Abstract
Benzing (2004) classified epiphytes as either ‘obligate’, ‘facultative’ or ‘accidental’ to describe variability in the tendency of epiphytic plants to grow arboreally. However, no method currently exists to quantitatively categorize epiphytes according to this classification system. Here, I derive a null model to test whether epiphytes are obligate, facultative or accidental, and then apply it to local epiphyte assemblages in two similar latitude forests on either side of the equator to test for hemispherical asymmetries in the relative abundance of different functional guilds of epiphytes. Results from the null model showed that the northern hemisphere site was comprised mostly of facultative epiphytes, which usually occurred on the forest floor. Conversely, the southern hemisphere site was comprised mostly of obligate epiphytes, which rarely occur on the forest floor, if at all, supporting previous speculation that epiphyte communities are structured in fundamentally different ways in the northern and southern hemispheres.
Introduction
A significant portion of the floristic diversity in many forested ecosystems is distributed epiphytically (Benzing Citation1990, Nadkarni et al. Citation2001, Nieder et al. Citation2001). Yet our understanding of vascular epiphyte communities lags far behind that of terrestrially rooted plant communities. One reason why epiphytes are poorly understood is that their spatial distributions are much more complicated. In addition to varying through space in the same way as terrestrially rooted plant communities (Ellis & Coppins Citation2009; Wolf Citation1993; Zapfack & Engwald Citation2007), epiphytes have an added dimension to their distribution resulting from their occurrence on discrete host trees. Epiphytes form metacommunities among individual hosts and metacommunity structure can differ substantially among host species, geographic locals and through time (Burns Citation2007a; Löbel et al. Citation2006; Roberts et al. Citation2005; Snäll et al. Citation2005; Zotz & Schultz Citation2008).
Another complexity of epiphyte distributions is their degree of dependence on an arboreal lifestyle. Some epiphytes have specialized life histories to help them survive in tree canopies, whereas others are adapted to the forest floor and grow epiphytically only occasionally (Bennett Citation1991). In an effort to describe this dichotomy, Benzing classified epiphytes into three functional guilds (Benzing Citation2004, see also Benzing Citation1989 ,Citation1990; Nadkarni et al. Citation2001). ‘Obligate’ epiphytes are those that occur almost exclusively as epiphytes. ‘Facultative’ epiphytes occur both epiphytically and on the forest floor, and ‘accidental’ epiphytes are almost exclusively rooted to the forest floor. Benzing's categories provide a useful way to conceptualize variation in the reliance of epiphytes on tree canopies as substrate. However, an objective, quantitative method to establish which category best describes the distribution of individual epiphyte species could be used in a variety of empirical circumstances to better understand the evolution and ecology of epiphytism. Here, I derive a null model for Benzing's functional guilds, which I then apply to a pair of similar latitude forests in the northern and southern hemispheres, both to illustrate how the null model can be used and to provide a preliminary test for hemispherical asymmetries in the structure of epiphyte assemblages.
Null model
To determine whether an epiphyte is obligate, facultative or accidental, its distribution needs to be quantified both arboreally (i.e. among host trees) and on the forest floor beneath host trees. If we assume that epiphytes are equally distributed between these two habitats, the expected epiphytic distribution () of species i based on its observed terrestrial distribution () is given by:
and the exact probability of obtaining an observed epiphytic distribution is given by the associated derivation of the binomial distribution:
where is the observed abundance of epiphyte species i in the entire metacommunity and there are n epiphyte species that occur on the forest floor.
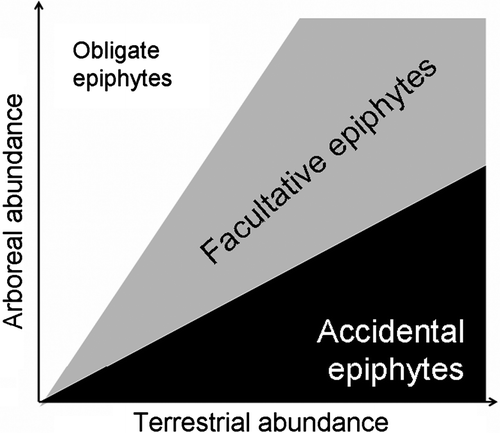
There are three statistical outcomes to the null model, which correspond to Benzing's functional guild classifications (). First, species can occur more frequently as epiphytes than expected from their terrestrial distribution (obligate epiphytes, white region). Second, they can occur less frequently in tree tops (accidental epiphytes, black region). Third, their epiphytic distribution can be predicted by their distribution on the forest floor (facultative epiphytes, grey region).
