Abstract
A field study was conducted to determine whether tap root survival of white clover could be improved by hybridisation with Trifolium uniflorum. Tap root fragmentation and percentage of surviving tap roots were measured in 13, 16 and 19–20 month old plants. There were no intact healthy tap roots in white clover or second backcross (BC2) hybrids (12.5% T. uniflorum genes) post 13 months, but these were still present in T. uniflorum and first backcross (BC1) hybrids (25% T. uniflorum genes). Survival of T. uniflorum tap roots was higher than BC1, BC2 and white clover—30% of plants had intact, healthy tap roots at 19–20 months. The BC1 generation (31%) also had higher tap root survival than BC2 (13%) and white clover (11%) at 13 months. Although improved survival was not expressed as strongly in older BC1 plants, tap root deterioration was slower than in white clover and BC2. There is potential for targeted selection of specific genotypes and traits to further increase tap root lifespan in BC1 hybrids as there has been no previous selection for root traits in this material. The relationships between root diameter, leaf size and persistence in T. uniflorum and hybrids may differ from those expected for white clover cultivars. Characteristics of nodal rooting would also be expected to play a part in longer-term productivity and persistence.
Introduction
The productivity, nutritive value and fixation of atmospheric nitrogen (N) by white clover (Trifolium repens L.) provide high-value herbage in temperate grasslands. However, loss of the white clover tap root is a pivotal stage in the decline of the white clover component of pastures (Woodfield & Caradus Citation1996; Brock et al. Citation2000). Young white clover plants develop a tap root through the secondary thickening of the seedling seminal root. Nodal roots develop once the primary shoot axis branches to form a network of radiating stolons. Between 12 and 18 months after establishment, tap root death occurs due to a combination of factors, such as natural senescence, pathogen and insect attack, and carbon allocation to nodal roots (Westbrooks & Tesar Citation1955; Brock et al. Citation2000). This is followed by loss of the primary shoot, leading to fragmentation of the plant into several smaller clonal units, each dependent upon its nodal root systems. Nodal roots are generally smaller than the primary tap root and clonal plants are, therefore, more susceptible to drying of the soil surface and attack by root pests and pathogens.
Interspecific hybridisation can be used to improve white clover through the introduction of characteristics from related Trifolium species (Williams Citation1987; Abberton Citation2007; Williams & Nichols Citation2011). Target characteristics have included increased flowering, drought resistance and pest resistance (Hussain et al. Citation1997; Marshall et al. Citation1998; Marshall et al. Citation2001). Trifolium uniflorum L. is a close, wild relative of white clover (Ellison et al. Citation2006) found in the Mediterranean region. It is of no agronomic value, and little information about it is reported in the literature. It has been considered for use in the dryland wheatbelt of southern Australia where rising water tables, salinisation and acid soils are agronomic issues, and ranked poorly for productivity but highly for persistence among the species investigated (Li et al. Citation2008). Nichols et al. (Citation2014a,b,c) have reported increased drought resistance and tolerance to low P supply in T. repens × T. uniflorum first backcross (BC1) hybrids, suggesting that hybridisation with T. uniflorum may provide a valuable route to reducing some of the current limitations of white clover.
Based on the thick, woody nature of the T. uniflorum tap root reported in the literature (Pandey & Petterson Citation1978; Dymock & Hunt Citation1989), we hypothesised that tap roots of T. uniflorum would survive longer than those of white clover. Given the expected intermediate characteristics of hybrids, between the two parents, we also hypothesised that tap roots of the hybrids would survive longer than those of white clover. The nature of the T. uniflorum tap root suggested that a potential increase in tap root survival may be attributed, at least partially, to increased root diameter. In a grazing experiment, Nichols et al. (Citation2014d) investigated the effect of hybridisation with T. uniflorum on important white clover characteristics. This experiment presented the opportunity to quantify tap root survival, as outlined above, in the T. repens × T. uniflorum hybrids compared with the white clover and T. uniflorum parents.
