Abstract
The basidiomycete decay fungi present within stems of five rimu trees (Dacrydium cupressinum) uprooted 10 years earlier in southern Westland were investigated by isolating onto a selective culture medium. The most common fungi obtained were Armillaria novae-zelandiae and Ganoderma applanatum, sensu Wakef., while species isolated less frequently included Rigidoporusconcrescens, Hypochnicium polonense and Irpex sp. There were few trends in overall percentage yields of basidiomycetes among trees, discs or radial depths, signifying that colonisation was uniform throughout stems, but A. novae-zelandiae was isolated more frequently from near the surface, while G.cf. applanatum had penetrated right to stem centres. Cultural pairing demonstrated that some vegetative compatibility groups of A. novae-zelandiae extended more than 4.5 m, indicating invasion by mycelial growth along stems, while those of G.cf. applanatum were found in no more than one disc, implying multiple basidiospore colonisation. Results from this and previous studies in the central North Island suggest that these patterns may be typical for fallen rimu in podocarp–hardwood forests over much of New Zealand.
Introduction
Significant research has been undertaken to determine the principal basidiomycete fungi decomposing coarse woody debris of dominant tree species in indigenous forests in New Zealand's central North Island (Hood et al. Citation1989, Citation2004, Citation2008; Beets et al. 2008; Hood & Gardner Citation2009). The species isolated most frequently from fallen stems in podocarp–hardwood and Nothofagus (beech) forests were Ganodermaapplanatum (Pers.) Pat., sensu Wakef. [formerly also referred to as G. mastoporum (Lév.) Pat. in New Zealand], Armillaria novae-zelandiae (G. Stev.) Herink. and Armillaria limonea (G. Stev.) Boesew. (noting that for A. limonea in beech forest, prevalence was demonstrated by fruiting incidence rather than isolation) (Hood et al. Citation2008). A fourth species, Cyclomyces tabacinus (Mont.) Pat., was also commonly isolated from fallen stems only in beech forest [N. fusca (Hook. f.) Oerst. and N. menziesii (Hook. f.) Oerst.]. But this work was conducted at two locations separated by a distance of no more than 70 km, and the question remains as to whether its outcomes are more widely applicable. Do other decay fungi predominate elsewhere where environmental conditions are different? If so, this might influence national estimates of the amounts of carbon released into the atmosphere from decomposing coarse woody debris in indigenous forests. Beets et al. (2008) found that the rate of decrease in wood density due to decomposition was partly dependent on the nature of the main colonising fungi, which may vary in different regions. An opportunity to address this question eventuated for one tree species, Dacrydium cupressinum Lamb. (rimu), at a site 880 km distant in southern Westland in the South Island where a storm had caused damage a decade earlier. Although destructive, it was advantageous that this occurred in dense podocarp forest, a type closely related to that in which the North Island work with this species had been carried out, providing an appropriate basis for comparison (Holloway Citation1954; Franklin Citation1968; Nicholls Citation1974; Newsome Citation1987; Norton et al. Citation1988; Franklin & Nicholls Citation1989; Halkett Citation1991). A study was therefore undertaken to investigate the basidiomycetes prevalent in fallen D. cupressinum stems at the South Island site. This article presents the results of this study, draws comparisons with the results of the earlier work in the central North Island, and considers the wider implications.
Materials and methods
The study was conducted in dense podocarp forest 1.5 km southwest of Hannah's Clearing, adjacent to the inland edge of the coast road between Haast and Jackson Bay. The site selected is located c. 200 m from the shoreline on an even, level, coastal terrace (latitude 43°57′9′′S, longitude 168°50′40′′E). The stand composition is comparable with that used in the central North Island studies except that D. cupressinum is even more predominant as the principal canopy species and there is no subcanopy of Beilschmiedia tawa (A. Cunn.) Kirk (Lauraceae), whose distribution does not extend to the southern South Island (Knowles & Beveridge Citation1982). Following a severe storm which caused extensive windfall in August 2000 (Jane Marshall, DOC, pers. comm.), a dense shrub layer composed of tree ferns and pioneer hardwood species such as Weinmannia racemosa Linn. f. (Cunoniaceae) and Pseudopanax colensoi (Hook. f.) Philipson (Araliaceae) has regenerated in the clearings. This shrub vegetation completely covers the fallen stems (A), in contrast to the earlier central North Island podocarp site where stand gaps are smaller. There, although the larger uprooted trees lie in the open on the forest floor, they are still completely shaded by the tall, residual, canopy trees which have prevented the growth of a dense understorey vegetation. Rainfall at the Hannah's Clearing study site averages around 3200 mm per year, with monthly means ranging between c. 220 and 340 mm, while mean annual temperature is 11.2 °C (monthly means ranging from 7.5 to 15 °C; source, climate data from station Haast Aws, agent number 4097, latitude 43°51′39.6′′S, longitude 169°0′25.2′′E, National Institute of Water and Atmospheric Research). Rainfall is therefore more than double that at the central North Island podocarp forest site, but temperatures are, if anything, very slightly cooler, particularly during summer.
