Abstract
Coprosma dumosa and C. tayloriae were compared to test whether they are two species as proposed by Jane (2005). Length of visible midvein and depth of corolla sinuses, characters used by Jane (2005) to differentiate the pair, were not significantly different between herbarium specimens determined by Jane as C. dumosa and C. tayloriae. No significant difference in visible midvein length for the five fruit colours recorded on specimens was found. However, fruit colour correlated with altitude, red-fruited specimens coming from higher altitudes than white-fruited specimens. Representative specimens of C. dumosa and C. tayloriae cited by Jane (2005) included specimens we redetermined as C. ciliata and C. pseudociliata. These and similar misidentifications of herbarium specimens suggests that Jane's (2005) concept of C. dumosa is based on a mixture of three species. We believe Jane (2005) has failed to reject the hypothesis that C. dumosa and C. tayloriae are conspecific.
Introduction
The Coprosma ciliata–parviflora complex is a name adopted for a group of species by Jane (Citation2005). Jane's concept of this group includes C. ciliata Hook.f., C. dumosa (Cheeseman) G.T.Jane, C. parviflora Hook.f., C. pseudociliata G.T.Jane and C. tayloriae G.T.Jane. Possibly C. decurva Heads and C. pedicellata Molloy et al. should also be regarded as part of this group of species, but were not dealt with in Jane's Citation2005 revision.
The taxonomic history of species in this group is convoluted. C. ciliata and C. myrtillifolia were described by Hooker (Citation1844) from the Auckland Islands. C. myrtillifolia was made a synonym of C. ciliata by Hooker (1867). C. parviflora was described by Hooker (Citation1853) from North and South Islands.
Cheeseman described two varieties in C. parviflora, var. pilosa (1887) and var. dumosa (1906). Cheeseman (1925) made var. pilosa a synonym of Hooker's C. ciliata. For var. dumosa, confusion over this variety arose because Allan (Citation1961) adopted a description of this variety that did not agree with Cheeseman's 1906 publication of the name. Perhaps because of the confusion caused by Allan's concept, A. P. Druce adopted informal names for Cheeseman's C. parviflora var. dumosa: Coprosma sp. “t” (e.g. in Eagle Citation1982) and on herbarium sheets as Coprosma “taylorae” (corrected by Jane Citation2005 to ‘tayloriae’).
Jane (Citation2003) outlined the taxonomic history of C. parviflora var. dumosa.
Jane's account makes it clear that Cheeseman's concept of this variety changed over time and was quite broad, encompassing what is now C. decurva Heads. However, the identity of the variety is fixed by its publication in Cheeseman (Citation1906) and Oliver's (Citation1935) lectotypification using a Cheeseman collection from Red Hills, Wairau. Oliver's lectotypification is in accord with the protologue and the Botanical Code of Nomenclature.
Jane (Citation2005) recognized five species belonging to what he called the C. ciliata and parviflora complex: C. ciliata, C. dumosa, C. parviflora, C. pseudociliata and C. tayloriae. One of these species, C. tayloriae, was newly described and in Jane's view is distinct from C. parviflora var. dumosa. He raised Cheeseman's variety to species rank as C. dumosa. He raised Cheeseman's C. parviflora var. pilosa to species rank as C. pseudociliata G.T.Jane as the name C. pilosa was not available at that rank (non C. pilosa Endl., a Norfolk Island species).
In 2007 we were contracted by the Terrestrial and Freshwater Biodiversity Information System fund to prepare an online key to all the New Zealand Coprosma species (Glenny et al. Citation2010). In the course of preparing the key we needed to decide which of Jane's (Citation2005) names to recognise in the key and examined his revision and specimens annotated by him in CHR and some in AK. C. ciliata, C. pseudociliata and C. parviflora presented no problems, although the distribution of C. ciliata seemed underestimated: we found the distribution extends into the North Island. However, we found specimens of C. dumosa and C. tayloriae could not be keyed out with any confidence using the key in Jane (Citation2005) and careful examination and testing of the species pair was needed.
