Abstract
The status of Schizaea australis and S. fistulosa in New Zealand has been uncertain. The results of a morphological analysis of 66 herbarium collections assigned to S. australis or S. fistulosa are presented here to show that two separate species are found in New Zealand, and that hybrids may occur in a few places where their distributions overlap. Lectotypes are also chosen for four basionyms relevant to New Zealand representatives of the Schizaeales—Lygodium articulatum A.Rich., L. gracilescens Colenso, S. australis Gaudich. and S. fistulosa Labill. This article is a contribution towards clarifying the taxonomic and nomenclatural status of New Zealand plants for the plant names database (Ngā Tipu Aotearoa) and the electronic Flora of New Zealand.
Introduction
The order Schizaeales includes Anemiaceae, Lygodiaceae and Schizaeaceae (Smith et al. Citation2006), of which only the latter two families are represented in New Zealand. These have been treated for the electronic Flora of New Zealand (Brownsey & Perrie Citation2012a, Citationb).Footnote Lectotypes are required for four basionyms in these two families and are selected here for Lygodium articulatum A.Rich. and L. gracilescens Colenso (Lygodiaceae), and Schizaea australis Gaudich. and S. fistulosa Labill. (Schizaeaceae).
The fern family Schizaeaceae includes two genera, Actinostachys and Schizaea, and c. 30 species widely distributed in tropical and south temperate regions, with one extending also to North America (Smith et al. Citation2006). In New Zealand the family is represented by four species of Schizaea—S. australis Gaudich., S. bifida Willd., S. dichotoma (L.) Sm. and S. fistulosa Labill. (Brownsey et al. Citation1985; Brownsey & Smith-Dodsworth Citation2000). Of these, S. dichotoma and S. bifida are quite distinctive and usually easily recognized by the dichotomously branching sterile portions of their fronds. Diminutive forms of S. bifida with unbranched sterile portions of the frond are sometimes confused with S. fistulosa, but S. bifida can be distinguished by its usually scabrid rather than smooth sterile portions of the frond, by having longer fertile branches (3–13 mm cf. 2–5 mm long) and by the presence of hairs amongst the sporangia (Brownsey & Perrie Citation2012b).
The status of the other two species, S. australis and S. fistulosa, is more contentious. Earlier authors either recognized only S. fistulosa in New Zealand (e.g., Allan Citation1961), or at best treated S. australis as a variety of S. fistulosa (e.g., Hooker Citation1867; Cheeseman Citation1925). In Australia, Chinnock (Citation1998) recognized only S. fistulosa and concluded that ‘a detailed study of the S. fistulosa complex in Australasia and elsewhere is desirable before a subdivision is attempted’.
Lash (Citation1966) carried out a morphological and cytological investigation of the S. fistulosa complex for an MSc thesis. He obtained chromosome counts of n=94 from populations at Rockville in northwest Nelson, Charleston, Denniston, Stockton, Westport and Woodpecker Bay, Nelson, supporting the earlier finding of n=94 in plants identified as S. fistulosa var. australis from the upper Waimakariri (Brownlie Citation1965). He also found n=c. 150 in six plants from populations at Rockville, Tākaka and Westport (see Dawson et al. Citation2000). He found no evidence to support the earlier count of n=270 in S. fistulosa (Brownlie Citation1965) which he suggested was inadvertently made from a tapetal cell. His counts of n=150 were generally from larger plants, but he concluded that the cytological discontinuity could not be related to any clear morphological continuity, and therefore he did not support the recognition of two separate taxa. Subsequently, Brownsey (in Brownsey et al. Citation1985; Dawson et al. Citation2000, ) obtained a count of n=190 in a plant of S. fistulosa from Waikumete Cemetery, Auckland. This suggested that two distinct species were present in New Zealand—S. australis with n=94 and S. fistulosa with n=190, probably reflecting diploid and tetraploid levels of ploidy. It also raised the possibility that the plants found by Lash with n=c. 150 were hybrids between the two species, an idea supported by examination of Fig. 17 in Lash's thesis which appeared to show at least some univalents (Brownsey et al. Citation1985).
Based on this evidence Brownsey & Smith-Dodsworth (Citation2000) recognized two distinct species in New Zealand. Schizaea fistulosa was said to be ‘common from North Cape to the Waikato, occurring also in north-west Nelson and on the Chatham Islands’ growing ‘in lowland areas on poor clay soils, either in the open, under light scrub or in open kauri forest’. However, S. australis was ‘local in mountain districts of the North Island from Mt Moehau southwards; confined largely to areas west of the main divide in the South Island; subalpine down to lowland areas in the far south, favouring boggy ground in short grassland or turf vegetation’. However, there is a substantial overlap in the range of morphological variation exhibited by the two species with no clear differentiation between them ().
Figure 1 Twelve different specimens of the S. australis/fistulosa aggregate collected between Rabbit Gully and Appos Flat, Rockville, Nelson, by Ian Lash, 12 May 1964 (CHR 343596). Three of the specimens are included in this morphological analysis (fourth from the left, S. australis; fifth from the right, putative hybrid; third from right, S. fistulosa). Copyright Allan Herbarium (CHR), Landcare Research, New Zealand.