Figure 1 Basidiomycete decay study in a South Island west coast dense lowland rimu forest. A, Study site 1.5 km southwest of Hannah's Clearing 10 years after the destructive storm. B, Fallen rimu study tree with a partially cut sample disc quadrant. C, Portion of extracted sample quadrant showing characteristic decay texture of Ganoderma cf. applanatum over most of the face (stem exterior is at bottom of image); brown zone lines (transected pseudosclerotial plates) delineate boundaries of separate vegetative compatibility groups (vcgs, treated as discrete mycelia) within the decayed wood. D, E, Cultures of basidiomycete decay fungi isolated from study trees paired on 2% malt agar in order to distinguish vcgs (plates are 9 cm in diameter). D, Armillaria novae-zelandiae: an incompatibility separation zone accompanied by a brown line (arrowed) indicates that the isolates in each of these two pairings belong to separate groups (those belonging to a common group merged evenly without a barrier line). E, Ganoderma cf. applanatum: the dense barrier line denotes two groups (individual mycelia) among these three mutually paired cultures.
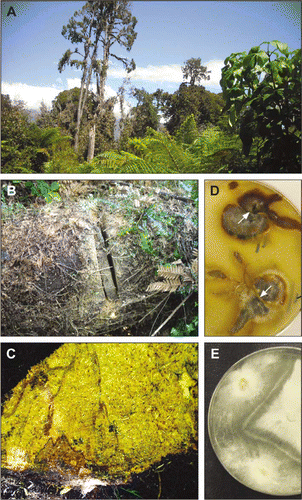
Five uprooted D. cupressinum trees were arbitrarily selected c. 20–200 m in from the road, over an area c. 200 m across. Identity was validated by the nature of the bark (still intact on all trees), the appearance of the wood when exposed by cutting, and by microscopic examination of a wood sample taken from each stem. Stems averaged c. 63 cm diameter breast height (dbh, diameter at 1.4 m above ground level when tree standing upright), with values ranging between 53 and 75 cm. In December 2010, samples were cut from three positions along each available length of exposed stem, the first at breast height and the remainder at arbitrary but measured intervals above original ground level, these being on trees 1–5, respectively: 9, 13 m; 3.5, 6 m; 8, 12 m; 8, 13 m; 6, 10 m. At each position, two elongated, radially orientated blocks were cut as sectors c. 4 cm wide from part of a disc c. 5 cm thick, each sector extending directly in from the external bark surface to the stem centre. Blocks were selected on mutually opposite sides of the stem and aligned along the disc diameter parallel to the ground surface. Sampling was performed by first cutting and extracting an upper disc quadrant on each side of the stem, from which the block was then removed from along the lower edge (Fig. B,C). Sampling (and isolation, see below) was therefore undertaken essentially as depicted in Hood & Gardner (Citation2009). A 15-cm radial depth datum was marked on each block with an indelible pencil at the time of sampling to assist in positioning later isolation points. Each block was sealed individually in a polythene bag and stored chilled, either in a cooler at 4 °C, or during transit in an insulated polystyrene box together with a freeze pack. During sampling, the locations of any fungal fruitbodies were recorded along each stem in relation to sample positions.
Isolation of decay fungi was attempted in the laboratory 13–14 days after field sampling. Each block was split aseptically down the radial longitudinal plane and, as previously described (Hood et al. Citation2008), 20 small chips were cut from the freshly exposed surface at measured intervals along a radial line, six from the outer 0–6.0 cm zone, seven from the 6.1–12.0 cm zone and seven at even intervals from along the radius deeper than 12.0 cm that extended to the stem centre. Chips were plated onto a medium selective for basidiomycetes consisting of 2% malt agar supplemented with 100 ppm streptomycin sulfate and 10 ppm benomyl. In all, 600 isolation attempts were made from the five trees (5 trees×3 discs×2 sectors×20 attempts). Plates were incubated at 20 °C for up to 6 weeks or sometimes longer, and all emergent mycelial colonies were subcultured on tubes of 2% malt agar (apart from four of Armillaria sp. that were identified without subculturing).