Jane (Citation2005) distinguishes these two species in his key to species with the differences shown in . In comparison notes Jane (Citation2005) adds that C. tayloriae in Canterbury and Otago is found at lower altitudes than C. dumosa, it has a planar branchlet arrangement, an obovate leaf, with a short stout midvein. The midvein in dried specimens of C. tayloriae is not visible on the upper surface except sometimes as a lightly impressed groove. In C. dumosa, he says, shaded leaves are often marginally ciliate and can be densely ciliate [sic.] on the upper surface. The leaf margin hairs of C. dumosa, he says, are fine, straight and violet-tinged, while in C. tayloriae, when present, the hairs are stout, translucent to yellow, curved to appressed. In his species descriptions he says that the female corolla of C. tayloriae is shallowly lobed (0.3–0.5 of mature bud length), while in C. dumosa it is deeply lobed, 0.6–0.8 of mature bud length.
Table 1 Differences between Coprosma dumosa and C. tayloriae in the dichotomous key in Jane (Citation2005).
Results
Misidentifications
We examined all herbarium specimens at CHR determined by Jane as these two species. Among those he determined as C. dumosa, we identified 12 specimens from Canterbury and Otago as C. ciliata and 5 as C. pseudociliata. Among those he determined as C. tayloriae, we identified 13 specimens as C. ciliata and 2 as pseudociliata. When the misidentified specimens were removed from C. dumosa and C. tayloriae there was no longer a difference in hair distribution between C. dumosa and C. tayloriae. This action removed all specimens from C. dumosa that were densely hairy on the upper surface or with hairs on the leaf margins, features mentioned for C. dumosa in Jane's description and comparison notes.
Visible midvein length
We tested C. tayloriae and C. dumosa for a difference in length of visible midvein on the lower leaf surface, as the key in Jane (Citation2005) states for C. tayloriae that the midvein is ‘not reaching the leaf tip beneath’ while for C. dumosa the key states: ‘midvein…extending straight to leaf tip beneath’. No actual measurement data are presented by Jane (Citation2005). This difference is the one most easily tested statistically and without observer bias. We examined two leaves chosen at random from each specimen cited by Jane (Citation2005) as representative of C. tayloriae and C. dumosa that are at CHR, plus others at CHR that Jane determined in 2006, and excluding any specimens that we considered to be C. ciliata or C. pseudociliata. Midvein length was measured as a proportion of the leaf length, for example, 4.5/8.0 mm, and was measured under a dissecting microscope. In some cases the extent of the midvein was difficult to estimate, but this was the case in both C. dumosa and C. tayloriae specimens. The ratios for the two groups were not normally distributed, and a one-way ANOVA using 5000 random permutations in Genstat (Citation2008) was used to test if the ratios differed. The null hypothesis of no difference in leaf length ratios between the two groups was not rejected (F 1,67=0.183). What is claimed in Jane's (Citation2005) key—that the midvein extends to the leaf tip in C. dumosa—is true more often for the C. dumosa leaves, but in only 17% of the leaves. Using this character would allow C. dumosa to be identified in only a small proportion of specimens.
Altitude range
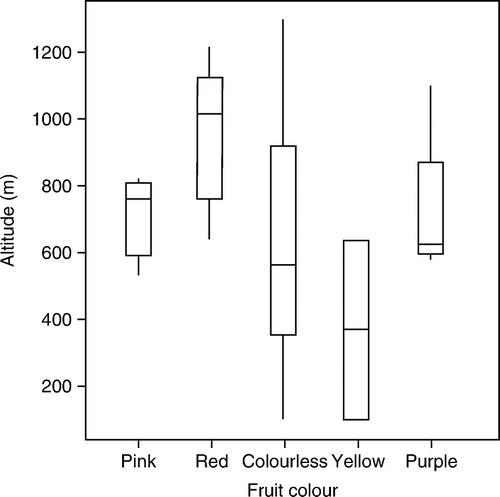
Jane (Citation2005) described a habitat difference between C. dumosa and C. tayloriae: ‘Coprosma tayloriae is close to C. dumosa which occupies high altitude, open habitats in the same areas of the eastern South Island’. For C. dumosa the altitude range is given as ‘600–1500 m in the north [of the South Island], to as low as 300 m in Otago’, while for C. tayloriae the altitude range is ‘500–1100 m a.s.l. in North Island, descending to sea level–500 m at about Harihari in Westland’. Using all specimens in CHR from Canterbury Province where an altitude was specified on the label, and using Jane's determinations on the specimens, we tested for a difference in altitude for the two species in Canterbury. For C. dumosa, the mean altitude was 946 m, the range 150–1436 m (n=37 specimens), and for C. tayloriae the mean altitude was 609 m and the range 250–1220 m (n=21 specimens). The altitudes were not normally distributed, and a one-way ANOVA using 5000 random permutations was used in Genstat (Citation2008). The null hypothesis of no difference in altitude between the two groups was rejected (F 1,56<0.01).
