ABSTRACT
The extent to which the wetted soil area of a urine patch influences surrounding pasture is relatively unknown. The study objective was to use 15N tracer to quantify pasture N uptake in the ‘wetted’ and periphery areas of a spring deposited bovine urine patch over 311 days. Ruminant 15N enriched urine was applied to soil creating a circular wetted area, ‘zone A’ (800 kg N ha−1), with and without urea fertiliser (35 kg N ha−1). Pasture yields, 15N recovery and soil inorganic-N dynamics were monitored from zone A and two peripheral zones, B and C. Fertiliser had no effect on cumulative urinary 15N recovery in pasture (50%–52%). Average cumulative pasture 15N recovery in zones A, B and C were 30.6%, 17.3% and 4.2%, respectively. Soil inorganic-15N recovery occurred in zones A and B, declining with distance from the wetted area. The results suggest an effective urine patch area of 0.95 m2 or 3.4 times the wetted area.
KEYWORDS:
Introduction
Nitrogen (N) excreta from grazing animals plays a key role in cycling N through pastoral systems (Haynes & Williams Citation1993; Selbie et al. Citation2015). Ruminant urine deposition accounts for nearly 70% of the N returned to pasture at rates of 600–1000 kg N ha−1 (Haynes & Williams Citation1993; Selbie et al. Citation2015). Dairy cow urination events approximate 2 L in volume (Doak Citation1952), occur 10–12 times a day (Jarvis et al. Citation1995) and cover an average surface area of 0.26 m2, with a range of 0.16–0.49 m2 (Petersen et al. Citation1956; Davies et al. Citation1962; Hogg Citation1968; Richards & Wolton Citation1976; Haynes & Williams Citation1993). These values vary significantly and are influenced by grazing conditions and environmental factors (Haynes & Williams Citation1993). The volume of soil wetted by a urine patch varies with surface area affected, antecedent soil moisture, soil surface water repellency, surface compaction, microtopography, vegetation cover, slope and wind (Williams & Haynes Citation1994). Urine patches are estimated to cover 20%–30% of the grazed paddock area per year, with this percentage varying with the stocking rate (Silva et al. Citation1999; Moir et al. Citation2011).
Some of the urinary N returned to soil may be volatilised as NH3, but much is eventually nitrified, resulting in the accumulation of nitrate () in the soil. Pasture N uptake from a urine patch has been estimated at 300–700 kg N ha−1 yr−1 (During & McNaught Citation1961; Ball et al. Citation1979; Ledgard Citation2001). Thus, the N loading under a urine patch far exceeds immediate plant demand. Surplus
in the soil is vulnerable to leaching and this is recognised as a significant N loss pathway from dairy farming systems (Silva et al. Citation1999). Loss of such reactive N has environmental implications, since
can potentially contribute to the eutrophication of surface waters, indirect losses of nitrous oxide, the formation of hypoxic zones and contamination of potable waters (Galloway et al. Citation2004). As a result, significant research has focused on understanding N loss dynamics under urine patches within dairy farming systems.
A urine patch is comprised of a ‘wetted area’ (where urine is directly deposited) and an ‘effective area’ which includes both the wetted area and the peripheral area outside it that is affected by the urinary N, primarily via plant root extension and soil N diffusion (Lantinga et al. Citation1987; Tinker & Nye Citation2000). Cattle urination events can create wetted areas of 0.014–0.49 m2 (Selbie et al. Citation2015). The effective area for cattle is reported to range from 0.03–1.1 m2 (Lotero et al. Citation1966; Moir et al. Citation2011). However, data are limited. Decau et al. (Citation2003) measured urinary N diffusion up to 20 cm from the wetted area of 0.4 m2 urine patches. Lotero et al. (Citation1966) reported effective areas of 0.9–1.2 m2 and observed a linear decrease in dry matter (DM) yields from the centre of a urine patch to the periphery of the effective area. Moir et al. (Citation2011) also observed seasonal variation in the effective area whereby urine-affected areas tended to be larger from spring/summer deposited urine, and smaller in winter and autumn. These differences are mainly attributed to rapid winter leaching when soils are draining (less N available for plant uptake), but other factors may include seasonal differences in both animal N and water intake.
Many N leaching studies have used lysimeters to quantify losses from urine patches (Clough et al. Citation1996, Citation1998; Silva et al. Citation1999; Di & Cameron Citation2002, Citation2004, Citation2007; Decau et al. Citation2003, Citation2004; McDowell & Houlbrooke Citation2009; Ledgard et al. Citation2014; Shepherd et al. Citation2014; Buckthought et al. Citation2015). In many cases, urine treatments cover the whole area of the lysimeter. However, a lysimeter is a confined area of soil and its casing represents a physical barrier to the surrounding soil. Therefore, urine patch leaching data obtained under such conditions are reflective of the wetted area of a urine patch and do not account for the full effective area. This may have implications for extrapolating lysimeter study results to field situations and the use of such data to understand N cycling in farm system models. Clearly, further research is needed to compare the wetted and effective areas in terms of their relative responses to urinary-N deposition. Thus, the objective of this study was to use a 15N tracer methodology to quantify the pasture response, N uptake and 15N recovery in the ‘wetted’ and ‘effective’ urine patch areas after a simulated spring urine deposition event. This was assessed in combination with, and without, an application of urea fertiliser which often follows deposition on New Zealand dairy farms.
Materials and methods
Experimental site and preparation
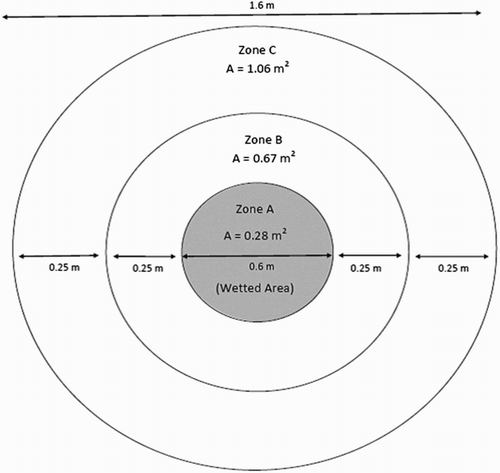
This field study took place at Scott Farm, owned and operated by DairyNZ, and located in the Waikato Region of New Zealand (−37.70°S; 175.36°E). The soil type was a Horotiu Silt Loam (Typic Orthic Allophanic) (Hewitt Citation1998) with a pH of 5.6 and a bulk density of 0.87 Mg m−3. Four months before the start of the study, the plot area was fenced off and any visible dung patches removed. The site was regularly cut to a pasture residual of 1600 kg DM ha−1 (c. 3 cm height). In early October 2011, due to insufficient late winter rain, the trial area was irrigated with 350 mm of water to remove any residual antecedent urine patch effects. The study site consisted of 16 circular plots (area 2.0 m2), each comprising three concentric rings (). The central ring, termed ‘zone A', was 0.28 m2 (0.6 m diameter); the middle ring, ‘zone B', was 0.67 m2 (0.25 m from the edge of zone A) and the outer ring, ‘zone C', was 1.06 m2 (0.25 m from the edge of zone B). Zone A was designated as the wetted area, and zones A, B and C, collectively, comprised the potential effective area ().