Isolates were sorted into groups as outlined earlier (Hood et al. Citation2008). Basidiomycete cultures were recognised as those with clamps or that were clampless but of known identity, and treatment with a-naphthol was used to distinguish any additional laccase positive isolates also indicative of decay fungi (Stalpers Citation1978). Basidiomycete species were identified where possible by monitoring weekly growth of representative cultures on 2% malt agar plates at 20 °C and documenting macro- and microscopic features after 6 weeks (Nobles Citation1965; Stalpers Citation1978; Hood et al. Citation2008). Isolates were matched with stock cultures obtained previously from fruitbodies of confirmed identity, by simultaneous culturing and reference to earlier laboratory description notes. Armillaria species were determined by diploid–haploid pairing to ascertain the compatibility of field isolates with selected single-spore tester cultures obtained from fruitbodies of A. novae-zelandiae (two haploid isolates: A.4.8, A.16.1) and A. limonea (three haploid isolates: A.33.3, A.33.5, A.33.9; Hood & Sandberg Citation1987). Stock diploid isolates (two of A. limonea: NZFS 142, K.21.014; and one of A. novae-zelandiae: L.227.1) were run simultaneously as standards. The identity of Ganoderma isolates was inferred from the associated fruitbodies of this genus present along each stem from which isolates were obtained. Fruitbody identity was determined by comparing with fresh fruitbody collections of G.cf. applanatum and G. australe (Fr.:Fr.) Pat. taken from podocarp–hardwood and Nothofagus forests [N. fusca and/or N. solandri var. cliffortioides (Hook. f.) Oerst.] in the southern South Island during December 2010, paying particular attention to basidiospore size and morphology, which unambiguously distinguish these species (Hasnain et al. Citation1984; Buchanan & Wilkie Citation1995; collections NZFRI 5572M–5589M; Forest Research Institute Mycology Herbarium, Rotorua, New Zealand). The percentage of isolation attempts yielding different fungi was calculated for each ‘unit’, units consisting of each of the three radial depth zones in all sectors. Total number of units was 90 (5 trees×3 discs×2 sectors×3 depth zones). Data were subjected to analysis of variance.
Cultural pairing of a selection of Armillaria and Ganoderma isolates was undertaken to distinguish vegetative compatibility groups (vcgs) and determine their distribution within stems. One pure (axenic) isolate was chosen from each sector of either two or three discs per tree, depending on availability, and selected cultures were paired in all combinations. For Ganoderma, mutual pairing was also conducted with a more comprehensive assortment of isolates from each of two discs. It was noted whether or not a distinct barrier zone formed between cultures after 3–6 weeks. The formation of such a zone denotes incompatibility, whereas an even merging indicates that cultures belong to the same vcg (Fig. D,E).
Results
Isolation from wood samples yielded a range of different bacteria and fungi, the latter including mucoraceous species, hyphomycetes and filamentous yeasts, but basidiomycete species predominated (). The most commonly isolated basidiomycetes were species of Armillaria and Ganoderma, which were identified by cultural pairing with tester isolates or association with naturally occurring fruitbodies, respectively. Among 21 axenic Armillaria isolates from different positions along three trees, 13 were determined positively as A. novae-zelandiae by diploid–haploid pairing, and the remainder were judged to be most likely of the same species (they displayed some signs of transforming an A. novae-zelandiae tester isolate to the diploid form and/or forming a barrier zone with an A. limonea but not A. novae-zelandiae tester isolate). Among all the Armillaria isolates tested (those from the study trees plus the diploid standard cultures), only the two A. limonea standards were compatible with the haploid testers of that species. Ganoderma fruitbodies were present singly or in loose clusters along the stems of trees 1–3 and 5. Five collections taken from these four trees were all of G.cf. applanatum, when examined in the laboratory (NZFRI 5572M–5576M), implying that isolates obtained were also of this species. No fruitbodies of G. australe were seen at the study site. Armillaria fruitbodies were also not seen during sampling (fruiting of Armillaria species occurs predominantly during May and June), but white mycelial fans of Armillaria were noted beneath the bark on tree 5 (disc 1).
Table 1 Means1 of percentage isolation attempts from each sector yielding fungi, by radial depth zone (with 95% confidence limits).