Materials and methods
Experimental arrangement
A field experiment was conducted under sheep grazing from November 2008 to May 2010 (Nichols et al. Citation2014d). The experimental area was located in a paddock at the AgResearch farm in Lincoln, on a Wakanui silt loam (Cox Citation1978) (Udic Ustochrept, USDA soil taxonomy). Twenty-seven clover entries of five clover types were used. These included two T. uniflorum accessions, eight white clover cultivars of a range of leaf sizes, and a red clover cultivar as a standard control (). Hybrid entries comprised 10 BC1 families and six second backcross (BC2) families (). On average, T. uniflorum contributes 25% of the genes in BC1 hybrids and 12.5% of the genes in BC2 hybrids. Red clover was included to allow comparison with a species that is currently used in dryland environments, to which T. repens × T. uniflorum hybrids may also be well suited. Scarified clover seed was germinated on damp filter paper, and established in root trainers containing a sand/peat potting mix in a temperature-controlled glasshouse in September 2008.
Table 1 Clover entries used in the experiment.
The existing perennial ryegrass/white clover vegetation in the paddock was removed by spraying with 2.5 L ha−1 Roundup® (glyphosate). The tall fescue (Festuca arundinacea Schreb.) cultivar ‘Advance’ with MaxP endophyte was then direct drilled into the paddock at a rate of 20 kg ha−1 on 10 November 2008, and the clover plants were transplanted 2–8 days later at 1 m spacing. The experimental area was positioned in the centre of the paddock to avoid high stock traffic areas. After drilling and the transplanting of clovers, the paddock was irrigated to aid establishment, and then no further irrigation was applied.
The experimental design contained five blocks (replicates), with four sub-plots within each replicate to allow for four tap root harvests over time. Within each sub-plot, the 27 clover entries were randomised in a 5 × 6 arrangement, with the extra spaces filled with spare plants to eliminate gap effects.
A row of pilot plants was established along one edge of the experimental area to provide material for monitoring the onset of tap root death. A mixture of clover types was used for this, including white clover cultivars, BC1 hybrids, BC2 hybrids and T. uniflorum accessions. These plants were established and maintained in the same manner as the experimental material.
The whole paddock was grazed by a mob of 150–200 ewes and hoggets at 5–12 week intervals, depending on the season. Grazing was carried out over 24–48 hours at 2000–2500 kg ha−1 total dry matter (DM), to a post-grazing residual of 800–1000 kg ha−1 total DM.
Measurements
Tap root lifespan was monitored by destructive harvesting of plants, beginning after the expected period of time at which tap root death occurs in white clover (12–18 months old) (Westbrooks & Tesar Citation1955). This was confirmed by harvesting pilot plants from each of the clover types to identify the onset of tap root loss. Age of the tap roots was measured from the time of seedling germination. Three harvests were conducted on 12–21 October 2009 (13 month old plants), 25–26 January 2010 (16 month old plants) and 22–30 April/24–28 May 2010 (19–20 month old plants). The third harvest combined plants from the harvest 3 and 4 sub-plots, due to low numbers of surviving tap roots.
At all harvests, turves of one spade square (180 × 180 mm) and 150–200 mm deep were dug out around the centre of each plant. The original centre of the plant was identified, and the fragmentation of the tap root was scored on a scale from 1 to 6 (1 = intact and healthy; 2 = intact with lesions; 3 = early fragmentation; 4 = advanced fragmentation; 5 = root remnants only; 6 = not present). The number of plants with intact, healthy tap roots was then used to calculate the percentage tap root survival for each of the five clover types.
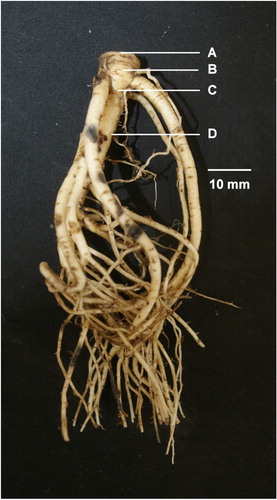
At harvest 1 only (13 month old plants), intact and healthy tap roots were collected and washed to remove the soil (). Measurements of tap root diameter were then made at the base (below the crown of the plant), 10 mm below the base, 20 mm below the base, and at the point immediately distal to the zone of basal thickening (). The latter is a zone of thickened root at the base of the tap root, the length of which varies from plant to plant. The number and diameter of lateral roots greater than 1 mm in diameter, arising from the top 20 mm of the tap root, were also measured. The top 100 mm of the tap roots (including lateral roots) were then dried for approximately 15 h at 80 ˚C and weighed.
Figure 1 Intact, healthy tap roots present in replicate 5 of the field experiment (top 100 mm of the root system). Plants are 13 months old.