Fig. 1 A graphical representation of relationships between the arboreal and terrestrial distributions of epiphytes. The white region illustrates obligate epiphytes that are distributed disproportionately in tree canopies. The grey region represents facultative epiphytes whose arboreal distribution can be predicted by their terrestrial distribution. The black region represents accidental epiphytes that are distributed disproportionately beneath tree crowns on the forest floor.
Field data
In the northern hemisphere, epiphyte distributions were quantified in Barkley Sound, which is located on the west coast of Vancouver Island, British Columbia, Canada (48°80′N, 125°20′W). In the southern hemisphere, epiphyte distributions were quantified in Otari-Wilton's Bush, which is located on the southern tip of the North Island of New Zealand (41°14′S, 174°45′E). The same sampling protocol was employed in both geographic locales, and detailed descriptions of the sample sites are given elsewhere (Burns 2007a, 2008; Burns & Dawson Citation2005).
Vascular epiphytes were inventoried with binoculars from the forest floor and the occurrence of all species growing epiphytically was quantified on the most abundant conifer species in either site (Canada: Thuja plicata, n=26; New Zealand: Dacrydium cupressinum, n=28). All trees of each host species growing within 10 m of a 1.0 km trail traversing both forests were sampled. Host trees that lacked epiphytes, usually smaller trees, were not considered. Host trees that lacked sufficient visual access into their canopies were also omitted to ensure adequate inventories. The surrounding vegetation usually blocked visual access to the omitted trees, so the host trees included in the analyses did not appear to be a morphologically biased sample from the total pool available.
Several sampling criteria were adopted to help ensure accurate inventories. The time taken to inventory each host tree was recorded and when it seemed that all epiphyte species inhabiting each tree had been found, a further search was made for one third of the time already spent searching. If a new species was encountered during this time, an additional one third of the total survey time was again spent searching in an attempt to ensure an adequate, consistent survey of each tree. To identify the accuracy of ground-based surveys, complete inventories of several host trees were made from a series of permanent canopy research platforms in both sites. Previous comparisons between ground-based and canopy-based inventories indicated that in several instances epiphytes were missed in ground-based surveys, but that these sampling discrepancies did not confound community-level analyses of epiphyte distributions (see Burns Citation2007a ,Citation2008).
Analogous terrestrial-based surveys were conducted beneath the host-specific metacommunity at each site. Line-intercept methods were used to quantify the frequency of occurrence of all plant species found growing epiphytically along the same forest trail used to census the epiphyte metacommunities. A straight line transect was established at regular intervals along each trail, and all terrestrially rooted plant species touching the vertically projected plane of each transect were enumerated. The size of the line transect varied between sites to match between-site differences in the average number of species found growing epiphytically on each host tree. Otherwise, differences between the total number of occurrences observed on the forest floor and in the forest canopy could bias null model conclusions. In Canada, transects were 3 m long, and the average number of plant species (±SE) found growing epiphytically (4.23±2.05) and in ground-based transects (3.69±1.19) did not differ (t=1.160, p=0.253). In New Zealand, transects were 7 m long and the average number of plant species (±SE) found growing epiphytically (4.96±2.95) and in ground-based transects (4.64±1.50) did not differ (t=0.514, p=0.610).