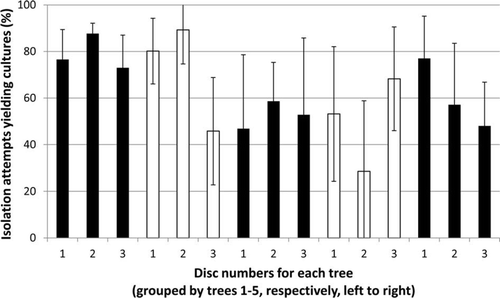
Basidiomycete species were cultured from an average of 63% of isolation attempts (mean of sample unit values) in all radial depth zones (; only one additional laccase positive isolate, indicating a white rot species, was obtained in addition to confirmed basidiomycetes). Ganoderma cf. applanatum was obtained from 30% of isolation attempts (sample unit mean; present in all five trees) and A. novae-zelandiae from 22% of isolation attempts (sample unit mean; also present in all trees; ). Other basidiomycete species recognised, all obtained from < 3% of isolation attempts, were Hypochnicium polonense (Bres.) Å. Strid [synonym, Hyphodermopsis polonensis (Bres.) Jülich; from three trees], Rigidoporus concrescens (Mont.) Rajchenb. [previously referred to R. catervatus (Berk.) Corner; from two trees; associated with a honeycomb pocket rot; Hood et al. Citation1989], and Irpex sp. (from two trees; culturally identical to a species with a white basidiocarp obtained from rimu stems in the central North Island; Hood et al. Citation1989; ). Another species isolated from an average of < 2% of attempts closely resembled R. concrescens both macro- and micromorphologically apart from the presence of single clamps at some septa (present in two trees). Four other unidentified basidiomycete species were cultured at very low frequencies (< 1% of attempts, sample unit means), each from just one tree (). Representative cultures of most basidiomycete species isolated in this study were lodged in the New Zealand Forest Research Institute Culture Collection, Rotorua (NZFS 3566–3577, 3580–3585, 3587–3598).
There was no significant variation (P > 0.05) in frequency of isolation among trees or discs for all decay fungi taken together (), or for G. cf. applanatum and A. novae-zelandiae, separately, except for a marginally significant difference between trees for G. cf. applanatum (P ~ 0.05). There was also no difference in isolation yield between radial depths for all decay fungi as a whole. However, isolation frequency increased significantly with radial depth for G. cf. applanatum (P<0.05), but decreased correspondingly for Armillaria sp. (P < 0.01; ). Outcomes were unchanged after arcsine transformation of data prior to analysis; inclusion, or not, of the single laccase positive isolate additional to confirmed basidiomycetes also did not affect the outcomes from the analyses.
Figure 2 Yields of decay fungi (confirmed basidiomycetes plus additional laccase positive) by disc and tree. Means of six sample units (2 sector blocks×3 depth classes) per disc (error bars: 95% confidence limits).
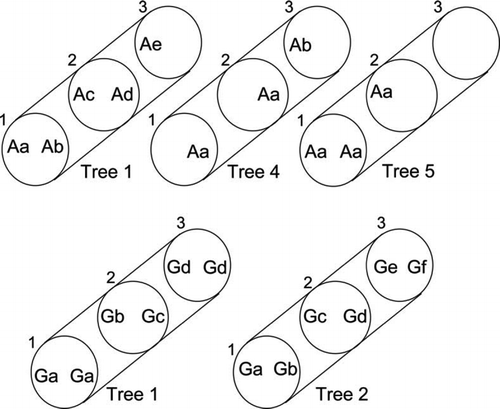
Pairing of selected isolates of A. novae-zelandiae and G. cf. applanatum was undertaken to investigate the distribution of vegetative compatibility groups within fallen stems. A separate vcg of A. novae-zelandiae was present in each sector in tree 1 (). However, one vcg extended between discs 1 and 2 in each of trees 4 and 5, distances of 6.6 and 4.6 m, respectively, along the same side of the stem. No vcgs of G. cf. applanatum were common to more than one disc, among isolates tested, and for four of six discs were unique to each sector in the same disc (Fig. ). However, for discs 1 and 3 in tree 1, one vcg was shared between each pair of sectors. This was investigated more comprehensively, and pairings in all combinations among 22 and 5 isolates from both sectors in disc 1 and 3, respectively (nearly 250 pairings, not counting replicates) were all compatible, confirming the detection of only one vcg in the sectors in each of discs 1 (Ga) and 3 (Gd; Fig. ). Self-pairings of isolates were all compatible.
Figure 3 Diagrammatic representation of selected trees showing distribution of vegetative compatibility groups (vcgs) of Armillaria novae-zelandiae (top row) and Ganoderma cf. applanatum (bottom row) within sector blocks (left and right) from sample discs (numbered 1–3 up stem). Letter symbols denote the vcg of one isolate tested per sector. Within each tree, vcgs coded by different letters are incompatible, as indicated by cultural pairing. Spaces indicate sectors in which A. novae-zelandiae was not present or from which axenic cultures were not obtained.