Experimental design and treatments
There were four treatments: (1) a single bovine urine application (800 kg N ha−1); (2) a single bovine urine application (800 kg N ha−1) with a single concurrently applied urea fertiliser application (35 kg N ha−1); (3) a fertiliser-only application (35 kg N ha−1); and (4) a control that received no urine or fertiliser. Treatments were applied only to zone A. Four replicates of each treatment were established in a Latin square design. All treatments were applied in spring on 17 October 2011. Herbage and soil samples were taken on 17 October prior to treatment application.
Fresh dairy cow urine was collected on 16 October 2011 during the morning milking at the AgResearch dairy farm at Tokanui. The urine was standardised to a concentration of 11.2 g N L−1 and a 15N enrichment of 5 atom% using a combination of urea at natural abundance and 99 atom% 15N enriched urea. Two litres of urine were applied to appropriate plots with a watering can with particular care taken to avoid urine spreading outside zone A. Plots receiving fertiliser then had urea fertiliser prills applied. All treatments were washed in with 10 mm of irrigation to minimise NH3 volatilisation (Black et al. Citation1987). An equivalent volume of water was applied to the control treatments.
Sample collection and analysis
Sampling began the day after treatment application (18 October 2011). To ensure the plots were sampled representatively, each concentric zone was divided into six equal segments that were designated as either soil or pasture sampling areas. This was to prevent anomalies in yield measurements occurring as a potential result of soil coring damaging plants, and to reduce spatial variability within each plot. Samples from each segment within a plot-zone were then bulked together to create a single sample for each zone for analysis. Pasture was cut to a residual of 1600 kg DM ha−1 (c. 3 cm height) with electric clippers. A total of 10 pasture harvests were performed on days 14, 32, 58, 85, 106, 133, 165, 203, 239 and 311 post treatment applications.
Harvested pasture was oven dried at 55 °C for 48–72 h. Dried samples were then weighed and finely ground using a Cyclone Sample Mill with a 1 mm screen (UDY Corporation). Analyses for total N and 15N enrichment were performed on an isotope ratio mass spectrometer (EA-CF/IRMS; PDZ Europa GSL/20–20).
Soil sample collection was carried out weekly for the first 4 weeks. After this, sampling was reduced to fortnightly, monthly and then bimonthly intervals before ceasing at the end of January 2012. Soil cores (2.5 cm diameter) were collected from two depths (0–7.5 cm and 7.5–20 cm) from all zones. The number of cores taken from each segment was limited due to the small area available (particularly in zone A). Totals of two, four and six cores were taken from zones A, B and C, respectively. Soil samples were stored in plastic bags at 4 °C. Preparation of the soils for analysis began within 24 h of collection. All soil samples were passed through a 4 mm sieve and any herbage material was removed. Soil gravimetric moisture content (θg) was calculated as the difference between the field moist and oven dried (105 °C for 24 h) subsamples.
Soil inorganic N was determined using a potassium sulphate (K2SO4) extraction procedure (1:5 soil to extractant ratio) (Mulvaney Citation1996), with analyses for ammonium (NH4-N) and nitrate-N + nitrite-N (NO3-N + NO2-N) performed on a Skalar SAN++ segmented flow analyser (Skalar Analytical). Soil inorganic N extracts were prepared for 15N enrichment analysis using the diffusion method as described by Brooks et al. (Citation1989). Soils were later dried and sieved and analysed for total N and 15N enrichment. Soil organic 15N recovery was calculated as the difference between total soil 15N recovery and inorganic 15N recovery. All soil 15N analysis was carried out using isotope ratio mass spectrometry (EA-CF/IRMS; PDZ Europa GSL/20–20).
Daily weather data were obtained over the duration of the experiment from the Ruakura climate station (via the NIWA Virtual Climate Station dataset [Tait & Turner Citation2005]), located 7 km from the field site.
Statistical analyses
Statistical analyses were performed using the ANOVA directive of GenStat (15th edition). The least significant difference (LSD) was used to determine statistical variation between the sample means at the 95% significance level.
When comparing treatment effects on pasture yield, N content and N uptake between whole plots, a weighted mean was calculated to account for the differences in surface area between zones A, B and C. (Equation 1 using pasture yield as an example).(1) Where Yielda, Yieldb and Yieldc are the measured yields from zones A, B and C; Areaa, Areab and Areac are the surface areas (m2) of zones A, B and C; and Areaa+b+c is the area of the total plot.
Results
Climate data
Rainfall was evenly distributed and totalled 1088 mm for the experimental period (Figure S1A). Average air temperature over the experimental period was 13.9 °C and the average soil temperature (10 cm depth) was 14.8 °C (Figure S1B). There was a total of 15 frost days which occurred between 23 May and 19 Jul 2012. There were no significant differences in gravimetric moisture (θg) between zones or treatments.