Results
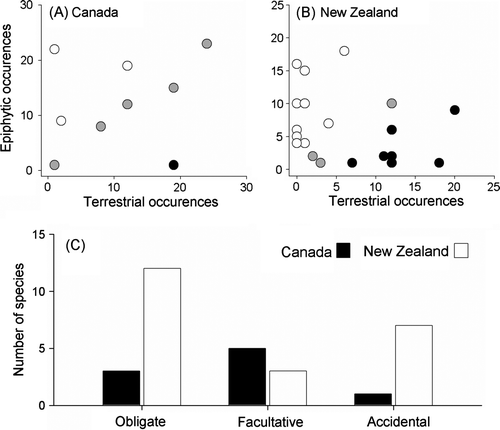
Nine species were found growing epiphytically on the 26 Thuja plicata trees sampled in Canada, and 22 species were found growing epiphytically on the 28 Dacrydium cupressinum trees sampled in New Zealand. In Canada (A), three species occurred epiphytically more often than expected from their distribution on the forest floor (obligate epiphytes). One species occurred less frequently as an epiphyte (an accidental epiphyte) and the epiphytic distribution of the remaining five species could be predicted by their distribution on the forest floor (facultative epiphytes). In New Zealand (B), 12 species were classified as obligate epiphytes, 7 species were accidental epiphytes and 3 species were facultative. A contingency table test showed that the number of obligate, facultative and accidental epiphytes differed between regions (x 2=7.87, df = 2, p<0.05), with Canada having more facultative epiphytes and New Zealand having more obligate and accidental species (C).
Fig. 2 Results from community-level analyses of epiphyte distributions in two study sites located in (A) New Zealand and (B) Canada. Each point in (A) and (B) represents the distribution of a single plant species. Obligate epiphytes are shown in white, facultative epiphytes are shown in grey and accidental epiphytes are shown in black. (C) Overall differences in the tendency of plants to occur as obligate, facultative and accidental epiphytes in the Canadian study site (black bars) and the New Zealand study site (white bars).
Discussion
Epiphyte assemblages showed pronounced differences in their reliance on tree canopies between hemispheres. In the northern hemisphere, the terrestrial distribution of most species accurately predicted their distribution in tree canopies. By contrast, most species in the southern hemisphere were either restricted to the forest floor or occurred more frequently as epiphytes.
Ibisch (Citation1996) proposed a different quantitative approach to characterize an epiphyte's dependency on an epiphytic lifestyle. Under their classification system, obligate epiphytes are those that occur >95% of the time arboreally, whereas accidental epiphytes occur arboreally <5% of the time (see, Zotz Citation2005). However, Ibisch's (Citation1996) method is arbitrary and lacks an analytical procedure to test whether epiphyte distributions differ statistically between arboreal and terrestrial habitats.
Fischer (Citation1960) was the first to recognize that species diversity can differ strongly between hemispheres and several recent studies have confirmed Fischer's early findings. Dunn et al. (Citation2009) showed that ant diversity is higher in the southern hemisphere, which they attribute to both present-day climatic differences and historical processes. Burns (Citation2007b) showed that tree species diversity differs between hemispheres, but that the directionality of diversity asymmetries depends on how species diversity is measured. The results presented here suggest that the degree of evolutionary specialization towards an epiphytic lifestyle might also differ between hemispheres.
Although we are far from an accurate understanding of why ecological phenomena vary between hemispheres, historical processes might be important in generating hemispherical asymmetries in the relative abundance of epiphyte functional guilds. Large portions of New Zealand escaped glaciation in the Pleistocene, whereas most of Canada was heavily glaciated. Therefore, both sites may have supported similar communities of obligate epiphytes at some point in the past, but they have yet to recolonize Canada from lower latitudes (cf. ‘time since disturbance’ hypothesis, Rosenzweig Citation1995). Southern Chile supports diverse communities of vascular epiphytes that are closely related taxonomically to New Zealand (Muñoz et al. Citation2003). Therefore, obligate epiphytism may have evolved more frequently in the ancient southern landmass Gondwana, which contained both New Zealand and South America, than in the ancient northern landmass Laurasia.
When species identified as obligate epiphytes by the null model occurred on the forest floor, they sometimes appeared to have been dislodged from the canopy above. This suggests that obligate epiphytes might ‘colonize’ the forest floor from the canopy above – the forest floor may simply constitute a ‘sink’ population (sensu Pulliam Citation1998). However, their sporadic occurrence on the forest floor does not necessarily suggest that they can successfully persist there (see Matelson et al. Citation1993). A similar, but directionally opposite situation may occur with accidental epiphytes, which inhabit the forest floor and occasionally disperse into tree canopies. However, incorporating such sinks directly into null model calculations does not bias its conclusions. The transient nature of obligate epiphytes on the forest floor and the transient nature of accidental epiphytes arboreally lower the probability of observing ephemeral individuals in sink populations, thereby accurately depicting their dependence on either habitat.
