20–32 µm long; often on soilB. rhypariza var. lamaina
8′. Ascospores (14–)16–23(–26) µm long; usually growing on Grimmia or AndreaeaB. rhypariza var. rhypariza
9. Ascospores less than 25 µm long B. castanea var. castanea
9.′ Ascospores more than 25 µm long 10
10. Ascospores (25–)29–39(–42) × (4-)4.5–7(−7.5) µm; widely distributed B. curvescens
10′. Ascospores (22–)25–33(–40) × (6–)7–10(–12) µm; Himalaya B. castanea var. euryspora
11. Thallus of distinctly elongated, podetium-like units 12
11′. Thallus crustose, squamulose, or indistinct 15
12. Medulla KC−; thallus units capitate; apothecia unknown B. corallina
12′. Medulla KC+ reddish (soon disappearing); thallus units not capitate; usually richly fertile 13
13.Thallus with punctiform or slightly elongate pseudocyphellae, strongly shiny, of rod-shaped units B. stipitata
13′.Thallus without pseudocyphellae but with deep furrows, matt to somewhat shiny, dwarf fruticose or with somewhat flattened units 14
14. Ascospores simple or 1(–3)-septate, (16–)20–25(–28) µm long B. pulvinar var. pulvinar
14′. Ascospores simple, 12(–15) µm long B. pulvinar var. microspora
15.Thallus effuse on soil, of flattish areoles that break down into ± sorediate granules; apothecia 0.3–0.4 mm diam; with perlatolic acid; South America B. granulata
15′.Thallus poorly developed to granulose, not breaking into sorediate granules; apothecia normally larger, up to 4 mm diam; without perlatolic acid 16
16.Ascospores 19–39 µm long B. septentrionalis
16′.Ascospores up to 19 µm long 17
17. Thallus with protocetraric acid; Southern Hemisphere B. peltata
17′.Thallus without protocetraric acid; Northern Hemisphere 18
18.Apothecium margin darker than the disc; disc often pruinose; without lobaric acid; widely distributed in the Northern Hemisphere B. pruinosa
18′.Apothecium margin paler than the disc; disc not pruinose; with lobaric acid; Himalaya B. yeti
DISCUSSION
This is the first study to assess the phylogenetic placement and the circumscription of Bryonora in a multilocus, phylogenetic context. Although uncertainties remain concerning the delimitation of the Lecanoraceae, as well as relationships within the family, we found strong support for the inclusion of Bryonora. Conversely, we found no indication of the previously hypothesized, close relationship between Bryonora and Protoparmelia.
Bryonora was recovered as a highly supported, monophyletic group that is sister to a clade that includes species of Lecanora in the strict sense (L. allophana, L. horiza, and L. imshaugii), thus representing the core of the family. Although relationships within the Lecanoraceae are not always supported, it is highly unlikely that future revisions of family boundaries would result in the transfer of Bryonora to another family. This is in contrast to the position of the genus Miriquidica in our analysis, which, although not fully supported, is well outside the Lecanoraceae. This genus has been viewed as a member of this family ever since its description (Hertel and Rambold Citation1987), but it is notable that when not “forced” into the Lecanoraceae by the choice of outgroup (as in, e.g., Zhao et al. Citation2016), Miriquidica has also ended up outside the family in other phylogenies (Miadlikowska et al. Citation2014; Spribille et al. Citation2020). The phylogenetic position of Miriquidica deserves further study, preferably using more markers, as its position is usually unresolved.
The presence or absence of a zeorine apothecial margin has been an important character for the delimitation of Bryonora. Although Poelt (Citation1983) included B. rhypariza in Bryonora, he explicitly excluded two other species (Lecanora castaneoides and L. vicaria) from the genus mainly due to their having a zeorine apothecial margin. Subsequently, Poelt and Obermayer (Citation1991) introduced an infrageneric division of Bryonora where the two species with a zeorine margin, B. rhypariza and B. selenospora (described in 1988), formed their own section (sect. Rhyparizae), which was later accommodated in the new genus Bryodina (Hafellner and Türk Citation2001). The argument in favor of recognizing Bryonora species with a zeorine apothecial margin as a lineage separate from the rest of the genus received some support in our analysis, as B. rhypariza is sister to a clade containing one of the previously excluded zeorine species, B. castaneoides (plus B. microlepis, with unknown apothecia). At the same time, the type species of Bryonora (B. castanea) and Bryodina (B. rhypariza) are both part of the same highly supported subclade within Bryonora. Recognizing Bryodina as genus distinct from Bryonora would thus imply describing a new, third genus for Bryonora pruinosa and B. septentrionalis, creating a generic division that would have low information content and unclear boundaries. In our opinion, Bryodina is therefore best treated as a synonym of Bryonora.