Pasture dry matter yields and N uptake
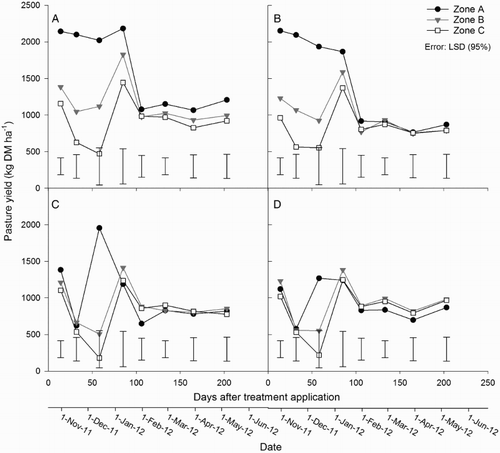
Mean DM yields for individual harvests ranged from 530–2200 kg DM ha−1 in zone A, 500–1800 kg DM ha−1 in zone B and 180–1500 kg DM ha−1 in zone C (). Yields of DM were greater in the urine treatments than the non-urine treatments at each harvest until day 85, however, these differences were only statistically significant (P < 0.001) on the second and third harvests (days 32 and 58). In the urine treatments, DM yields from zone A were greater than zone B (P < 0.001) up to day 58; and greater than zone C (P < 0.05) up to day 85. Pasture DM yields from zone B were greater than zone C (P < 0.05) on only two harvests (). In the non-urine treatments, DM yields from zone A were greater than zones B and C on day 58 only. There was no fertiliser effect on DM yields in any treatment.
Figure 2. Mean pasture yields in zones A, B and C for treatments A, urine + fertiliser; B, urine only; C, fertiliser only; D, control. Error bars = LSD (95%), n = 4.
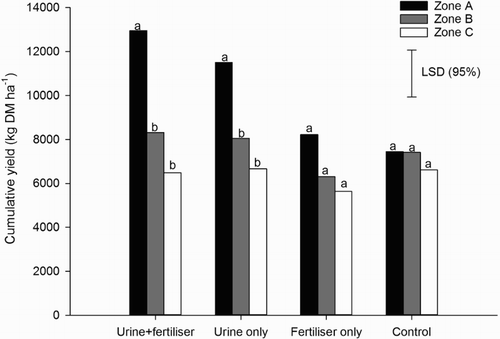
Cumulative DM yields ranged from 7500–13,000 kg DM ha−1, 6300–8300 kg DM ha−1 and 5600–6700 kg DM ha−1 in zones A, B and C, respectively. In the urine treatments, cumulative DM yields from zone A were greater (P < 0.001) than those from zone B or C, with no effect of fertiliser addition (). In the non-urine treatments, there were no significant differences in cumulative DM yields between zones A, B or C ().
Figure 3. Cumulative pasture yields in zones A, B and C for each treatment. Different letters represent a statistically significant difference between yields within treatments. Error bar = LSD (95%), n = 4.
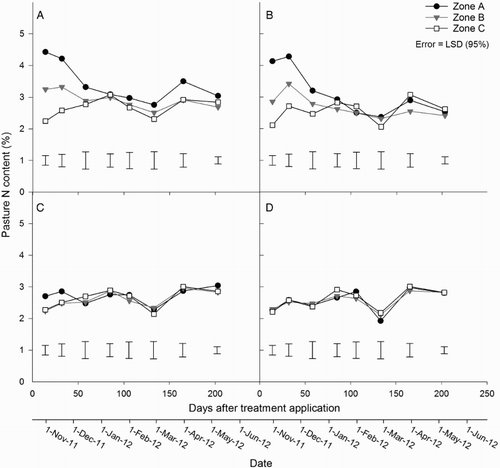
Pasture N concentrations ranged from 1.9%–4.4% in zone A, 2.1%–3.4% in zone B and 2.1%–3.1% in zone C (). After urine application, pasture N concentrations in zone A were greater (P < 0.05) than in zones B and C on days 14 and 32 (). Pasture N concentrations in zones A and B of the urine treatments were greater (P < 0.05) than in any of the zones within non-urine treatments from day 14 to 58 (zone A) and day 14 to 32 (zone B). Pasture N concentrations in zone C did not differ between treatments ().
Figure 4. Mean pasture N content in zones A, B and C for the A, urine + fertiliser; B, urine only; C, fertiliser only; and D, control treatments. Error bars = LSD (95%), n = 4.
After urine application, pasture N uptake generally decreased with time in zones A and B, but remained relatively constant in zone C (). In the urine treatments, pasture N uptake in zone A was greater (P < 0.001) than in zones B and C on days 14, 32 and 58 (). Pasture N uptake from zone B was greater (P < 0.001) than from zone C on days 14, 32 and 58 in the urine + fertiliser treatment, and on days 14 and 32 in the urine-only treatment (). In non-urine treatments, N uptake remained relatively constant over time with no differences in N uptake between zones, apart from day 58, when an increase in pasture N uptake was observed in zone A only of both non-urine treatments. Fertiliser had no effect on N uptake at the zone scale, neither was there any interaction between fertiliser and urine treatments ().
Figure 5. Mean pasture N uptake in zones A, B and C for the A, urine + fertiliser; B, urine only; C, fertiliser only; and D, control treatments. Error bars = LSD (95%), n = 4.
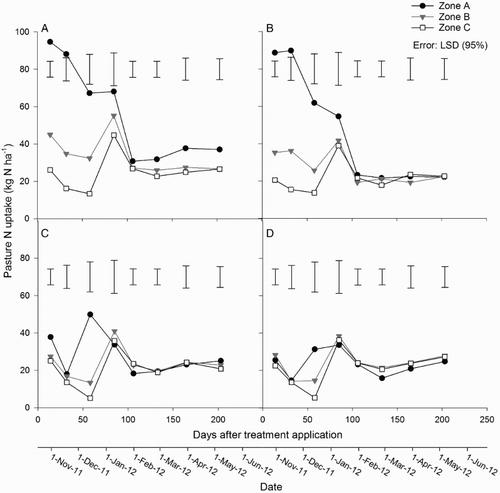
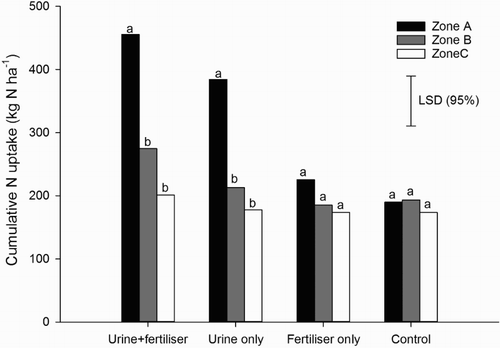
Cumulative N uptake in the urine-affected treatments was greater in zone A than in zones B and C (P < 0.001); however, cumulative N uptake from zone B did not differ from zone C (). There was no difference in cumulative N uptake between zones in the non-urine treatments ().