Discussion
Basidiomycete decay fungi were isolated at comparatively high rates from decomposing wood in fallen rimu stems at the Hannah's Clearing site. The overall isolation frequency was, if anything, slightly greater at this South Island west coast location after 10 years than was obtained in the central North Island trees after 20 years (Hood et al. Citation2004), possibly due to the lesser time needed to fully colonise the medium-sized stems. Certainly, the comparable yields between depth zones and along stems indicates that invasion of the whole stem was complete within one decade at Hannah's Clearing. In the North Island, this situation was reached somewhere between 4 and 20 years after trees were wind thrown (Hood et al. Citation2004), and is likely to have taken a little longer in the larger stems.
However, the species of decay fungi and their proportions within rimu stems were broadly comparable at both locations. At Hannah's Clearing, as in the central North Island, the predominant basidiomycetes were species of Ganoderma and Armillaria. This conclusion seems genuine, because although numbers are small, the study trees were selected arbitrarily before fruitbodies or other fungal signs were noted along each stem, much of which was hidden in the undergrowth. Fruitbodies of G. cf. applanatum were present on most of the study trees associated with a characteristic soft, dry, fibrous, brittle decay, interspersed with brown pseudosclerotial plates, as was also observed in the North Island stems. These fructifications were the basis for identifying Ganoderma isolates to G. cf. applanatum, as in the North Island. Only G. cf. applanatum has been observed fruiting on fallen rimu study trees at both locations, even though collections of G. australe (as well as G. cf. applanatum) were made elsewhere in the southern South Island at the same time, some from standing dead snags or living trees in Nothofagus forests. All Armillaria isolates behaved culturally as belonging to A. novae-zelandiae. This is consistent with the proportionately high incidence of records of this species from the southern South Island. All clearly identified Armillaria collections from this region held in national mycology repositories are of A. novae-zelandiae, apart from one of A. limonea (PDD 57695, New Zealand Fungal Herbarium, Landcare Research New Zealand). The two other New Zealand Armillaria species (Kile & Watling Citation1983; Hood Citation1992; Coetzee et al. Citation2001) have not yet been recorded in this region and are found more often in beech forests, although not exclusively on Nothofagus hosts (NZFRI, PDD records). Cultures of basidiomycetes detected less frequently in this study, such as H. polonense, R. concrescens and Irpex sp., also matched those obtained from fallen rimu stems in the central North Island. Other species found there, such as Sistotrema sp., were not isolated in this study, but the sample size was insufficient to draw any conclusions about the absence of less common basidiomycetes. Cultures were also isolated that resemble those of R. concrescens, but which produce occasional clamps. As with R. concrescens, these isolates appeared to be associated with an incipient honeycomb pocket rot with anastomosing angular brown zone lines visible on the cut surface during the early stages of decomposition. Further study will be needed to determine if these cultures belong to a related species or alternatively may also be of R. concrescens, and whether, if the latter, R. concrescens is correctly placed in a genus which lacks clamps. Cultures resembling R. concrescens both macro- and microscopically, but with clamps, were also isolated from N. menziesii in the central North Island (Hood et al. Citation2008).
The distributions of the predominant basidiomycete species within the fallen rimu stems were similar to those observed in fallen trees in podocarp–hardwood and Nothofagus forests in the earlier central North Island studies (if B. tawa, whose stems were more deeply colonised by Armillaria, is excluded; Hood & Gardner Citation2009). Armillaria novae-zelandiae was again isolated most frequently from the outer 6 cm of these stems, whereas G. cf. applanatum had penetrated right to the centre. Moreover, results from the cultural pairing studies conformed to earlier central North Island patterns even if testing was generally less exhaustive (pairing involved only one isolate per sector for most discs, so that not all vcgs would have been detected). Two vcgs of A. novae-zelandiae had extended more than 6 m and at least 5 m, respectively, in each of two trees, indicative of vegetative mycelial growth along the stem beneath the bark, as observed previously (Hood et al. Citation2004, Citation2008). By contrast, no vcgs of G. cf. applanatum were found in more than one disc, supporting the earlier implication of multiple colonisation by means of basidiospores with more limited vegetative growth (despite the occurrence of only one vcg in both sectors from each of two discs where testing was more comprehensive). With respect to both A. novae-zelandiae and A. limonea, it is noteworthy that the incompatibility zone between two unlike diploid isolates generally takes the form of a brown line whether pairing is within or between species. This behaviour appears to differ from that of some Armillaria species where a brown line is reported only for between-species pairing (Korhonen Citation1978; Anderson et al. Citation1979; Rishbeth Citation1982; Kile Citation1983; Dunne et al. Citation2002).