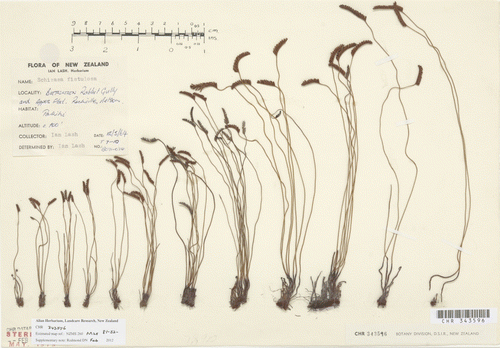
In preparing the treatment of Schizaeaceae for the electronic Flora of New Zealand (Brownsey & Perrie Citation2012b), these two species were examined in more detail. The results presented here show that two species are present in New Zealand, and that hybrids may occur in places where their distributions overlap.
Materials and methods
All material of Schizaea in AK, CHR and WELT was examined. Type material in K and P, and online images of material in B, FI and MEL, were also examined. Herbarium abbreviations follow Thiers (Citation2012).
Schizaea australis and S. fistulosa have relatively simple frond morphology with few good characters for discriminating the two species. Previous treatments (e.g., Brownsey & Smith-Dodsworth Citation2000) have used the lengths of the sterile and fertile portions of the frond to distinguish the two species. In addition, the stomata form long vertical lines on the sterile portions of the frond of these two species, and the guard cells were measured as a predictor of ploidy, since cell size generally increases with ploidy level (Barrington et al. Citation1986).
Mean lengths of stomatal guard cells were calculated for 63 collections of S. australis, S. fistulosa and putative hybrids, from throughout their distributions but with an emphasis on areas where they are believed to overlap. Measurements were made on dried herbarium specimens. Five stomata from one frond in each collection were measured under the highest power (×63) of an Olympus SZ60 dissecting microscope. This is a minimal number based on what can be accurately measured on normal herbarium specimens. Because the stems tend to twist when they dry, not all stomata are perfectly aligned to allow precise measurement when viewed under high magnification. Measurements were also made of the length of the sterile portion of the frond (from the junction with the rhizome to the junction with the fertile portion of the frond), and the length of the fertile portion of the frond (referred to here as sterile lamina length and fertile lamina length respectively). Measurements were taken from the longest frond on each sheet. A list of the specimens examined for morphological analysis is available as supplementary material.
An attempt was made to also measure the sizes of spores, but Schizaea plants shed their spores very quickly and most herbarium collections examined contained insufficient spores to make reliable measurements. Adequate numbers of spores were obtained from just 9 of the 66 collections examined, of which four were S. fistulosa and five were S. australis. The length and width of 20 spores were measured for each collection using the×40 objective of a compound microscope. Specimens examined are indicated in the supplementary material.
Results
The ranges of variation observed in lengths of the guard cells, and sterile and fertile laminae for 63 collections of S. australis, S. fistulosa and putative hybrids are given in . A clear difference in guard cell length was recorded between the two species, but there is significant overlap in the ranges of the sterile and fertile lamina lengths.
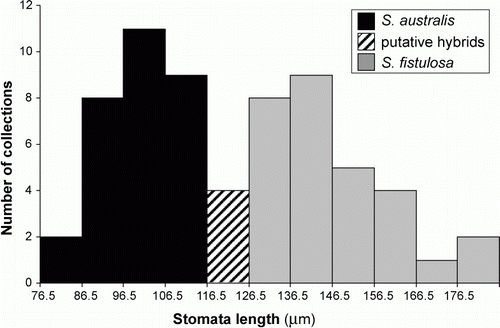
Table 1 Summary of the range of variation in guard cell length, sterile lamina length and fertile lamina length in 63 collections of S. australis, S. fistulosa and their putative hybrids.
The mean length of the guard cells ranged from 127–180 µm in 30 collections of S. fistulosa, from 81–114 µm in 29 collections of S. australis, and from 117–126 µm in 4 collections of intermediate plants (), indicating a clear difference between the two species. When plotted as a bar graph (), the range of variation in guard cell length is bimodal corresponding to different sizes in S. australis and S. fistulosa. The longer guard cells in S. fistulosa are presumed to be a reflection of its higher level of ploidy. The small number of collections with guard cells of intermediate length corresponds to collections with intermediate sterile and fertile lamina lengths and which are of possible hybrid origin.
Figure 2 Variation in guard cell length for 63 collections of S. australis, S. fistulosa and their putative hybrids.