15N recovery in urine-treated pasture
During individual harvests, the recovery of 15N in zone A of the urine-treated pasture was greatest following harvests on days 14 and 32, and then declined relatively quickly until day 106 (A–B). By day 106, pasture 15N recovery at individual harvests had decreased to be less than 1.5% of 15N applied, and by day 311 was only 0.35% of the 15N applied (A–B). In both urine treatments, pasture 15N recovery from zone A was greater (P < 0.001) than from zones B and C at every harvest. Pasture 15N recovery from zone B was greater (P < 0.001) than zone C at every harvest (A–B). Pasture 15N recovery in zone C occurred until day 106. Within zones, there was no interaction between the urine and fertiliser treatments on pasture 15N recovery at any sampling occasion.
Figure 7. Pasture 15N recovery in zones A, B and C for the A, urine + fertiliser; B, urine only; C, fertiliser only; and D, control treatments. Error bars = LSD (95%), n = 4.
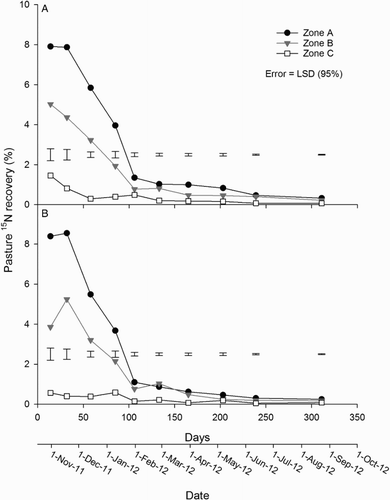
Cumulative pasture 15N recovery increased rapidly from day 14 to day 106 (). Total cumulative pasture 15N recoveries from all zones did not differ between the urine plus fertiliser (52.1%) and urine-only (49.7%) treatments (). Similarly, urine treatment, with or without fertiliser, had no effect on cumulative 15N recoveries when comparing individual zones A, B and C (). However, within urine treatments, the cumulative 15N recovery from zone A was greater (P < 0.05) than from zones B and C, and the cumulative recovery from zone B was greater (P < 0.05) than from zone C at every harvest ().
Table 1. Cumulative recovery of 15N in pasture and soil at day 106. There was no effect of fertiliser on 15N recovery.
Soil N concentrations and soil 15N recovery
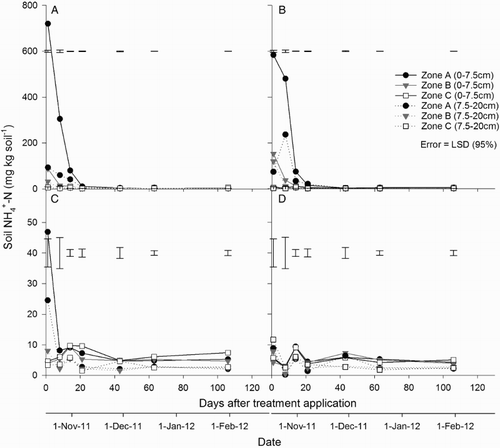
At both soil depths (0–7.5 and 7.5–20 cm), soil concentrations were greater (P < 0.001) in the urine-affected treatments than the non-urine treatments from day 1 to 21. At both depths, soil
concentrations under the urine treatments were greater (P < 0.001) in zone A than in zones B or C, from day 1 to 21; and were greater in zone B compared with zone C from day 1 to 14 (A–B). After this time, soil
concentrations equalled those in the control, with no difference between zones. In the fertiliser treatment, soil
concentrations were greater (P < 0.05) in zone A, compared with zones B and C, only on day 1 (A–C). There was no difference in soil
concentrations between zones in the control at either depth (D).
Figure 8. Soil concentration at two depths (0–7.5 and 7.5–20 cm) in zones A, B and C for the A, urine + fertiliser; B, urine only; C, fertiliser only; and D, control treatments. Error bars = LSD (95%), n = 4. Note different scales on the y axes.
In the 0–7.5 cm depth, soil concentrations peaked in zone A on day 21 in the two urine treatments, and on day 1 in the fertiliser-only treatment (A–C). In the 7.5–20 cm depth, peaks in soil
concentration were observed only in the urine treatments (also day 21) (A–B). Under the urine treatments, at 0–7.5 cm depth, soil
concentrations were greater (P < 0.001) in zone A compared with zones B and C until day 63. They were greater (P < 0.05) in zone B compared with zone C until day 43 (A–B).
Figure 9. Soil concentrations at two depths (0–7.5 and 7.5–20 cm) in zones A, B and C for the A, urine + fertiliser; B, urine only; C, fertiliser only; and D, control treatments. Error bars = LSD (95%), n = 4. Note different scales on the y axes.
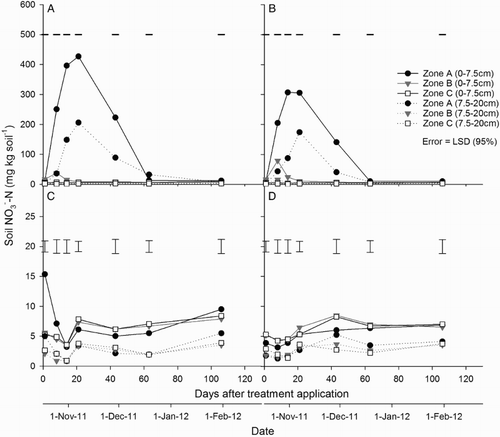
In the non-urine treatments, there was no difference in soil concentrations between zones, with the exception of day 1 in the fertiliser-only treatment, where there was a greater concentration in zone A at the 0–7.5 cm depth (C–D).
Inorganic-15N was recovered up to day 43 () after which soil inorganic-N concentrations were too low to allow for analysis. shows the temporal pattern of 15N recovery, as inorganic-N , by treatment, zone and soil depth at each soil sampling event during the first 43 days.
Figure 10. Soil inorganic 15N recovery for the A, urine + fertiliser at 0–7.5 cm depth; B, urine only at 0–7.5 cm depth; C, urine + fertiliser at 7.5–20 cm depth; and D, urine-only treatments at 7.5–20 cm depth. Error bars = LSD (95%), n = 4.