The conclusions in the previous paragraph partly assume that vcgs (also known as somatic incompatibility groups) represent clones or individual mycelia (previously referred to as ‘colonies’). However, in more recent work by Dodd et al. (Citation2006) molecular differences were found between isolates within the same vcgs of both A. novae-zelandiae and A. limonea. An equivalent situation has been demonstrated, at least to some degree, with certain Armillaria species in the northern hemisphere (Rizzo et al. Citation1995; Guillaumin et al. Citation1996) and also for other basidiomycetes (e.g. Jacobson et al. Citation1993; Stenlid & Vasiliauskas Citation1998). Dodd et al. (Citation2006) worked with archival stock cultures from a former study in modified podocarp–hardwood forest in the Raungaehe Range east of Te Teko in the North Island, where it was shown that for both Armillaria species, isolates belonging to the same vcg tend to be spatially grouped at the landscape scale (Fig. in Hood & Sandberg Citation1987). Therefore, one explanation for multiple genotypes within a vcg may be that the member isolates are partially sib-related as a result of localised dispersal of a proportion of basidiospores from the fruitbodies of one parent shared in common (Rizzo et al. 1995; Guillaumin et al. Citation1996). This interpretation also accounts for the occasional isolation of cultures belonging to the same vcg at locations slightly more dispersed from the main cluster (Hood & Sandberg Citation1993). If this reasoning is correct, it might be expected that in certain exotic pine plantations, where fruiting is less common and evidence indicates that Armillaria infection centres are derived from external basidiospore sources (Hood et al. Citation2002; Power et al. Citation2008), more frequent out-crossing might lead to less molecular variation between isolates belonging to the same vcgs. Alternatively, it is possible that somatic mutation has occurred over time (Guillaumin et al. Citation1996), either within expanding mycelia of the same vcg in the field, or among stock cultures while stored under oil, although this rationale conflicts with an earlier assumption that mycelia of A. novae-zelandiae and A. limonea are genetically stable, at least in the short-term (Hood & Sandberg Citation1993). Whichever explanation is correct, it seems reasonable to assume that isolates of the same vcg taken from a mycelium growing vegetatively along a fallen stem will all be of the same genotype, although this remains to be tested at this level for A. novae-zelandiae and A. limonea. The degree to which vcgs of G. cf. applanatum may be clonal has also not been explored by molecular means.
The comprehensive nature of these studies with wood-decomposing basidiomycetes in North and South Island indigenous forests necessitated a large number of isolation attempts in order to understand fungal distributions satisfactorily. Despite this effort, the sheer size of these stems means that less common species with localised mycelia are still likely to have eluded sampling, and in addition, the selective isolation medium would have excluded any ascomycete decay fungi present (Hood & Gardner Citation2009). However, this work may progress more readily using molecular techniques by constructing primers from isolates or directly from authenticated fruitbodies. This procedure could enable rapid and efficient sampling of stems leading to a more detailed picture of internal fungal distribution, by circumventing the intermediate isolation step, even if this approach would not distinguish active from any residual mycelia that are no longer alive. Rapid molecular identification of specific basidiomycetes is already a reality in parts of the northern hemisphere (Johannesson & Stenlid Citation1999; Guglielmo et al. Citation2007).
In this South Island study, as in previous work, fruitbodies of G. cf. applanatum were generally present on those lengths of the stems from which cultures of this species were isolated. However, sampling at Hannah's Clearing was not extensive enough to confirm this association statistically, as was done elsewhere. The numerous collections in national mycology herbaria, together with other data, show that Ganoderma and Armillaria species are common and widely distributed throughout New Zealand. It is therefore reasonable to assume that the pattern of results from these studies at two widely separated locations is broadly applicable to rimu and probably other indigenous tree species (Hood et al. Citation2004, Citation2008; Hood & Gardner Citation2009) over a much more extensive area, in lowland podocarp–hardwood forests, if not elsewhere. Stems colonised by G. cf. applanatum were previously found to have decomposed and decreased in density more rapidly than those occupied by other decay fungi (Beets et al. Citation2008). Hence, it is likely that this finding is also more generally applicable to fallen trees in indigenous forests throughout a large part of New Zealand.