Statistical analysis
For analysis, clover entries were grouped into the five broad clover types (white clover, T. uniflorum, BC1, BC2, red clover). Tap root diameter, tap root dry weight (DW) and diameter of lateral roots (log-transformed to satisfy normality assumptions) were analysed using analysis of variance (ANOVA) and Tukey's method, to account for multiple comparisons and unbalanced data. The tap root diameter analyses presented here primarily excluded red clover, due to its larger size compared with the other clover types, which hindered comparisons between white clover, T. uniflorum and the hybrids, the objective of this study. However, a second ANOVA was also conducted to compare red clover diameters with those of the other clovers, and any significant differences are also described in the text, along with the red clover means. Back-transformed means are presented for lateral root diameters, with the estimated standard error of the mean (SEM) (back-transformed mean × log SEM). The percentage of intact tap roots was compared among the five clover types with Fisher's exact test, as the percentages were ratios of counted values (which are binomially distributed). A generalised linear modelling (GLM) approach, with negative binomial distributions and log link function, was used to analyse the number of lateral roots arising from the top 20 mm of the tap root, in order to account for the observed large variation of these counted values. All analyses were carried out using Minitab version 15 (Minitab Inc), apart from GLM (glm.nb function in package MASS in R version 2.8.1 [R Core Team Citation2012]). The analyses took into account block and clover type effects, plus their interactions where appropriate.
Results
Tap root fragmentation
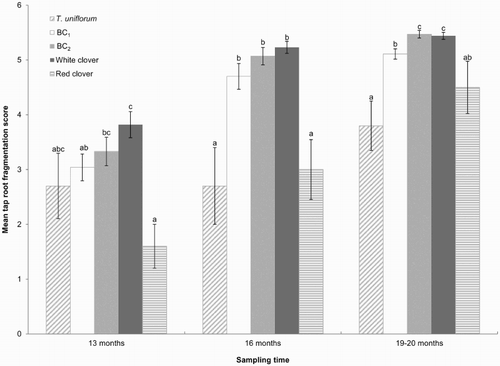
The fragmentation scores of T. uniflorum were lower than those of BC1, BC2 and white clover on all occasions (i.e. less tap root deterioration). These differences were significant for 16 (January 2010) and 19–20 month old (April/May 2010) plants (P < 0.05), with a similar trend at 13 months when compared with white clover (P = 0.069) (). Compared with white clover, deterioration of the BC1 tap root was lower in 13 (P = 0.017) and 19–20 month old plants (P < 0.001). The BC1 generation also had lower tap root deterioration at the final harvest (19–20 month old plants) (P < 0.009) when compared with BC2. Tap root fragmentation scores of the BC2 generation and white clover did not differ from each other at any time.
Tap root fragmentation of red clover did not differ from T. uniflorum but was lower than BC2 (P < 0.018) and white clover (P < 0.019). There was also less tap root deterioration in red clover than in BC1 at 16 months old (P = 0.02).
Tap root survival
Tap root survival of the T. uniflorum parent and BC1 was higher than that of white clover and BC2 (). At 13 months, 50% of T. uniflorum tap roots were still intact compared with just 11% for white clover (P = 0.012) and 13% for BC2 (P = 0.029). Survival of BC1 tap roots (31%) was not different from T. uniflorum at that time, but was higher (P = 0.032) than in white clover.
Table 2 Percentages of intact healthy tap roots (fragmentation scores of 1) for the five clover types in 13, 16 and 19–20 month old plants.
No healthy intact tap roots of white clover or BC2 were found after the first sampling date, but some T. uniflorum and BC1 tap roots were still present in 16 and 19–20 month old plants (). Tap root survival of T. uniflorum was higher than BC1 at both 16 (P = 0.003) and 19–20 months (P < 0.001). Survival of red clover tap roots did not differ from BC1 at any time, and was different from T. uniflorum only in 16 month old plants (P = 0.044), when the percentage of surviving red clover tap roots was significantly lower.
Tap root diameter
There was a trend for decreasing tap root diameter in the order of: T. uniflorum > BC1 > BC2 > white clover. The exception was the very base of the tap root, at the crown of the plant, where the diameter of BC1 was the largest (). Differences between the T. uniflorum and white clover parents were not significant at the base of the tap root but white clover was 43% smaller (P = 0.001) 20 mm below the base (C) and 46% smaller (P = 0.001) below the basal thickening (D). This indicates that the white clover tap root tapered more rapidly than that of T. uniflorum. The intermediate diameters of the two hybrid generations were also approximately 30% smaller (P < 0.05) than T. uniflorum 20 mm below the base of the tap root (C) and 22%–25% smaller (P < 0.05) below the basal thickening (D). Tap root diameter of BC2 did not differ from white clover at any point, but in BC1 it was 37% greater (P = 0.026) than white clover below the basal thickening (D).