The two overlap extensively in their distributional and altitudinal ranges, and specimens from the same locality, collector and date have been determined by Jane as belonging to two species. For instance, H. Talbot, Nov. 1947, Otarama, CHR 300679 was determined as C. dumosa and CHR 300755, with the same label details, determined as C. tayloriae. We cannot see differences in the leaves of these two specimens, although the first is sterile and the second has female flowers, suggesting they are from different plants. We consider these two specimens to be conspecific.
Female corolla sinus depth
We tested the difference between the depths of the sinus of the female corolla as a proportion of corolla length (termed here ‘sinus depth’) in all specimens of C. dumosa and C. tayloriae present at AK and CHR, using Jane's determinations. The descriptions in Jane (Citation2005) give a sinus depth ratio for the corolla of ‘mature buds’. We were unsure as to why this stage was chosen, as it is not a well-defined stage in corolla development. A comparison of mature female corolla buds of C. dumosa and C. tayloriae was not possible using specimens at AK and CHR because there are too few specimens at this developmental stage.
We investigated sinus depth ratio in a series of flowers from a single specimen, CHR 300697, determined by Jane as C. dumosa that had female flowers in all degrees of development, to gain an understanding of how sinus depth varies with developmental stage. Sinus depth was only 0.15 of corolla length in the youngest unopened corolla with style arms contained within the bud and only 1.5 mm long. But for three flowers examined with the style arms projecting from the corolla (with style arms 1.8 mm, 3.0 mm and 4.0 mm long, indicating increasing maturity) the sinus depth was between 0.48 and 0.55, with no trend towards increasing depth. Nor was there a trend towards increasing corolla length once the corolla was open and the style arms exserted (corolla length was 1.6 mm long for the flower in bud, and 1.7–1.9 mm long for the three flowers that had opened, with no trend of increasing length). Sinus depth therefore appears to be independent of age of flower after corolla opening. We decided to test for a difference in sinus depth in mature flowers with exserted style arms. Although this was not the stage of development specified by Jane (Citation2005), it was the only way to achieve a sample size sufficient for a statistical comparison.
We measured sinus depth of female corollas taken from 11specimens of C. tayloriae and 5 specimens of C. dumosa, cited as representative specimens in Jane (Citation2005), the types of C. dumosa and C. tayloriae, or determined by Graeme Jane in 2006 (). All female specimens available in AK and CHR were used for this comparison. Because of the paucity of specimens with female flowers (especially C. dumosa), we measured 2–4 flowers per specimen and pooled the scores for all flowers. For each flower we measured sinus depth on one side of the lobe, and length of the corolla at that lobe (lobes can have a shallow sinus on one side and a deep sinus on the other). The ratio of lobe length to corolla length is referred to here as sinus depth. Because the corolla has to be split at one of the sinuses to flatten it for examination, only three of the usually four sinuses can be measured. In some corollas sinus depths were almost equal, while in others they varied a great deal, usually because three of the sinuses were deep but one was shallow (e.g. in the type of C. tayloriae, one flower had lobe depths 0.17, 0.45, 0.74, while another flower had lobe depths of 0.30, 0.62, 0.74). This was equally the case in both C. dumosa and C. tayloriae specimens. All sinus depths were used to derive the average for a flower.
Table 2 Mean corolla lobe depths in female flowers o f Coprosma dumosa and C. tayloriae, using specimens cited by Jane (Citation2005) or determined by Jane in 2006.
For C. dumosa, average sinus depth for individual flowers ranges between 0.27 and 0.78, while in C. tayloriae it ranges between 0.29 and 0.76. Mean sinus depths for flowers of the two species were 0.525 and 0.549, respectively. Sinus depths were not normally distributed and were tested with a one-way ANOVA using 5000 random permutations in Genstat (Citation2008). The null hypothesis of no difference in means between the two groups was not rejected (F 1,39=0.455). The ranges do not match the ranges given by Jane (Citation2005) of 0.6–0.8 for C. dumosa, and 0.3–0.5 for C. tayloriae, but it must be borne in mind that our comparison was of mature flowers, not flowers in bud. Our observations of corolla depth in flowers with an open corolla give no support to there being two species.