Acknowledgements
Help with isolation and subculturing was provided by Rachel Hood, other laboratory or technical assistance was given by Judy Gardner, John Smith, Pam Smith and Rita Tetenburg (Scion), and Lloyd Donaldson (Scion) identified wood samples from the study trees. Thanks, also, to Joy Hood for field support. Entry to the study site was facilitated through Jane Marshall (DOC, Hokitika) and Andrew Harper (NIWA) enabled access to climate data. The manuscript was improved by useful suggestions from two anonymous referees and partial funding was provided by the former New Zealand Foundation for Research, Science and Technology.
References
- Anderson , JB , Ullrich , RC , Roth , LF and Filip , GM . 1979 . Genetic identification of clones of Armillaria mellea in coniferous forests in Washington . Phytopathology , 69 : 1109 – 1111 .
- Beets , PN , Hood , IA , Kimberley , MO , Oliver , GR , Pearce , SH and Gardner , JF . 2008 . Coarse woody debris decay rates for seven indigenous tree species in the central North Island of New Zealand . Forest Ecology and Management , 256 : 548 – 557 .
- Buchanan PK , Wilkie JP 1995 . Taxonomy of New Zealand Ganoderma – two non-laccate species . In: Buchanan PK , Hseu RS , Moncalvo JM Ganoderma: systematics, phytopathology and pharmacology . Proceedings of Contributed Symposium 59A,B, Fifth International Mycological Congress, Vancouver, Canada 14–21 August 1994 . National Taiwan University , Taipei . Pp. 7 – 17 .
- Coetzee , MPA , Wingfield , BD , Bloomer , P , Ridley , GS , Kile , GA and Wingfield , MJ . 2001 . Phylogenetic relationships of Australian and New Zealand Armillaria species . Mycologia , 93 : 887 – 896 .
- Dodd SL , Shah FA , Hood IA 2006 . Molecular tools to determine the mode of spread of the root pathogen Armillaria in commercial pine forests . Poster presentation, American Phytopathological Society, Canadian Phytopathological Society, Mycological Society of America Joint Meeting , Quebec City , Canada 29 July–2 August 2006 . http://lib.crop.cri.nz/scripts/public/Files/DoddS-21420.pdf (accessed 5 November 2011). Phytopathology 96 (6: Supplement): S30 .
- Dunne , CP , Glen , M , Tommerup , IC , Shearer , BL and Hardy , GEStJ . 2002 . Sequence variation in the rDNA ITS of Australian Armillaria species and intra-specific variation in A. luteobubalina . Australasian Plant Pathology , 31 : 241 – 251 .
- Franklin , DA . 1968 . Biological flora of New Zealand 3. Dacrydium cupressinum Lamb. (Podocarpaceae) rimu . New Zealand Journal of Botany , 6 : 493 – 513 .
- Franklin DA , Nicholls JL 1989 . Haast forest class map. Mapping Series 6, Sheet No. 22, 4 p . with 1 map sheet . Forest Research Institute, New Zealand Forest Service . Wellington , A.R. Shearer, Government Printer .
- Guglielmo , F , Bergemann , SE , Gonthier , P , Nicolotti , G and Garbelotto , M . 2007 . A multiplex PCR-based method for the detection and early identification of wood rotting fungi in standing trees . Journal of Applied Microbiology , 103 : 1490 – 1507 .
- Guillaumin , J-J , Anderson , JB , Legrand , P , Ghahari , S and Berthelay , S . 1996 . A comparison of different methods for the identification of genets of Armillaria spp . New Phytologist , 133 : 333 – 343 .
- Halkett J 1991 . The native forests of New Zealand . Wellington , GP Publications Ltd . 149 p .
- Hasnain , SM , Newhook , FJ , Wilson , JD and Corbin , JB . 1984 . First report of Ganoderma allergenicity in New Zealand . New Zealand Journal of Science , 27 : 261 – 267 .
- Holloway , JT . 1954 . Forests and climates in the South Island of New Zealand . Transactions of the Royal Society of New Zealand , 82 : 329 – 410 .
- Hood IA 1992 . An illustrated guide to fungi on wood in New Zealand . Auckland University Press in association with the New Zealand Forest Research Institute . 424 p .
- Hood , IA and Gardner , JF . 2009 . Fungi decaying stems of fallen tawa (Beilschmiedia tawa) trees in the central North Island of New Zealand . New Zealand Journal of Botany , 47 : 115 – 119 .
- Hood , IA and Sandberg , CJ . 1987 . Occurrence of Armillaria rhizomorph populations in the soil beneath indigenous forests in the Bay of Plenty, New Zealand . New Zealand Journal of Forestry Science , 17 : 83 – 99 .