Although the null model can identify facultative epiphytes statistically, rather than being a single homogeneous category, facultative epiphytes incorporate a continuum of generalist species, often with weak affinities for either terrestrial or epiphytic habitats. The continuous nature of epiphyte distributions can be incorporated into future studies by evaluating the ratio between the number of epiphytic and terrestrial occurrences. For example, ratios of arboreal and terrestrial occurrences can be compared with measurements of plant physiology to identify life history adaptations to promote an arboreal lifestyle. Comparisons could also be made within a phylogenetic context to investigate the evolutionary transitions towards epiphytism (cf. Tsutsumi & Kato Citation2006).
Only one host-specific metacommunity was quantified at each site, mostly because host tree diversity is low in Canada and Thuja plicata is the only host species that consistently houses significant assemblages of vascular epiphytes. However, the structure of epiphyte metacommunities may often differ among host species (Burns & Zotz Citation2010), and an epiphyte's distributional classification could differ strongly depending on the host species. Documenting potential shifts in the distributional classification of epiphyte species among host species could yield important information on the habitat requirements and life history adaptations of epiphytes, in addition to the outcome of interactions with host trees and other epiphytes. Future studies should also take care to adequately sample epiphyte communities, both arboreally and terrestrially. For example, individual- or sample-based rarefaction can be used to ensure both habitats have been adequately characterized (Gotelli & Colwell Citation2001). Terrestrial-based sampling in this application was restricted to species that were observed arboreally. If all species occurring terrestrially were quantified, the null model could also be employed to evaluate the tendency of terrestrially based species to occur epiphytically.
Epiphytism was once considered to be a hallmark of tropical rainforests (Janzen Citation1975). However, Dawson (Citation1980) was the first to point out that this generalization stems from a northern-latitude perspective and is not entirely accurate. Instead of being a tropical phenomenon, vascular epiphytes are particularly depauperate in north–temperate forests, and recent reviews of epiphytism now explicitly recognize the apparent latitudinal asymmetry in the diversity of vascular epiphytes (e.g. Benzing Citation2004; Nadkarni et al. Citation2001; Zotz Citation2005). However, latitudinal gradients in epiphyte diversity have yet to be quantified properly. Aside from the two data points presented here, geographic gradients in the relative abundance of different functional guilds of epiphytes have also not been quantified and could be strongly asymmetric hemispherically. Benzing (Citation1990) hypothesized that epiphytes become increasingly facultative as environmental conditions in tree canopies converge on terrestrial environmental conditions. Although it was postulated two decades ago, this hypothesis has never been tested. Quantitative analyses of Benzing's classifications of epiphytism may aid empirical tests of these hypotheses and help to narrow the gap between our understanding of epiphytic and terrestrially rooted plant assemblages.
References
- Bennett , BC . 1991 . Comparative biology of Neotropical epiphytic and saxicolous Tillandsia species: population structure . Journal of Tropical Ecology , 7 : 361 – 371 .
- Benzing DH 1989 . The evolution of epiphytism . In: Luttge U Vascular plants as epiphytes: evolution and ecophysiology . Berlin , Springer Verlag . 167 199 .
- Benzing DH 1990 . Vascular epiphytes . Cambridge , Cambridge University Press .
- Benzing DH 2004 . Vascular epiphytes . In: Lowman MD Rinker HB Forest canopies . Berlin , Springer Verlag . 175 211 .
- Burns , KC . 2007a . Network properties of an epiphyte metacommunity . Journal of Ecology , 95 : 1142 – 1151 .
- Burns , KC . 2007b . Is tree diversity different in the Southern Hemisphere? . Journal of Vegetation Science , 18 : 307 – 312 .
- Burns , KC . 2008 . Meta-community structure of vascular epiphytes in a temperate rainforest . Botany , 86 : 1252 – 1259 .
- Burns , KC and Dawson , JW . 2005 . Patterns in the diversity and distribution of epiphytes and vines in a New Zealand forest . Austral Ecology , 30 : 891 – 899 .
- Burns , KC and Zotz , G . 2010 . A hierarchical framework to investigate epiphyte assemblages: networks, metacommunites and scale . Ecology , 91 : 377 – 385 .
- Dawson , JW . 1980 . Middle-latitude rainforests in the Southern hemisphere . Biotropica , 12 : 159 – 160 .