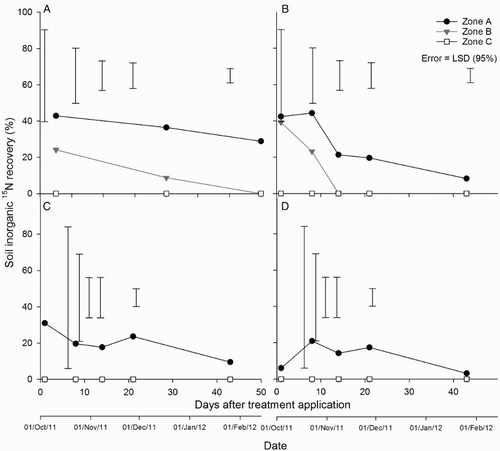
At 0–7.5 cm depth, soil inorganic 15N recovery in zone A of the urine plus fertiliser treatment declined from 43% on day 1 to 13% on day 43. In zone A of the urine-only treatment, inorganic 15N recovery declined from 44% to 8% over the same time. Inorganic 15N recovery only occurred on days 1 and 8 in zone B. In this time, inorganic 15N recovery declined from 25% to 0% and 38% to 0% in the urine plus fertiliser and urine-only treatments, respectively (A–C). No inorganic 15N recovery occurred in zone C at 0–7.5 cm depth.
At 7.5–20 cm depth, soil inorganic 15N recovery in zone A of the urine plus fertiliser treatment declined from 32% on day 1 to 10% on day 43. In zone A of the urine-only treatment, inorganic 15N recovery declined from 21% to 4% over the same time (B–D). No inorganic 15N recovery occurred in zones B and C at 7.5–20 cm depth.
By day 106, at 0–7.5 cm depth, the recovery of total soil 15N (as organic-15N) in zone A was 12.0% and 13.5% for the urine plus fertiliser and urine-only treatments (). These recoveries were greater (P < 0.01) than those measured for both zone B and zone C (). At the 7.5–20 cm depth, the recoveries ranged from 0.7% to 3.2% in the urine + fertiliser treatment and 1.6% to 5.4% in the urine-only treatment (). There were no treatment or zone effects at this depth.
Discussion
The observed increase in pasture 15N recovery under the urine treatments was a function of increased pasture N uptake which in turn was a product of increases in pasture DM yield and N content.
Under the urine treatments, the total pasture 15N recoveries (49%–52%) from all zones were similar to those reported previously (Decau et al. Citation2003; Leterme et al. Citation2003), where urine-15N recoveries ranged from 30%–65% when urine was applied in spring or summer. Recovery of spring-applied urinary N is generally higher because conditions are usually more favourable for pasture growth for a longer period that precedes the following winter (Ball & Field Citation1982). Pasture 15N recoveries tend to be lower after autumn or winter urine application due to greater leaching and denitrification losses of N (Fraser et al. Citation1994; Williams & Haynes Citation1994; Clough et al. Citation1996, Citation1998; Di et al. Citation2002; Silva et al. Citation2005). Although summer rainfall in the current experiment was higher than average in December 2011 and January 2012, this occurred c. 1 month after urine application and did not appear to cause a significant reduction in 15N recovery by the pasture.
Greater pasture 15N recovery occurred in zone A because the urine application, and thus the total N loading, was confined to zone A. The fact that 22% of the urinary 15N (18% in zone B and 4.2% in zone C) was recovered by pasture outside the wetted area may have been due to NH3 deposition on to soil or plants outside the wetted zone A, plant uptake of volatilised NH3, lateral movement of soil inorganic-N, or N uptake by plants in zones B and C that had rooting structures within zone A.
Ammonia volatilised from the soil following 15N enriched urine application can be directly absorbed through the stomata of plants (Farquhar et al. Citation1980; Sommer & Jensen Citation1991; Schjoerring & Mattsson Citation2001; van Hove et al. Citation2002). A number of studies have assessed NH3 absorption and emission from crop plant canopies such as maize (Denmead et al. Citation1982; Harper et al. Citation2000), sugarcane (Denmead et al. Citation2008), rapeseed, barley, wheat and peas (Schjoerring & Mattsson Citation2001). However, little is known about the extent of volatilised NH3 that is redeposited and absorbed by pasture following urine deposition, or the distances that NH3 travels from the urine patch (Ross & Jarvis Citation2001). Sommer & Jensen (Citation1991) measured foliar absorption of NH3 by ryegrass at various distances from a dairy farm yard and measured deposition of 3.0 and 0.7 g N m−2, at 10 m and 130 m from the source, respectively. Ross & Jarvis (Citation2001) measured NH3 emission and deposition, using wind tunnels, from ryegrass swards affected by urine (227 kg N ha−1) and found that 20%–60% of NH3 emitted was deposited within a distance of 2 m. Thus NH3 deposition rates and absorption by nearby pasture can be significant. The exchange of NH3 between the plant canopy and the atmosphere may be bidirectional and pastures have been shown to be a source of NH3 emissions (Harper et al. Citation1996; Plantaz Citation1998). However, under intensive farm management conditions, van Hove et al. (Citation2002) found gaseous NH3 concentrations inside ryegrass leaves were always lower than atmospheric concentrations, and concluded that the ryegrass pasture canopy was unlikely to be a significant NH3 source. Negating the potential for plants to be a source of NH3 in the current experiment is the fact that the pasture canopy's leaf area was relatively small due to the pasture having been harvested prior to urine application. Thus, emission of NH3 from plants within zone A, with subsequent plant uptake in zones B or C, is unlikely to have made a significant contribution to plant 15N recovery in zones B and C. Direct emission of NH3 from the soil and its subsequent deposition in zones B and C should also have been limited due to the fact that 10 mm of water was applied to suppress NH3 volatilisation (Black et al. Citation1987; Fraser et al. Citation1994; Clough et al. Citation1996; Saarijarvi et al. Citation2006) thus volatilisation of NH3 from soil in zone A, with subsequent plant absorption in zones B and C, would also be expected to be minimal. Plant 15N uptake resulting from potential diffusion of NH3 through the soil pore network should also have been minimal due to water occupying soil pores. It is clear, however, that more research is required to determine the role that NH3 absorption and emission from pastoral systems play in determining the fate of N in a urine patch.