Figure 4 Mean diameter of intact tap roots (tap root fragmentation scores of 1) (±SEM) in 13 month old plants (October 2009). Measurements were made A, at the base of the tap root; B, 10 mm below the base; C, 20 mm below the base; D, below the basal thickening. Means with the same letters show no significant differences at the 5% level.
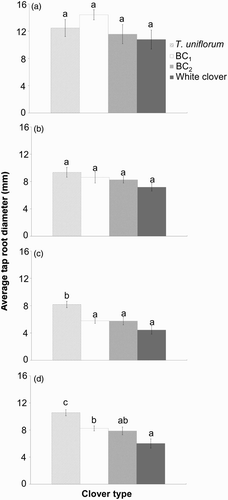
Tap root diameter of red clover was 18.9 mm at the base, 12.4 mm at 10 mm, 9.3 mm at 20 mm, and 11.3 mm below the basal thickening. These measurements were higher than those for T. uniflorum, but the differences were only significant (P = 0.035) at the base of the tap root (+52%) (data not shown). Red clover tap root diameter was also greater than the diameters of BC2 (P = 0.018) and white clover (P = 0.008) at the base of the tap root; greater than BC1 (P = 0.033) and white clover (P = 0.011) at 20 mm; and greater than BC1 (P = 0.022), BC2 (P = 0.035) and white clover (P = 0.001) below the basal thickening (data not shown).
Tap root dry weight
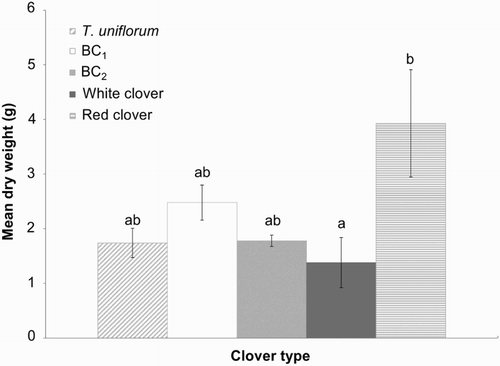
Red clover had the greatest mean DW for intact tap roots (fragmentation scores of 1) of the five clover types, and was 2.9 times larger (P = 0.033) than white clover (). There were also trends towards greater tap root DW in red clover (2.2 times larger) compared with BC2 (P = 0.093) and T. uniflorum (P = 0.066), but there were no differences among the hybrids and parents.
Lateral roots
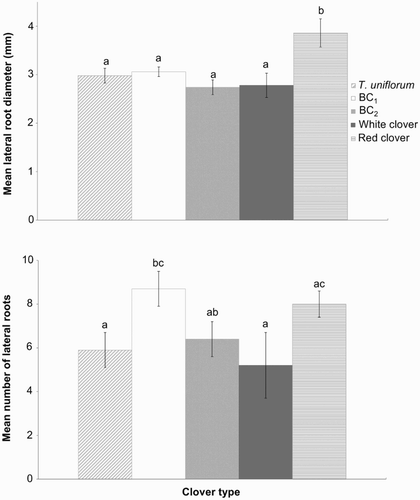
The mean diameter of lateral roots (>1 mm in diameter) arising from the top 20 mm of the tap root was 26%–41% higher (P < 0.05) in red clover (A), but its lateral root numbers were the same as for the other clover types (B). Lateral roots of T. uniflorum and BC1 were slightly thicker than those of BC2 and white clover but there were no significant differences among the hybrids and parents. However, BC1 had 47% more (P = 0.015) lateral roots than the T. uniflorum parent and 67% more (P = 0.016) than the white clover parent (B).
Figure 6 Measurements on lateral roots arising from the top 20 mm of intact healthy tap roots, in 13 month old plants (October 2009). A, back-transformed mean lateral root diameter (± estimated SEM); B, mean number of lateral roots larger than 1 mm in diameter (±SEM). Means with the same letter show no significant differences at the 5% level.