Fruit colour
Herbarium records (at CHR) indicate that predominant fruit colours in specimens determined as C. dumosa and C. tayloriae are translucent white (33 specimens) and orange-red to red (20 specimens), with a minority of other fruit colours recorded: pink (three specimens), yellow (two specimens from Prices Valley, and Mt Sinclair, both Banks Peninsula), purplish-red (two specimens from Mt Tihia near Lake Rotoaira, and Ruahine Ra.), wine-coloured (one specimen from Arahura Valley). The distribution of fruit colours for all specimens at CHR which state fruit colour, and using Jane's determinations, are presented in . In some populations, a mixture of these colours can be seen. An example is CHR 519946, K. H. Platt, Glendhu Forest, Otago (det. C. dumosa by Jane in 2006), where the label states, ‘white to pale-magenta to red, pale-yellow to yellow to orange (on different plants), often darkening on ripening’.
Table 3 Fruit colour in Coprosma dumosa and C. tayloriae as recorded on herbarium specimen labels at CHR, using determinations of Jane (Citation2005) but excluding specimens redetermined by us as C. ciliata or C. pseudoc iliata.
Fruit colour was tested for correlation with the ratio of visible midvein length/total leaf length on the lower surface of leaves. Thirty-nine herbarium specimens where the label specified fruit colour had two leaves measured for midvein length ratio and the average of the two ratios used to represent that specimen. Twenty-one specimens were determined by Jane in 2006 as C. tayloriae, and 18 as C. dumosa. All available specimens of C. dumosa at CHR were used. C. tayloriae specimens were chosen from throughout its geographical range.
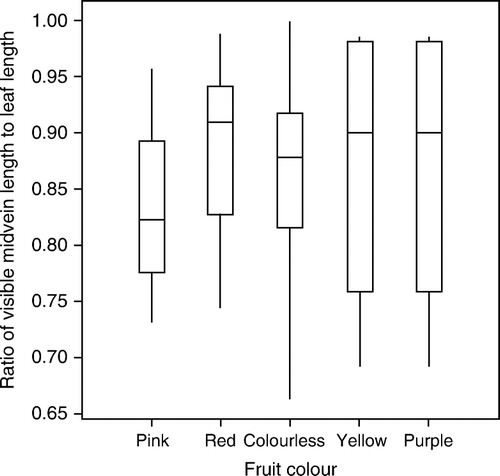
As the vein length ratio was not normally distributed, a one-way ANOVA was done using 5000 random permutations in Genstat (Citation2008) using visible vein length ratio as the variate and fruit colour as factor. The null hypothesis of no difference between mean visible vein length ratio for the five fruit colours was not rejected (F 4,36=0.692). A box plot of mean vein length ratios for each colour is presented in .
Figure 1 Box-plot summary of the ratio of visible midvein length to total leaf length for different fruit colours in Coprosma dumosa. Numbers of samples are pink 4, red 15, colourless 18, yellow 2, purple 2.
Fruit colour was also tested for correlation with altitude. Forty-one specimens at CHR were used where fruit colour and altitude were both specified. Twenty were determined by Jane as C. dumosa and 21 as C. tayloriae. Altitude was not normally distributed and a one-way ANOVA was done using 5000 random permutations in Genstat (Citation2008) using altitude as variate and fruit colour as factor. The null hypothesis of no difference between altitude for the four fruit colours was rejected (F 4,37=0.013). The mean altitude for white-fruited specimens (n=19) was 624 m, while that for red-fruited specimens (n=14) was 970 m. Pink-fruited specimens (n=3) had a mean altitude of 706 m, purple fruited (n=4) 732 m and yellow-fruited (n=2) 370 m. A box plot of altitude for each colour is presented in .