- Dunn , RR , Agnosti , D , Andersen , AN , Arnan , X , Bruhl , CA , Cerda , X , Ellison , AM , Fisher , BL , Fitzpatrick , MC , Gibb , H , Gotelli , NJ , Gove , AD , Guenard , B , Janda , M , Kaspari , M , Laurent , EJ , Lessard , JP , Longino , JD , Majer , D , Menke , SB , McGlynn , TP , Parr , CL , Philpott , SM , Pfeiffer , M , Retana , J , Suarez , AV , Vasconcelos , HL , weiser , MD and Sanders , NJ . 2009 . Climatic drivers of hemispheric asymmetry in global patterns of ant species richness . Ecology Letters , 12 : 324 – 333 .
- Ellis , CJ and Coppins , BJ . 2009 . Integrating multiple landscape-scale drivers in the lichen epiphyte response: climatic setting, pollution regime and woodland spatial–temporal structure . Diversity and Distributions , 16 : 43 – 52 .
- Fischer , AG . 1960 . Latitudinal variations in organic diversity . Evolution , 14 : 64 – 81 .
- Gotelli , NJ and Colwell , RK . 2001 . Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness . Ecology Letters , 4 : 379 – 391 .
- Ibisch PL 1996 . Neotropische Epiphytendiversität – das Beispiel Bolivien . Wiehl, , Germany , Martina Galunder-Verlag . 357 .
- Janzen DH 1975 . Ecology of plants in the tropics . London , Edward Arnold .
- Löbel , S , Snäll , T and Rydin , H . 2006 . Metapopulation processes in epiphytes inferred from patterns of regional distribution and local abundance in fragmented forest landscapes . Journal of Ecology , 94 : 856 – 868 .
- Matelson , TJ , Nadkarni , NM and Longino , JT . 1993 . Longevity of fallen epiphytes in a neotropical montane forest . Ecology , 74 : 265 – 269 .
- Muñoz , AA , Chacón , P , Pérez , F , Barnert , ES and Armesto , JJ . 2003 . Diversity and host tree preferences of vascular epiphytes and vines in a temperate rainforest in southern Chile . Australian Journal of Botany , 51 : 381 – 391 .
- Nadkarni NM , Merwin MC , Nieder J . 2001 . Forest canopies, plant diversity . In : Levin SA Encyclopaedia of biodiversity 3 . London , Academic Press . 27 40 .
- Nieder , J , Prosperí , J and Michaloud , G . 2001 . Epiphytes and their contribution to canopy diversity . Plant Ecology , 153 : 51 – 63 .
- Pulliam , HR . 1998 . Sources, sinks, and population regulation . American Naturalist , 132 : 652 – 661 .
- Roberts , NR , Dalto , PJ and Jordan , GJ . 2005 . Epiphytic ferns and bryophytes of Tasmanian tree-ferns: a comparison of diversity and composition between two host species . Austral Ecology , 30 : 146 – 154 .
- Rosenzweig M 1995 . Species diversity in space and time . Cambridge , Cambridge University Press .
- Snäll , T , Ehrlén , J and Rydin , H . 2005 . Colonization–extinction dynamics of an epiphyte metapopulation in a dynamic landscape . Ecology , 86 : 106 – 115 .
- Tsutsumi , C and Kato , M . 2006 . Evolution of epiphytes in Davalliaceae and related ferns . Botanical Journal of the Linnaean Society , 151 : 495 – 510 .
- Wolf , JHD . 1993 . Diversity patterns and biomass of epiphytic bryophytes and lichens along an altitudinal gradient in the northern Andes . Annals of the Missouri Botanical Garden , 80 : 928 – 960 .
- Zapfack , L and Engwald , S . 2007 . Biodiversity and spatial distribution of vascular epiphytes in two biotopes of the Cameroonian semi-deciduous rain forest Plant Ecology , 195 : 117 – 130 .
- Zotz , G . 2005 . Vascular epiphytes in the temperate zones – a review . Plant Ecology , 176 : 173 – 183 .
- Zotz , G and Schultz , S . 2008 . The vascular epiphytes of a lowland forest in Panama-species composition and spatial structure . Plant Ecology , 195 : 131 – 141 .