Discussion
Results confirmed the hypothesis that tap root survival of T. uniflorum was higher than that of white clover. Survival of BC1 tap roots was also greater than white clover, but this trait was lost by a second backcross to white clover. This confirms previous work showing that traits from T. uniflorum can be introduced to white clover (Nichols et al. Citation2014b,c), although these currently do not always persist past the first backcross generation (Nichols et al. Citation2014b). The latter may be improved with ongoing breeding. Tap root death in white clover generally occurs 12–18 months after establishment (Westbrooks & Tesar Citation1955; Brock & Tilbrook Citation2000), but has been recorded as early as 6 months and as late as 2 years after sowing (Brock et al. Citation2000). In the current study, all white clover tap roots had disintegrated by 16 months, but T. uniflorum and BC1 tap roots were still present at 19–20 months. The percentage of T. uniflorum tap roots that were still intact at that time (30%) suggests they would have survived for at least several months more.
First generation hybrids have been shown to have improved drought resistance compared with white clover under controlled field conditions (Nichols et al. Citation2014b). They may, therefore, be better adapted than white clover to dryland environments, where red clover is often used as an alternative. Although tap root fragmentation was often lower than the hybrids, tap root survival of the red clover cultivar ‘Sensation’ was generally no better than T. uniflorum or the two hybrid generations, except in 13 month old BC2 plants. This suggests there would be no disadvantage to tap root longevity in using hybrids rather than red clover, although only one red clover cultivar was studied here. Dry matter scores measured over 18 months in the current experiment also suggested there was no disadvantage to productivity of hybrids compared with red clover, at least in this dryland Canterbury environment (Nichols et al. Citation2014d). A definitive comparison between red clover and T. repens × T. uniflorum hybrids was not the focus of this study, and would be better performed at a later stage in the breeding programme, utilising a wider range of red clover cultivars, particularly for lower rainfall areas.
Although the percentage of surviving BC1 tap roots at the end of the experiment was small, survival was much higher in younger plants and significantly greater than white clover at 13 months. In addition, although the percentage of intact, healthy tap roots of BC1 did not differ significantly from BC2 and white clover at 19–20 months, the tap root fragmentation scores showed that deterioration of BC1 tap roots was still lower at that time. The longer-term impact of the timing of tap root losses is important, as smaller clonal plants are more vulnerable to environmental stresses (Woodfield & Caradus Citation1996). Small increases in tap root duration may, therefore, have significant effects on the impact of seasonal dry, or drought, conditions if these coincide with the period of ‘normal’ white clover plant fragmentation. Second backcross and, particularly, white clover plants had lower tap root survival in October (13 months), and may therefore have suffered from greater moisture stress later on in spring and summer. While white clover had higher dry matter yields than the hybrids throughout most of the experiment, this difference was no longer present in 19–20 month old plants (Nichols et al. Citation2014d). It is unknown how other traits related to drought resistance in T. repens × T. uniflorum hybrids influenced relative growth differences at this time. There may also be traits in the clonal component of the population, such as those related to nodal roots rather than tap roots, which influenced growth during the summer period.
The hybrid families in this study were from early crosses in the hybridisation programme, and no selections have been made for rooting characteristics. If the differences observed between BC1 hybrids and white clover at 13 months could be improved, and tap root survival at 19 months and later increased, valuable impacts on plant survival and production could be achieved, perhaps leading ultimately to improved persistence. It appears there is scope for such selection, through crossing individuals with increased tap root survival at 13 and possibly 16 months, to increase the frequency of the genes responsible. The superior tap root survival and lower fragmentation of T. uniflorum suggests that useful traits exist within this species, and wider screening among hybrid families may identify populations in which these traits are more predominant. In white clover, response to selection for root characteristics is known to be high (Woodfield & Caradus Citation1990; Caradus & Woodfield Citation1998). For example, Caradus & Woodfield (Citation1998) found that the diameter of the seedling tap root increased by 2.4% per breeding cycle. To establish a breeding population, higher replication than that used in the current experiment would be necessary to obtain a sufficient number of plants with the target characteristics.