The seven Bryonora species included in our phylogeny include all species currently known from Europe, but sequence data for the additional eight species from the Himalayas and the Southern Hemisphere were not available. This includes the three species with fruticose to subfruticose thalli that were included in the section Stipitantes by Poelt and Obermayer (Citation1991). Whether the morphological characteristics of this group indicate a separate evolutionary trajectory within or outside Bryonora remains to be evaluated. Likewise, the status of the varieties of B. castanea, B. rhypariza, and B. pulvinar described by Poelt (Citation1983) and Poelt and Obermayer (Citation1991) warrants further study, as several of them may represent distinct species.
Supplemental Material
Download (322.4 KB)ACKNOWLEDGMENTS
We wish to thank Mika Bendiksby for providing us with 11 ITS and mrSSU sequences and Stefan Ekman for comments on the manuscript. We thank three anonymous reviewers for their constructive comments.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Supplementary material
Supplemental data for this article can be accessed on the publisher’s Web site.
Additional information
Funding
LITERATURE CITED
- Andersen HL, Ekman S. 2005. Disintegration of the Micareaceae (lichenized Ascomycota): a molecular phylogeny based on mitochondrial rDNA sequences. Mycol Res. 109(1):21–30.
- Andreev MP. 2004. Notes on the lichen genus Miriquidica (Lecanorales, Lecanoraceae) in Russia. Bibl Lichenol. 88:15–42.
- Arup U, Ekman S, Lindblom L, Mattsson J-E. 1993. High performance thin layer chromatography (HPTLC), an improved technique for screening lichen substances. Lichenologist. 25(1):61–71.
- Bhattacharya D, Lutzoni F, Reeb V, Simon D, Nason J, Fernandez F. 2000. Widespread occurrence of spliceosomal introns in the rDNA of ascomycetes. Mol Biol Evol. 17(12):1971–84.
- Ferencova Z, Rico VJ, Hawksworth DL. 2017. Extraction of DNA from lichen-forming and lichenicolous fungi: a low-cost fast protocol using Chelex. Lichenologist. 49(5):521–25.
- Fries TM. 1871. Lichenographia Scandinavica sive disposito lichenum in Dania, Suecica, Norvegica, Fennia, Lapponia Rossica hactenus collectorum. Pars Prima. Uppsala (SWE): Ed. Berling.
- Fryday AM. 2008. Three new species of lichenized fungi with cephalodia from the southern New Zealand shelf Islands (Campbell Plateau). Lichenologist. 40(4):283–94.
- Fryday AM, Øvstedal DO. 2012. New species, combinations and records of lichenized fungi from the Falkland Islands (Islas Malvinas). Lichenologist. 44(4):483–500.
- Gardes M, Bruns TD. 1993. TS primers with enhanced specificity for basidiomycetes – application to the identification of mycorrhizae and rusts. Mol Ecol. 2(2):113–18.
- Grube M, Baloch E, Arup U. 2004. A phylogenetic study of the Lecanora rupicola group (Lecanoraceae, Ascomycota). Mycol Res. 108(5):506–14.
- Hafellner J, Türk R. 2001. Die lichenisierten Pilze Österreichs - eine Checkliste der bisher nachgewiesenen Arten mit Verbreitungsangaben. Stapfia. 76:1–167.
- Hertel H, Rambold G. 1987. Miriquidica genus novum Lecanoracearum (Ascomycetes lichenisati). Mitt Bot Staatssamml München. 23:377–92.
- Holtan-Hartwig J. 1991. A revision of the lichens Bryonora castanea and B. curvescens. Mycotaxon. 40:295–305.
- Ihlen P, Ekman S. 2002. Outline of phylogeny and character evolution in Rhizocarpon (Rhizocarpaceae, lichenized Ascomycota) based on nuclear ITS and mitochondrial SSU ribosomal DNA sequences. Biol J Linn Soc. 77(4):535–46.
- Katoh K, Rozewicki J, Yamada KD. 2019. MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief Bioinform. 20(4):1160–66.
- Kirika P, Parnmen S, Lumbsch T. 2012. Two new species of Lecanora sensu stricto (Lecanoraceae, Ascomycota) from east Africa. Mycokeys. 3:37–47.
- Lanfear R, Calcott B, Ho SY, Guindon S. 2012. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 29(6):1695–701.
- Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B. 2017. PartitionFinder 2: new methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Mol Biol Evol. 34(3):772–73.
- Leavitt SD, Fernández-Mendoza F, Pérez-Ortega S, Sohrabi M, Divakar PK, Vondrák J, Lumbsch HT, St. Clair L. 2013. Local representation of global diversity in a cosmopolitan lichen-forming fungal species complex (Rhizoplaca, Ascomycota). J Biogeogr. 40:1792–806.