Lateral subsurface movement of urine-derived inorganic-15N is a potential alternative explanation for pasture 15N recovery outside of zone A. Cumulative pasture 15N uptake was essentially complete by the time that soil inorganic-N forms were at concentrations too low to be analysed (106 days) and it is also within this time period that major differences between zones were observed with respect to pasture 15N recovery at individual harvests. Inorganic soil 15N recovery was, as expected, greatest on day 1. The fact that urinary 15N was recovered in zone B on day 1 in both the urine + fertiliser and urine-only treatments indicates that urinary N moved from zone A into zone B within 24 h of application, via lateral macropore flow (Williams et al. Citation1990). Diffusion of along a concentration gradient (Wild Citation1972) cannot explain the rapid recovery of plant 15N in zone C and is unlikely to have contributed significantly to the pasture 15N recovery in zone B because the diffusion coefficient of nitrate through soil is relatively slow at 0.05–1.2 cm2 d−1 (Hagin & Tucker Citation1982). Instead, the lack of any detectable soil inorganic 15N recovery in zone C infers that pasture 15N uptake in zone C was potentially due to pasture roots translocating 15N from zones A and/or B. The main root axis of perennial ryegrass plants can exceed 40 cm (Robin et al. Citation2010) and thus plants in zone C could have readily accessed inorganic 15N in either zones A or B.
As 15N was recovered only in the pasture and soil fractions, a 15N mass balance was not the focus of this study; however, the potential fate of the unaccounted for 15N could vary widely. The field trial was set up in late spring to minimise N leaching risk; however, very high rainfall in December 2011 and January 2012 resulted in drainage, so it is very likely that urinary 15N was leached as -15N. This rainfall may have also temporarily increased denitrification and the subsequent loss of 15N as nitrous oxide (N2O-15N). Volatilisation of 15N as NH3 was unlikely to be significant because all treatments were washed in with 10 mm water (Black et al. Citation1987) but this pathway cannot be ruled out.
The increase in pasture N uptake observed in zone A only of the non-urine treatments suggests the water applied to all treatments to prevent NH3 emissions played a role, as this was the only difference in treatment between the zones. We suggest the antecedent soil moisture from the water applied to zone A on day 0, coupled with 12 mm rain on day 47 was enough to result in a flush of mineralisation and rapid plant uptake in zone A. The drier antecedent soil conditions in zones B and C resulted in a slower response to the rainfall that was observed the following harvest (). Under the conditions of this study, the effective area of the urine patch, based on total urinary-15N recovery, may have been up to 2.01 m2 (0.28 + 0.67 + 1.06 m2) or seven times the wetted area. However, as the results demonstrate, recovery of urinary-15N was not constant with distance from zone A. If we assume a total pasture 15N recovery of 51% with 30%, 17% and 4% from zones A, B and C, respectively, () and express these pasture recoveries as rates of recovery on a per unit area basis (0.28, 0.67 and 1.06 m2 for zones A, B and C, respectively) then pasture 15N recovery rates equate to 107, 25 and 3.8 % m−2. Thus while pasture in zone C is responsible for recovering some of the 15N it is far less efficient than pasture in zones A and B. Thus, the effective area is perhaps best described by the sum of zones A and B (0.95 m2), which in total is still 3.4 times the size of zone A. Such an effective area falls within the upper end of the range previously reported (Lotero et al. Citation1966; Moir et al. Citation2011).
Urinary N uptake from outside the ‘wetted area’, as demonstrated in this study, may have implications for the interpretation of data from lysimeter studies. Ruminant urine is generally applied to the entire surface of a lysimeter (Silva et al. Citation1999, Citation2005; Di & Cameron Citation2002; Di et al. Citation2002; Menneer et al. Citation2008; McDowell & Houlbrooke Citation2009; Ledgard et al. Citation2014; Shepherd et al. Citation2014; Buckthought et al. Citation2015) but the lysimeter casing creates a physical barrier between the plant root systems inside and outside the lysimeter, which is not representative of field conditions. Consequently, the ‘effective area’ in such lysimeter studies corresponds to the wetted area. The lack of a peripheral ‘zone B’ in these lysimeter studies may result in enhanced leaching or gaseous losses if it is assumed that pasture N uptake in the wetted area is constant regardless of whether a peripheral zone B is present or not. Conversely, the pasture roots confined within the lysimeter casing may be exposed to higher amounts of available N than in a field situation where subsurface lateral flow of urine may occur. This might result in higher dry matter yields and higher N uptake in lysimeters compared with the field conditions, and reduced losses.
Implications of these results on lysimeter studies will be influenced by many factors, not least the season of urine deposition and climatic conditions. Therefore, further research is clearly needed to determine and compare seasonal urinary-N losses and plant uptake dynamics from lysimeters and in the field, with and without a peripheral zone present.
Conclusion
The greatest recovery of urinary 15N occurred in the pasture fraction (51%). Most of this was from within the urine-wetted area (zone A); however, 33% of the total 15N recovered in pasture was from outside the wetted area. The effective area of pasture partaking in urinary-15N uptake was therefore at least 3.4 times the wetted area. Soil inorganic-N dynamics showed urinary-15N present outside the initially wetted area within the first 15 days of the experiment. However, the mechanism for 15N recovery by pasture outside the wetted area was likely to be due to plant root uptake from outside the wetted area, translocating 15N to the unwetted pasture zone. These results have implications for understanding the fate of N under a urine patch and the extent of urinary-N uptake by pasture, both spatially and temporally, and the methodologies used in obtaining such information. Further research is required to determine the fate of urinary-N from lysimeters with and without a peripheral zone present.
Supplementary data
Figure S1. A, Rainfall over the experimental period; B, air and soil temperatures (10 cm depth) over the experimental period.
Acknowledgements
The statistical analyses were carried out by Dr John Waller, formerly of AgResearch Ltd. All 15N analyses were carried out by Roger Cresswell at Lincoln University.
Disclosure statement
No potential conflict of interest was reported by the authors.
Figure S1. A, Rainfall over the experimental period; B, air and soil temperatures (10 cm depth) over the experimental period.
Download PDF (473.2 KB)Additional information
Funding
References
- Ball PR, Field TRO. 1982. Responses to nitrogen as affected by pasture characteristics, season and grazing management. In: Lynch PB, editor. Auckland: Ray Richards Publisher.