The general, positive relationships observed in white clover between leaf size and root diameter, and leaf size and tap root survival (Caradus & Woodfield Citation1986; Brock & Tilbrook Citation2000) did not appear to influence tap root survival in T. uniflorum. The leaf size of this species is significantly smaller than that of white clover (Nichols et al. Citation2014d), yet tap root survival was higher. In contrast, Brock & Tilbrook (Citation2000) observed that leaf size in white clover was positively associated with tap root diameter, and that loss of the tap root was faster in small-leaved cultivars. A different relationship from the standard white clover correlation may also be present in T. repens × T. uniflorum hybrids as, on average, leaf lamina area of BC1 hybrids was also smaller than that of white clover (Nichols et al. Citation2014d), but tap root survival was again higher. Such a relationship may be indicative of material adapted to low soil moisture, as Caradus & Woodfield (Citation1986) also observed that white clover dryland ecotypes had larger diameter roots than expected for their leaf size. In general, characteristics in ‘wild’ germplasm such as T. uniflorum, and in relatively unselected hybrids, may reflect adaptations to edaphic stresses, such as drought and low soil fertility. For example, Nichols & Crush (Citation2014) also observed that phosphate partitioning between above- and below-ground biomass in T. uniflorum and some hybrids was similar to that reported in white clover hill country ecotypes, and in cultivars developed from such material. Further studies would be necessary to determine the interaction between leaf size, tap root diameter and tap root death in T. repens × T. uniflorum hybrids, which could differ from the relationships seen in white clover.
Various factors contribute to tap root death, including natural ageing, disease and inadequate carbohydrate supply (Westbrooks & Tesar Citation1955; Thomas Citation2003). An alternative to selecting directly for tap root survival is to select for underlying factors. We hypothesised that increased tap root diameter could contribute to a longer lifespan in the hybrid tap root but it did not appear to be a strong contributing factor, at least for 13 month old plants in the families studied here. Although white clover tap root diameter tended to be smaller than both hybrid generations, the difference was only significant when compared with BC1, below the basal thickening. However, having a greater diameter away from the tap root base may have played a part in the higher survival of T. uniflorum compared with BC2 and white clover. It is not known how differences in diameter at different points along the tap root may influence tap root fragmentation and survival. When measured in 13 month old plants, most tap root diameter measurements, as well as tap root survival, were higher for red clover compared with white clover. However, higher diameters at several points along the tap root did not improve tap root survival of red clover compared with the BC1 generation. In general, the greater tap root diameters in red clover may reflect the fact that it relies solely on its tap root for survival.
Comparative anatomy of white clover, T. uniflorum and T. repens × T. uniflorum tap roots has not been studied and could provide useful information to explain tap root survival, as well as other ecophysiological differences, among clover types. The tap root of T. uniflorum is relatively woody (Dymock et al. Citation1989), which may play a part in increased survival through mechanical resistance to decay. We hypothesised that the roots of T. repens × T. uniflorum hybrids will be thicker than those of white clover due to the morphology of T. uniflorum roots reported in the literature (Pandey Citation1957; Pandey & Petterson Citation1978). Using root cross-sectional area at the base of thickest nodal root as a proxy for root diameter, we have recorded thicker nodal roots in a T. repens × T. uniflorum BC1 hybrid family than in the corresponding white clover cultivar parent, both overall and under water-stressed conditions (Nichols et al. Citation2014b). Following the theory outlined by Thomas (Citation2003), it may be expected that a stronger nodal root system would divert more carbon away from the tap root. Instead, tap root survival was higher in some hybrid plants, although a reduced carbohydrate supply could contribute to the eventual death of the tap root in these plants. It should be noted that a strong nodal root system is vital for persistence of clonal plants following the loss of the tap root. Given the relatively small differences in tap root lifespan which we observed in the current experiment—notwithstanding potential improvements with further selection and breeding—the potential ability of the hybrids to transition to strong, deeply rooted clonal plants may be more important for long-term persistence and drought resistance (Nichols et al. Citation2014b).
Westbrooks & Tesar (Citation1955) and Kilpatrick & Dunn (Citation1961) noted the presence of Rhizoctonia and/or Fusarium species on white clover tap roots, and concluded that they played some part in tap root loss. Discoloration of the outer layer of the tap root and rotting of lateral roots, as described by Westbrooks & Tesar (Citation1955), were noted in the current experiment. In addition, lesions were present on some tap roots, while others had split at the crown. The most severe blackening of the roots occurred in red clover plants. Other data from this experiment showed lower shoot fungal disease in T. uniflorum than in BC1 and BC2 hybrids and white clover (Nichols et al. Citation2014d). There was considerable variation among individual BC1 families for fungal disease scores, with some families decreasing relative to their white clover parent. It is therefore possible that T. uniflorum and some hybrids were also less susceptible to root diseases, which could have contributed to increased tap root survival. During tap root harvesting, clover root weevil larvae (Sitona lepidus Gyllenhal) and, particularly during later harvests, grass grub (Costelytra zealandica [White]) were present. However, Dymock & Hunt (Citation1989) and Dymock et al. (Citation1989) have reported tolerance of T. uniflorum to grass grub and reduced grass grub larval growth on T. uniflorum and some T. repens × T. uniflorum hybrids. The latter was related to differences in root diameter and the effect this had on larval feeding (Dymock & Hunt Citation1989). Decreased feeding by insect pests, on the thick roots of T. uniflorum and T. repens × T. uniflorum hybrids, may therefore reduce the incursion of fungal diseases into damaged tissue.