- Leavitt SD, Kraichak E, Vondrak J, Nelsen MP, Sohrabi M, Perez-Ortega S, St Clair LL, Lumbsch HT. 2016. Cryptic diversity and symbiont interactions in rock-posy lichens. Mol Phylogenet Evol. 99:261–74.
- Liu YJ, Whelen S, Hall BD. 1999. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol. 16(12):1799–808.
- Magnusson AH. 1946. Lichens from Lycksele Lappmark and adjacent parts of Norway. Arkiv för Botanik. 33A(1):1–146.
- Magnusson AH. 1952. Lichens from Torne Lappmark. Arkiv för Botanik. 2(2):49–249.
- Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lücking R, et al. 2006. New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia. 98(6):1088–103.
- Miadlikowska J, Kauff F, Högnabba F, Oliver JC, Molnár K, Fraker E, Gaya E, Hafellner J, Hofstetter V, Gueidan C, et al. 2014. A multigene phylogenetic synthesis for the class Lecanoromycetes (Ascomycota): 1307 fungi representing 1139 infrageneric taxa, 317 genera and 66 families. Mol Phylogenet Evol. 79:132–68.
- Mirarab S, Nguyen N, Guo S, Wang L-S, Kim J, Warnow T. 2015. PASTA: ultra-large multiple sequence alignment for nucleotide and amino-acid sequences. J Comput Biol. 22(5):377–86.
- Myllys L, Velmala S, Holien H, Halonen P, Wang L-S GT. 2011. Phylogeny of the genus Bryoria. Lichenologist. 43:617–38.
- Myllys L, Velmala S, Lindgren H, Glavich D, Carlberg T, Wang L-S GT. 2014. Taxonomic delimitation of the genera Bryoria and Sulcaria, with a new combination Sulcaria spiralifera introduced. Lichenologist. 46:737–52.
- Nguyen L-T, Schmidt HA, von Haeseler A, Minh BQ. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol Biol Evol. 32:268–74.
- Nordin A. 2015. Lecanora vicaria – en bortglömd art. Lavbulletinen. 2015:62–63.
- Nylander W. 1862. Ad lichenographiam Groenlandiae quaedam addenda. Flora (Regensburg). 45:81–83.
- Orange A, James PW, White FJ. 2010. Microchemical methods for the identification of Lichens. 2nd ed. London (UK): British Lichen Society.
- Øvstedal DO, Lewis Smith RI. 2001. Lichens of Antarctica and South Georgia: a guide to their identification and ecology. Cambridge: Cambridge University Press.
- Owe-Larsson B, Rambold G. 2001. The sorediate species of the lichen genus Miriquidica (Lecanorales, Lecanoraceae). Bibl Lichenol. 78:335–64.
- Parnmen S, Rangsiruji A, Mongkolsuk P, Boonpragob K, Elix J, Lumbsch HT. 2010. Morphological disparity in Cladoniaceae: The foliose genus Heterodea evolved from fruticose Cladia species (Lecanorales, lichenized Ascomycota). Taxon. 59(3):841–49.
- Pino-Bodas R, Martín MP, Burgaz AR, Lumbsch HT. 2013. Species delimitation in Cladonia (Ascomycota): a challenge to the DNA barcoding philosophy. Mol Ecol Resour. 13:1058–68.
- Poelt J. 1958. Die lobaten Arten der Flechtengattung Lecanora Ach. sensu ampl. in der Holarktis. Mitt Bot Staatssamml München. 2:411–573.
- Poelt J. 1983. Bryonora eine neue Gattung der Lecanoraceae. Nova Hedwigia. 38:73–111.
- Poelt J, Mayrhofer H. 1988. Über Cyanotrophie bei Flechten. Plant Syst Evol. 158:265–81.
- Poelt J, Obermayer W. 1991. Beiträge zur Kenntnis der Flechtenflora des Himalaya II. Die Gattung Bryonora (Lichenes, Lecanoraceae) zugleich eine Revision aller Arten. Nova Hedwigia. 53:1–26.
- Printzen C. 1995. Die Flechtengattung Biatora in Europa. Bibl Lichenol. 60:1–275.
- Reeb V, Lutzoni F, Roux C. 2004. Contribution of RPB2 to multilocus phylogenetic studies of the Pezizomycotina (euascomycetes, Fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol Phylogenet Evol. 32:1036–60.
- Rodriguez Flakus P, Printzen C. 2014. Palicella, a new genus of lichenized fungi and its phylogenetic position within the Lecanoraceae. Lichenologist. 46:535–52.
- Ronquist F, Teslenko M, Mark P, Ayres DL, Höhna S, Larget B, Liu L, Huelsenbeck J. 2012. MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol. 61:539–42.