- Ball PR, Keeney DR, Theobald PW, Nes P. 1979. Nitrogen balance in urine-affected areas of a New Zealand pasture. Agron J. 7:133–175.
- Black AS, Sherlock RR, Smith NP. 1987. Effect of timing of simulated rainfall on ammonia volatilization from urea, applied to soil of varying moisture content. J Soil Sci. 38:679–687. doi: 10.1111/j.1365-2389.1987.tb02165.x
- Brooks PD, Stark JM, McInteer BB, Preston T. 1989. Diffusion method to prepare soil extracts for automated nitrogen-15 analysis. Soil Sci Soc Am J. 53:1707–1711. doi: 10.2136/sssaj1989.03615995005300060016x
- Buckthought LE, Clough TJ, Cameron KC, Di HJ, Shepherd MA. 2015. Fertiliser and seasonal urine effects on N2O emissions from the urine-fertiliser interface of a grazed pasture. New Zeal J Agr Res. 58:311–324.
- Clough TJ, Ledgard SF, Sprosen MS, Kear MJ. 1998. Fate of N-15 labelled urine on four soil types. Plant Soil. 199:195–203. doi: 10.1023/A:1004361009708
- Clough TJ, Sherlock RR, Cameron KC, Ledgard SF. 1996. Fate of urine nitrogen on mineral and peat soils in New Zealand. Plant Soil. 178:141–152. doi: 10.1007/BF00011172
- Davies EB, Hogg DE, Hopewell HG. 1962. Extent of return of nutrient elements by dairy cattle: possible leaching losses. Transactions of International Society of Soil Science Palmerston North, NZ. p. 715–720.
- Decau ML, Simon JC, Jacquet A. 2003. Fate of urine nitrogen in three soils throughout a grazing season. J Environ Qual. 32:1405–1413. doi: 10.2134/jeq2003.1405
- Decau ML, Simon JC, Jacquet A. 2004. Nitrate leaching under grassland as affected by mineral nitrogen fertilization and cattle urine. J Environ Qual. 33:637–644. doi: 10.2134/jeq2004.6370
- Denmead OT, Freney JR, Dunin FX. 2008. Gas exchange between plant canopies and the atmosphere: case-studies for ammonia. Atmos Environ. 42:3394–3406. doi: 10.1016/j.atmosenv.2007.01.038
- Denmead OT, Freney JR, Simpson JR. 1982. Dynamics of ammonia volatilization during furrow irrigation of maize. Soil Sci Soc Am J. 46:149–155. doi: 10.2136/sssaj1982.03615995004600010028x
- Di HJ, Cameron KC. 2002. Nitrate leaching and pasture production from different nitrogen sources on a shallow stony soil under flood-irrigated dairy pasture. Aust J Soil Res. 40:317–334. doi: 10.1071/SR01015
- Di HJ, Cameron KC. 2004. Treating grazed pasture soil with a nitrification inhibitor, eco-n™, to decrease nitrate leaching in a deep sandy soil under spray irrigation—a lysimeter study New Zealand. J Agr Res. 47:351–361. doi: 10.1080/00288233.2004.9513604
- Di HJ, Cameron KC. 2007. Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor—a lysimeter study. Nutr Cycl Agroecosys. 79:281–290. doi: 10.1007/s10705-007-9115-5
- Di HJ, Cameron KC, Silva RG, Russell JM, Barnett JW. 2002. A lysimeter study of the fate of 15N-labelled nitrogen in cow urine with or without farm dairy effluent in a grazed dairy pasture soil under flood irrigation. New Zeal J Agr Res. 45:235–244. doi: 10.1080/00288233.2002.9513514
- Doak BW. 1952. Some chemical changes in the nitrogenous constituents of urine when voided on pasture. J Agric Sci. 42:162–171. doi: 10.1017/S0021859600058767
- During C, McNaught KJ. 1961. Effects of cow urine on growth of pasture and uptake of nutrients. New Zeal J Agr Res. 4:591–605. doi: 10.1080/00288233.1961.10431617
- Farquhar GD, Firth PM, Wetselaar R, Weir B. 1980. On the gaseous exchange of ammonia between leaves and the environment—determination of the ammonia compensation point. Plant Pathol. 66:710–714.
- Fraser PM, Cameron KC, Sherlock RR. 1994. Lysimeter study of the fate of nitrogen in animal urine returned to irrigated pasture. Eur J Soil Sci. 45:439–447. doi: 10.1111/j.1365-2389.1994.tb00529.x
- Galloway JN, Dentener FJ, Capone DG, Boyer EW, Howarth RW, Seitzinger SP, Asner GP, Cleveland CC, Green PA, Holland EA, et al. 2004. Nitrogen cycles: past, present and future. Biogeochemistry. 70:153–226. doi: 10.1007/s10533-004-0370-0
- Hagin J, Tucker B. 1982. Fertilization of dryland and irrigated soils. Advanced series in agricultural sciences, vol. 12. Berlin: Springer Science & Business Media.
- Harper LA, Bussink WB, van der Meer HG, Corré WJ. 1996. Ammonia transport in a temperate grassland: I. Seasonal transport in relation to soil fertility and crop management. Agron J. 88:614–621. doi: 10.2134/agronj1996.00021962008800040020x
- Harper LA, Denmead OT, Sharpe RR. 2000. Identifying sources and sinks of scalars in a corn canopy with inverse Langrangian dispersion analysis II. Ammonia. Agr Forest Meteorol. 104:75–83. doi: 10.1016/S0168-1923(00)00149-0
- Haynes RJ, Williams PH. 1993. Nutrient cycling and soil fertility in the grazed pasture ecosystem. Adv Agron. 49:119–199. doi: 10.1016/S0065-2113(08)60794-4
- Hewitt AE. 1998. New Zealand soil classification. 2nd ed. Lincoln: Manaaki Whenua, Landcare Research New Zealand Ltd.
- Hogg DE. 1968. Lysimeter studies of potassium losses from urine applied to pasture. International Congress of Soil Science 9th. p. 631–638.
- Jarvis SC, Scholefield D, Pain B. 1995. Nitrogen cycling in grazing systems. In: Bacon PE, editor. Nitrogen fertilisation in the environment. New York: Marcel Dekker; p. 381–420.