Discussion and conclusions
We conclude that C. tayloriae should be regarded as a synonym of C. dumosa. C. dumosa and C. tayloriae cannot be distinguished using the dichotomous key provided by Jane (Citation2005). Jane(Citation2005) has failed to make a convincing case for there being two species. Our testing of two morphological characters, vein length on the lower leaf surface and depth of corolla lobing, gives no support to there being two species. Fruit colour is predominantly orange to red in specimens Jane identified as C. dumosa, and white in specimens Jane determined as C. tayloriae, however fruit colour is not correlated with leaf midvein length, and Jane's determinations may simply reflect his belief that fruit colour could be used as a basis for distinguishing two species. There is a significant difference in mean altitude of specimens, but this may reflect a similar bias in Jane's determinations. However, red-fruited specimens are found at a higher altitude than white-fruited specimens, and this is evidence, independent of specimen determinations, in favour of Jane's (Citation2005) two species hypothesis. This evidence alone, is not, in our opinion, sufficient to establish that there are two species, nevertheless this evidence suggests a genetic difference between lower and high altitude plants that would be worth investigating. Wichmann (Citation2002) provides ITS DNA evidence that C. dumosa is of hybrid origin involving C. microcarpa as one of its parents, but this was based on a single sample. Further sampling may show more complex origins.
An illustration of the problem presented in this paper is provided by a specimen (CHR 358372, A. P. Druce, Mt Ben More, Marlborough, 3600 ft, shrub-tussockland) that has three pieces collected from the same plant, two from the crown of the plant and one from the inside and base of the plant. The two crown pieces have no leaf margin hairs and only short hairs on the young branchlets. The inside-base piece has hairs on the leaf margin and both long and short hairs on the young branchlets. This specimen, determined as C. dumosa by Jane in 2006, we believe is C. pseudociliata because of the presence of leaf margin hairs and long branchlet hairs on the piece from the shaded and protected part of the plant. The specimen shows that plants of C. pseudociliata growing in the open are very difficult to distinguish from C. dumosa s. l. The only indications that the crown pieces are of C. pseudociliata is that the leaf apex is quite acute and secondary veins on the underside of the leaf are visible. It is likely that other herbarium specimens determined as C. dumosa or C. tayloriae are in fact C. pseudociliata, but their true identity is difficult to establish. It is more parsimonious to hypothesise that CHR 358372 is C. pseudociliata than to suppose that it belongs to another species, C. dumosa sensu Jane (Citation2005).
Taxonomy
Coprosma dumosa (Cheeseman) G.T.Jane, New Zealand J. Bot. 43: 739, Citation2005.
= Coprosma parviflora var. dumosa Cheeseman, Man. New Zealand fl. 254, 1906. Lectotype (chosen by Oliver, Citation1935): Red Hills [Wairau Valley, Marlborough], 2500 ft, T. F. Cheeseman, Jan 1882, AK 8868!
= Coprosma tayloriae A.P.Druce ex G.T.Jane, New Zealand J. Bot. 43: 746–7, Citation2005. Holotype: Mt Hauhungatahi, Volcanic Plateau, 3500 ft, H. Carse 1369/1, 1 Jan 1921, CHR 328667!
Both C. dumosa and C. tayloriae were published as species simultaneously by Jane (Citation2005). Article 11.5 Note 2 of the Botanical Code (McNeill et al. Citation2006) specifies that a choice is possible between names with equal priority at corresponding rank, and the first such choice to be effectively published establishes the priority of the chosen name. This choice is made here. We have decided to retain Cheeseman's name, being the first published taxon name of this species.
Key
A key to the species of the species revised by Jane (Citation2005) is presented below that uses differences in leaf and branchlet hairs that we consider helpful in distinguishing them, to supplement differences in visibility of veins on the lower leaf surface that were used by Jane (Citation2005). However, as noted in the discussion above, specimens of C. ciliata and C. pseudociliata in exposed habitats in eastern provinces may be very difficult to identify using the hair characters used in this key.