- Hood , IA and Sandberg , CJ . 1993 . Armillaria populations in a Pinus radiata plantation on a former indigenous rainforest site . New Zealand Journal of Forestry Science , 23 : 62 – 77 .
- Hood , IA , Sandberg , CJ and Kimberley , MO . 1989 . A decay study of windthrown indigenous trees . New Zealand Journal of Botany , 27 : 281 – 297 .
- Hood , IA , Horner , IJ , Gardner , JF and Sandberg , CJ . 2002 . Armillaria root disease of Pinus radiata in New Zealand: 1. Basidiospore dispersal . New Zealand Journal of Forestry Science , 32 : 94 – 102 .
- Hood , IA , Beets , PN , Kimberley , MO , Gardner , JF , Oliver , GR and Pearce , S . 2004 . Colonisation of podocarp coarse woody debris by decomposer basidiomycete fungi in an indigenous forest in the central North Island of New Zealand . Forest Ecology and Management , 196 : 311 – 325 .
- Hood , IA , Beets , PN , Gardner , JF , Kimberley , MO , Power , MWP and Ramsfield , TD . 2008 . Basidiomycete decay fungi within stems of Nothofagus windfalls in a Southern Hemisphere beech forest . Canadian Journal of Forest Research , 38 : 1897 – 1910 .
- Jacobson , KM , Miller , OK Jr and Turner , BJ . 1993 . Randomly amplified polymorphic DNA markers are superior to somatic incompatibility tests for discriminating genotypes in natural populations of the ectomycorrhizal fungus Suillus granulatus . Proceedings of the National Academy of Sciences of the United States of America , 90 : 9159 – 9163 .
- Johannesson , H and Stenlid , J . 1999 . Molecular identification of wood-inhabiting fungi in an unmanaged Picea abies forest in Sweden . Forest Ecology and Management , 115 : 203 – 211 .
- Kile , GA . 1983 . Identification of genotypes and the clonal development of Armillaria luteobubalina Watling & Kile in eucalypt forests . Australian Journal of Botany , 31 : 657 – 671 .
- Kile , GA and Watling , R . 1983 . Armillaria species from south-eastern Australia . Transactions of the British Mycological Society , 81 : 129 – 140 .
- Knowles , B and Beveridge , AE . 1982 . Biological flora of New Zealand 9. Beilschmiedia tawa (A. Cunn.) Benth. et Hook. f. ex Kirk (Lauraceae) tawa . New Zealand Journal of Botany , 20 : 37 – 54 .
- Korhonen , K . 1978 . Interfertility and clonal size in the Armillariella mellea complex . Karstenia , 18 : 31 – 42 .
- Newsome PFJ 1987 . The vegetation cover of New Zealand. Water and Soil Miscellaneous Publication 112 . Wellington North, National Water and Soil Conservation Authority. Water and Soil Directorate, Ministry of Works and Development . 153 p . with 2 map sheets .
- Nicholls JL 1974 . Urewera forest class map. Mapping Series 6, Sheet No. 7. Forest Research Institute, New Zealand Forest Service . Wellington, A.R. Shearer, Government Printer. 4 p. with 1 map sheet .
- Nobles , MK . 1965 . Identification of cultures of wood-inhabiting Hymenomycetes . Canadian Journal of Botany , 43 : 1097 – 1139 .
- Norton , DA , Herbert , JW and Beveridge , AE . 1988 . The ecology of Dacrydium cupressinum: a review . New Zealand Journal of Botany , 26 : 37 – 62 .
- Power , MWP , Ramsfield , TD and Hood , IA . 2008 . Detection of Armillaria basidiospore dispersal . New Zealand Plant Protection , 61 : 35 – 40 .
- Rishbeth , J . 1982 . Species of Armillaria in southern England . Plant Pathology , 31 : 9 – 17 .
- Rizzo , DM , Blanchette , RA and May , G . 1995 . Distribution of Armillaria ostoyae genets in a Pinus resinosa–Pinus banksiana forest . Canadian Journal of Botany , 73 : 776 – 787 .
- Stalpers , JA . 1978 . Identification of wood-inhabiting Aphyllophorales in pure culture. Centraalbureau voor Schimmelcultures . Studies in Mycology , 16 : 1 – 248 .
- Stenlid , J and Vasiliauskas , R . 1998 . Genetic diversity within and among vegetative compatibility groups of Stereum sanguinolentum determined by arbitrary primed PCR . Molecular Ecology , 7 : 1265 – 1274 .