- Lantinga EA, Keuning JA, Groenwold J, Deenen PJAG. 1987. Distribution of excreted nitrogen by grazing cattle and its effects on sward quality, herbage production and utilization. In: van der Meer, HG, editor. Animal manure on grassland and fodder crops. Fertilizer or Waste? Dordrecht: Nartinus Nijhoff Publishers; p. 103–117.
- Ledgard SF. 2001. Nitrogen cycling in low input legume based agriculture, with emphasis on legume/grass pastures. Plant Soil. 228:43–59. doi: 10.1023/A:1004810620983
- Ledgard SF, Luo J, Sprosen MS, Wyatt JB, Balvert SF, Lindsey SB. 2014. Effects of the nitrification inhibitor dicyandiamide (DCD) on pasture production, nitrous oxide emissions and nitrate leaching in Waikato, New Zealand. New Zeal J Agr Res. 57:294–315. doi: 10.1080/00288233.2014.928642
- Leterme P, Barre C, Vertes F. 2003. The fate of 15N from dairy cow urine under pasture recieving different rates of N fertiliser. Agronomie. 23:609–616. doi: 10.1051/agro:2003038
- Lotero J, Woodhouse WW, Petersen RG. 1966. Local effect on fertility of urine voided by grazing cattle. Agron J. 58:262–265. doi: 10.2134/agronj1966.00021962005800030005x
- McDowell RW, Houlbrooke DJ. 2009. The effect of DCD on nitrate leaching losses from a winter forage crop receiving applications of sheep or cattle urine. Proceedings of the New Zealand Grasslands Association. 71:117–120.
- Menneer JC, Ledgard S, Sprosen M. 2008. Soil N process inhibitors alter nitrogen leaching dynamics in a pumice soil. Aust J Rural Health. 46:323–331.
- Moir JL, Cameron KC, Di HJ, Fertsak U. 2011. The spatial coverage of dairy cattle urine patches in an intensively grazed pasture system. J Agr Sci. 149:473–485. doi: 10.1017/S0021859610001012
- Mulvaney RL. 1996. Nitrogen—Inorganic forms. In: Sparks DL, Page AL, Halmke PA, Loeppert RH, Soltanpour PN, Tabatabai MA, Johnstone CT, Sumner ME, editors. Methods of soil analysis part 3: chemical methods. Madison, WI: Soil Science Society of America, Inc; p. 1123–1184.
- Petersen RG, Lucas HL Jr. WWW. 1956. The distribution of excreta by freely grazing cattle and its effect on pasture fertility. 1. Excretal distribution. Agron J. 48:440–444. doi: 10.2134/agronj1956.00021962004800100002x
- Plantaz MAHG. 1998. Surface/atmosphere exchange of ammonia over grazed pasture. [Unpublished thesis]. Wageningen, The Netherlands: Wageningen University.
- Richards IR, Wolton KM. 1976. The spatial distribution of excreta under intensive cattle grazing. Journal of the British Grassland Society. 31:89–92. doi: 10.1111/j.1365-2494.1976.tb01123.x
- Robin AHK, Matthew C, Crush JR. 2010. Time course of root initiation and development in perennial ryegrass— a new perspective. Proc New Zeal Grassl Assoc. 72:233–240.
- Ross CA, Jarvis SC. 2001. Measurement of emission and deposition patterns of ammonia from urine in grass swards. Atmos Environ. 35:867–875. doi: 10.1016/S1352-2310(00)00371-X
- Saarijarvi K, Mattila PK, Virkajarvi P. 2006. Ammonia volatilization from artificial dung and urine patches measured by the equilibrium concentration technique (JTI method). Atmos Environ. 40:5137–5145. doi: 10.1016/j.atmosenv.2006.03.052
- Schjoerring JK, Mattsson M. 2001. Quantification of ammonia exchange between agricultural cropland and the atmosphere: Measurements over two complete growth cycles of oilseed rape, wheat, barley and pea. Plant Soil. 228:105–115. doi: 10.1023/A:1004851001342
- Selbie DR, Buckthought LE, Shepherd MA. 2015. The challenge of the urine patch for managing nitrogen in grazed pasture systems. Adv Agron. 129:229–292. doi: 10.1016/bs.agron.2014.09.004
- Shepherd M, Welten B, Wyatt J, Balvert S. 2014. Precipitation but not soil texture alters effectiveness of dicyandiamide to decrease nitrate leaching from dairy cow urine. Soil Use Manage. 30:361–371. doi: 10.1111/sum.12127
- Silva RG, Cameron KC, Di HJ, Hendry T. 1999. A lysimeter study of the impact of cow urine, dairy shed effluent, and nitrogen fertiliser on nitrate leaching. Aust J Soil Res. 37:357–369. doi: 10.1071/S98010
- Silva RG, Cameron KC, Di HJ, Jorgensen EE. 2005. A lysimeter study to investigate the effect of dairy effluent and urea on cattle urine N losses, plant uptake and soil retention. Water, Air, and Soil Pollution. 164:57–78. doi: 10.1007/s11270-005-2249-7
- Sommer SG, Jensen ES. 1991. Foliar absorption of atmospheric ammonia by ryegrass in the field. J Environ Qual. 20:153–156. doi: 10.2134/jeq1991.00472425002000010024x
- Tait A, Turner R. 2005. Generating multiyear gridded daily rainfall over New Zealand. J Appl Meteorol. 44:1315–1323.
- Tinker PB, Nye PH. 2000. Solute movement in the rhizosphere. Oxford: Oxford University Press.
- van Hove LWA, Heeres P, Bossen ME. 2002. The annual variation in stomatal ammonia compensation point of rye grass (Lolium perenne L.) leaves in an intensively managed grassland. Atmos Environ. 36:2965–2977. doi: 10.1016/S1352-2310(02)00242-X
- Wild A. 1972. Nitrate leaching under bare fallow at a site in northern Nigeria. J Soil Sci Plant. 23:315–324.
- Williams PH, Gregg PEH, Hedley MJ. 1990. Use of potassium bromide solutions to simulate dairy cow urine flow and retention in pasture soils. New Zeal J Agr Res. 33:489–495. doi: 10.1080/00288233.1990.10428447
- Williams PH, Haynes RJ. 1994. Comparison of initial wetting pattern, nutrient concentrations in soil solution and the fate of 15N-labelled urine in sheep and cattle urine patch areas of pasture soil. Plant Soil. 162:49–59. doi: 10.1007/BF01416089