- Santesson R. 1993. The lichens and lichenicolous fungi of Sweden and Norway. Lund (SWE): SBT-förlaget.
- Santesson R, Moberg R, Nordin A, Tønsberg T, Vitikainen O. 2004. Lichen-forming and lichenicolous fungi of Fennoscandia. Uppsala (SWE): Museum of Evolution, Uppsala University.
- Schmitt I, Crespo A, Divakar PK, Fankhauser JD, Herman-Sackett E, Kalb K, Nelsen MP, Nelson NA, Rivas-Plata E, Shimp AD, et al. 2009. New primers for promising single-copy genes in fungal phylogenetics and systematics. Persoonia. 23:35–40.
- Schmull M, Miadlikowska J, Pelzer M, Stocker-Wörgötter E, Hofstetter V, Fraker E, Hodkinson BP, Reeb V, Kukwa M, Lumbsch HT, et al. 2011. Phylogenetic affiliations of members of the heterogeneous lichen-forming fungi of the genus Lecidea sensu Zahlbruckner (Lecanoromycetes, Ascomycota). Mycologia. 103(5):983–1003.
- Schneider K, Resl P, Westberg M, Spribille T. 2015. A new, highly effective primer pair to exclude algae when amplifying nuclear large ribosomal subunit (LSU) DNA from lichens. Lichenologist. 47:269–75.
- Schoch CL, Seifert KA, Huhndorf SM, Robert V, Spouge JL, Levesque CA, Chen W, Crous PW, Boekhout T, Damm U, et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for fungi. Proc Natl Acad Sci USA. 109:6241–46.
- Singh G, Dal Grande F, Divakar PK, Otte J, Crespo A, Schmitt I. 2017. Fungal-algal association patterns in lichen symbiosis linked to macroclimate. New Phytologist. 214:317–29.
- Singh G, Dal Grande F, Divakar PK, Otte J, Leavitt SD, Szczepanska K, Crespo A, Rico VJ, Aptroot A, Silva Cáceres ME, et al. 2015. Coalescent-based species delimitation approach uncovers high cryptic diversity in the cosmopolitan lichen-forming fungal genus Protoparmelia (Lecanorales, Ascomycota). PLoS One. 10(5):e0124625.
- Spribille T, Fryday AM, Pérez-Ortega S, Svensson M, Tønsberg T, Ekman S, Holien H, Resl P, Schneider K, Stabentheiner E, et al. 2020. Lichens and associated fungi from Glacier Bay National Park, Alaska. Lichenologist. 52:61–181.
- Svensson M, Westberg M. 2021. A new lichenicolous species of Carbonea (Ascomycota, Lecanoraceae) from northern Sweden. Phytotaxa. 522:221–30.
- Timdal E. 1992. (‘1991’). A monograph of the genus Toninia (Lecideaceae, Ascomycetes). Opera Botanica. 110:1–137.
- Timdal E. 1993. Miriquidica ventosa comb. nov., a rediscovered lichen. Bryologist. 96:616–18.
- Vainio E. 1883. Adjumenta add Lichenographiam Lapponiae fennicae atque Fenniae borealis. II. Meddeland. Soc. Fauna Flora Fenn. 53:1–340.
- Wedin M, Wiklund E, Crewe A, Döring H, Ekman S, Å N, Schmitt I, Lumbsch HT. 2005. Phylogenetic relationships of Lecanoromycetes (Ascomycota) as revealed by analyses of mtSSU and nLSU rDNA sequence data. Mycol Res. 109:159–72.
- White TJ, Bruns T, Lee S, Taylor J. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M, Gelfand D, Sinsky JJ, White T, editors. PCR protocols: a guide to methods and applications. New York (US): Academic Press New York; p. 315–32.
- Wijayawardene NN, Hyde KD, Lumbsch HT, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R. 2018. Outline of Ascomycota: 2017. Fungal Divers. 88:167–263.
- Wirth V. 1981. Zur flechtenkundlichen Durchforschung Süddeutschlands und angrenzender Gebiete. Stuttg Beitr Naturkd, A. 349:1–19.
- Zahlbruckner A. 1928. Catalogum Lichenis Universalis 5. Berlin (GER): Gebrüder Bornträger.
- Zhao X, Leavitt SD, Zhao ZT, Zhang LL, Arup U, Grube M, Pérez-Ortega S, Printzen C, Śliwa L, Kraichak E, et al. 2016. Towards a revised generic classification of lecanoroid lichens (Lecanoraceae, Ascomycota), based on molecular, morphological and chemical evidence. Fungal Divers. 78:293–304.
- Zoller S, Scheidegger C, Sperisen C. 1999. PCR primers for the amplification of mitochondrial small subunit ribosomal DNA of lichen-forming ascomycetes. Lichenologist. 31:511–16.