| 1. | Leaf margins nearly always with short to long hairs (80–900 µm long); branchlet hairs often markedly different in length: long (240–500 µm) and short (90–120 µm); leaves thin in texture, 90–280 µm thick . . . . . . . . . . . . . . . . . . . . . . . . . .2 Leaf margins nearly always glabrous, if hairs are pres ent they are confined to the apical third of the leaf margin; branchlet hairs uniformly short (60–160 µm long) [with the exception of some specimens of C. parviflora]; leaves thick in texture, (140)190–320(400) µm thick. . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3 | ||||
| 2. | Hairs on upper leaf surface confined to midvein, extending from lamina base to ⅓–⅔ the distance to leaf apex; branchlets straight; found in lowland forests, rarely in montane forests; fruit usually white, rarely pink or yellow . . . . . . . . . . . . .C. ciliata Hairs on upper leaf surface absent or evenly spread over the lamina surface; branchlets often curved; found in upper montane forests and penalpine scrub and tussocklands; fruit scarlet to crimson . . . . . . . . . . . . . . . . . . . . . .C. pseudociliata | ||||
| 3. | Lower surface of leaf always glabrous; midvein only, or midvein and 1–2 pairs of side veins visible on lower leaf surface, a network of veins rarely visible (in ca. 8% of specimens); fruit colour usually either translucent white or orange-red to red, rarely pink, yellow or magenta. . . . . . . . . . . . . . . . . . . . . . .C. dumosa Lower surface of leaf nearly always with evenly distributed semi-appressed short hairs, rarely glabrous (ca. 10% of specimens); a network of veins commonly visible on lower leaf surface (in ca. 70% of specimens); fruit colour usually violet, rarely red or orange . . . . . . . . . . . .C. parviflora | ||||
Note: hair lengths and leaf thicknesses are presented to give some objective basis to the comparative terms long/short, thick/thin. To identify specimens these lengths and thicknesses need not be measured. Leaf thickness was measured immediately to one side of the leaf midvein.
Acknowledgements
We wish to thank Peter de Lange and two anonymous reviewers for their constructive comments on a previous draft of this paper; Professors Phil Garnock-Jones and Shirley Pledger and a second anonymous reviewer for their reviews of the current version of the paper, and for advice on statistical tests; and Peter Heenan for reviewing this and an earlier draft. Thanks also to Guy Forrester for help with statistical tests and Cissy Pan for drafting figures. We thank Ewen Cameron (Auckland Museum) for selecting specimens of C. dumosa with female flowers for a loan. This research was supported by the Ministry of Science and Innovation through the Defining New Zealand's Land Biota Biosystematics contract.
References
- Allan HH 1961 . Flora of New Zealand Volume 1 Government Printer Wellington Pp 571 – 572
- Cheeseman , TF . 1887 . On the New Zealand species of Coprosma . Transactions of the New Zealand Institute , 19 : 218 – 252 .
- Cheeseman TF 1906 . Manual of the New Zealand flora Government Printer Wellington .
- Cheeseman TF 1925 . Manual of the New Zealand flora Government Printer Wellington .
- Eagle A 1982 . Eagle's trees and shrubs of New Zealand . Second series Auckland, Collins .
- Genstat 2008 . Genstat statistical package release 11.1. Rothamstad, Lawes Agricultural Trust .
- Glenny D , Cruickshank J , Rolfe J 2010 . Key to Coprosma species of New Zealand . Interactive online key accessible at http://www.landcareresearch.co.nz/research/biosystematics/plants/coprosmakey/ (accessed 1 July 2011).
- Hooker JD 1844 . Flora Antarctica . London, Reeve .
- Hooker JD 1853 . Flora Novae-Zelandiae . London, Reeve .
- Hooker JD 1867 . Handbook of the New Zealand flora . London, Reeve
- Jane , GT . 2003 . What is Coprosma parviflora var. dumosa? . Auckland Botanical Society Journal , 58 : 112 – 115 .
- Jane , GT . 2005 . An examination of Coprosma ciliata and C. parviflora complex . New Zealand Journal of Botany , 43 : 735 – 752 .
- McNeill J , Barrie FR , Burdet HM , Demoulin V , Hawksworth DL , Marhold K , Nicolson DH , Prado J , Silva PC , Skog JE , Wiersema JH , Turland NJ 2006 . International code of botanical nomenclature (Vienna code) adopted by the Seventeenth International Botanical Congress Vienna , 2005 July Austria . Regnum Vegetabile 146, A.R.G. Gantner Verlag KG, Ruggell, Liechtenstein .
- Oliver WRB 1935 . The genus Coprosma . Bulletin of the Bernice P. Bishop Museum 132 .
- Wichmann , SR , Wright , SD , Cameron , EK , Keeling , J and Gardner , RC . 2002 . Elevated genetic heterogeneity and Pleistocene climatic instability: inferences from nrDNA in New Zealand Coprosma (Rubiaceae) . Journal of Biogeography , 29 : 943 – 954 .