Conclusion
Overall, both T. uniflorum and the BC1 generation did have higher tap root survival and lower tap root deterioration than white clover, although the number of intact BC1 tap roots at the end of the experiment was lower than expected. Tap root survival of T. uniflorum and BC1 and older BC2 plants was as good as that of red clover which, for the most part, varied little from all the other clover types. As there had been no selection in the hybrids for any characteristics, including root traits, it is likely that tap root survival could be improved further by breeding and selection, especially as there are clearly superior traits present within T. uniflorum. Greater understanding of the factors contributing to tap root death would help to target secondary characteristics for selection. In this study tap root diameter did not appear to be a significant factor in tap root survival of T. repens × T. uniflorum hybrids, but there were indications that it may have some influence. Investigation of the interaction between tap root survival and factors such as root diameter, root anatomy, soil moisture and susceptibility to root diseases and pests may assist in further improving tap root survival in BC1 hybrids. The impact on important traits such as productivity and drought resistance should also be considered. Given the complexity of the interactions between the factors affecting tap root lifespan, and the difficulty of studying root traits in the field, selecting directly for tap root survival may be the most efficient approach. Greater understanding of the nodal root characteristics of the hybrids, and their interaction with tap roots, may also help to improve the long-term persistence of white clover.
Acknowledgements
We would like to thank Isabelle Verry of AgResearch for providing the hybrid material. Thanks also to Stephen Stilwell and Jackie Sammonds of Lincoln University and Gary Arnold from AgResearch for assistance with harvesting. Chikako van Koten of AgResearch assisted with statistical analysis.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abberton MT 2007. Interspecific hybridization in the genus Trifolium. Plant Breeding 126: 337–342. doi: 10.1111/j.1439-0523.2007.01374.x
- Brock JL, Albrecht KA, Tilbrook JC, Hay MJM 2000. Morphology of white clover during development from seed to clonal populations in grazed pastures. Journal of Agricultural Science 135: 103–111. doi: 10.1017/S0021859699008060
- Brock JL, Tilbrook JC 2000. Effect of cultivar of white clover on plant morphology during the establishment of mixed pastures under sheep grazing. New Zealand Journal of Agricultural Research 43: 335–343. doi: 10.1080/00288233.2000.9513432
- Caradus JR, Woodfield DR 1986. Evaluation of root type in white clover genotypes and populations. New Zealand Agronomy Society Special Publication No. 5. 322–325.
- Caradus JR, Woodfield DR 1998. Genetic control of adaptive root characteristics in white clover. Plant and Soil 200: 63–69. doi: 10.1023/A:1004296707631
- Cox JE 1978. Soils and agriculture of part Paparua County. Soil Bureau Bulletin 34. New Zealand Department of Scientific and Industrial Research. 128 p.
- Dymock JJ, Hunt VA 1989. Laboratory studies of Trifolium uniflorum root consumption by grass grub (Costelytra zealandica). Proceedings of the 42nd New Zealand Weed and Pest Conference. Pp. 86–87.
- Dymock JJ, Van Den Bosch J, Caradus JR, Lane GA 1989. Growth and survival of grass grub, Costelytra zealandica (White) (Coleoptera: Scarabaeidae) on Trifolium species and T. repens × T. uniflorum hybrids. New Zealand Journal of Agricultural Research 32: 389–394. doi: 10.1080/00288233.1989.10421757
- Ellison NW, Liston A, Steiner JJ, Williams WM, Taylor NL 2006. Molecular phylogenetics of the clover genus (Trifolium—Leguminosae). Molecular Phylogenetics and Evolution 39: 688–705. doi: 10.1016/j.ympev.2006.01.004
- Hussain SW, Williams WM, Mercer CF, White DWR 1997. Transfer of clover cyst nematode resistance from Trifolium nigrescens Viv. to T. repens L. by interspecific hybridisation. Theoretical and Applied Genetics 95: 1274–1281. doi: 10.1007/s001220050693
- Kilpatrick RA, Dunn GM 1961. Fungi and insects associated with deterioration of white clover taproots. Crop Science 1: 147–149. doi: 10.2135/cropsci1961.0011183X000100020017x
- Li GD, Lodge GM, Moore GA, Craig AD, Dear BS, Boschma SP et al. 2008. Evaluation of perennial pasture legumes and herbs to identify species with high herbage production and persistence in mixed farming zones in southern Australia. Australian Journal of Experimental Agriculture 48: 449–466. doi: 10.1071/EA07108
- Marshall AH, Holdbrook-Smith K, Michaelson-Yeates TPT, Abberton MT, Rhodes I 1998. Growth and reproductive characteristics in backcross hybrids derived from Trifolium repens L. × T. nigrescens Viv. interspecific crosses. Euphytica 104: 61–66. doi: 10.1023/A:1018687200252
- Marshall AH, Rascle C, Abberton MT, Michaelson-Yeates TPT, Rhodes I 2001. Introgression as a route to improved drought tolerance in white clover (Trifolium repens L.). Journal of Agronomy and Crop Science 187: 11–18. doi: 10.1046/j.1439-037X.2001.00495.x
- Nichols SN, Crush JR 2014. Phosphate response of Trifolium uniflorum compared with T. repens and some T. repens × T. uniflorum hybrids. Crop & Pasture Science 66: 857–863.
- Nichols SN, Crush JR, Ouyang L 2014a. Phosphate responses of some Trifolium repens × Trifolium uniflorum interspecific hybrids grown in soil. Crop and Pasture Science 65: 382–387. doi: 10.1071/CP14029
- Nichols SN, Hofmann RW, Williams WM 2014b. Drought resistance of Trifolium repens × Trifolium uniflorum interspecific hybrids. Crop and Pasture Science 65: 911–921. doi: 10.1071/CP14067
- Nichols SN, Hofmann RW, Williams WM 2014d. The effect of interspecific hybridisation with Trifolium uniflorum on key white clover characteristics. Field Crops Research 161: 107–117. doi: 10.1016/j.fcr.2014.03.004
- Nichols SN, Hofmann RW, Williams WM, Crush JR 2014c. Nutrient responses and macronutrient composition of some Trifolium repens × Trifolium uniflorum interspecific hybrids. Crop and Pasture Science 65: 370–381. doi: 10.1071/CP13446
- Pandey KK 1957. A self-compatible hybrid from a cross between two self-incompatible species in Trifolium. Journal of Heredity 48: 278–281.
- Pandey KK, Petterson GB 1978. Fertile interspecific hybrids between Trifolium repens and T. uniflorum: prospects for grasslands white clover improvement. Australian Plant Breeding and Genetics Newsletter 28: 114–116.
- R Core Team 2012. R: a language and environment for statistical computing. Vienna, R Foundation for Statistical Computing.
- Thomas RG 2003. Comparative growth forms of dryland forage legumes. In: Moot, DJ ed. Legumes for dryland pastures. Proceedings of a New Zealand Grassland Association (Inc.) Symposium. Grassland Research and Practice Series No. 11. Wellington, New Zealand Grassland Association. Pp. 19–25.
- Westbrooks FE, Tesar MB 1955. Tap root survival of ladino clover. Agronomy Journal 47: 403–410. doi: 10.2134/agronj1955.00021962004700090004x
- Williams WM 1987. Genetics and breeding. In: Baker MJ, Williams WM eds. White clover. Wallingford, CAB International. Pp. 343–419.
- Williams WM, Nichols SN 2011. Trifolium. In: Kole C ed. Wild crop relatives: genomic and breeding resources. Legume crops and forages. Berlin Heidelberg, Springer-Verlag. Pp. 249–272.
- Woodfield DR, Caradus JR 1990. Estimates of heritability for, and relationships between, root and shoot characters of white clover II. Regression and progeny on mid-parent. Euphytica 46: 211–215. doi: 10.1007/BF00027220
- Woodfield DR, Caradus JR 1996. Factors affecting white clover persistence in New Zealand pastures. Proceedings of the New Zealand Grassland Association 58: 229–235.