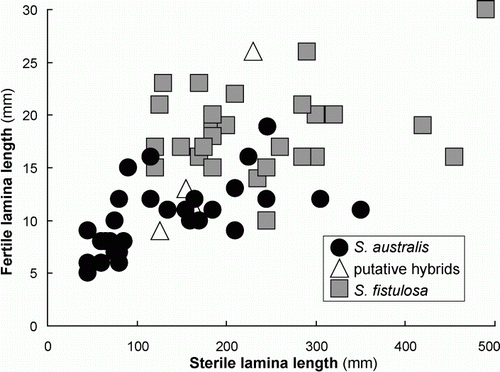
When guard cell length is plotted against length of the fertile lamina () there is a clear separation of the two species. Good separation of the two species is also evident when guard cell length is plotted against length of the sterile lamina (). When length of the fertile lamina is plotted against length of the sterile lamina (), separation of the two species is less clear. However, there is a comparatively small zone of overlap and most collections could, in fact, be distinguished on this basis.
Figure 3 Fertile lamina length and guard cell length for 63 collections of S. australis, S. fistulosa and their putative hybrids.
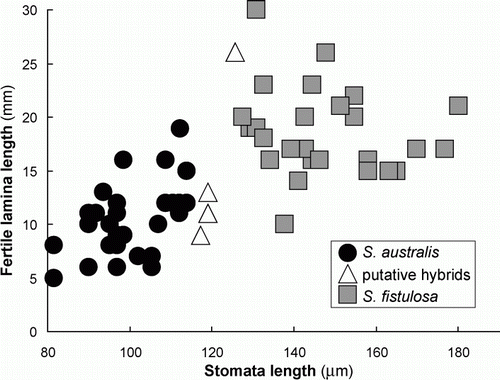
Figure 4 Sterile lamina length and guard cell length for 63 collections of S. australis, S. fistulosa and their putative hybrids.
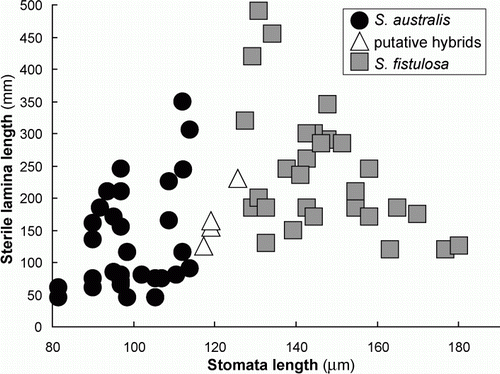
Figure 5 Fertile and sterile lamina lengths for 63 collections of S. australis, S. fistulosa and their putative hybrids.
The ranges of variation observed in spore length and width for nine collections of S. australis and S. fistulosa are given in . A clear difference in spore length and width was recorded between the two species for this limited number of samples. No unequivocal evidence of spore abortion was detected. However, distinguishing spores which are clearly aborted from the often poorly developed remnants of normal spores in sporangia from which most of the spores have been lost is an almost impossible task in this group of ferns.
Table 2 Summary of the range of variation in spore length and spore width in nine collections of S. australis and S. fistulosa.
Discussion
From these results it is clear that guard cell length is the best individual morphological character for discriminating S. australis and S. fistulosa. The longer guard cell length in S. fistulosa strongly suggests a difference in ploidy between the two species, reinforcing the cytological evidence which has previously been presented.
Spore size, albeit from only nine collections, also supports the cytological evidence, with larger spores evident in the tetraploid S. fistulosa.
Of the other two characters measured, the fertile lamina length is a much more reliable character than sterile lamina length, and most (but not all) collections can be identified on this character alone (Figs 3, 5). This is almost certainly because the very short fertile laminae are much less influenced by environmental conditions than the longer sterile laminae. In both species, the sterile laminae are likely to grow much longer in sheltered, lowland habitats amongst other vegetation than when they are in more exposed habitats. Hence there is a much greater overlap of size range in this character than for the fertile lamina length. The overlap is greatest in lowland habitats and is most evident in northwest Nelson and the west coast of the South Island where populations of S. australis, which mostly occur above 300 m elsewhere, descend almost to the coast on pakihi soils. Here, they grow much taller than at higher elevations and are difficult to distinguish from S. fistulosa.
Identifying hybrids by morphology is even more difficult and may only be possible if aborted spores can be detected. Morphological evidence from Figs 1–4 is equivocal but suggests that hybrids may occur in areas where the species overlap, thus reinforcing the earlier cytological evidence. Perhaps, fortunately, there are relatively few areas of geographic overlap (see distribution maps in Brownsey & Perrie Citation2012b). In the North Island, S. australis grows only above c. 450 m, and S. fistulosa generally occurs at much lower elevations. Only on Mt Hobson, Great Barrier Island, and Mt Moehau, northern Coromandel, are the two species known to overlap, and here morphological intermediates provide some evidence for hybridization. In the South Island, S. fistulosa only occurs along the northern coastline, but there is a substantial area of overlap in northwest Nelson where S. australis descends to lowland pakihi soils. Evidence for hybridization is greatest in this region, with a continuum of morphological variation, making accurate identification of individual collections extremely difficult.
Schizaea australis has been recorded from the Chatham Islands (de Lange et al. Citation2011) but the cited specimen (AK 888) has a mean stomatal length of 158 µm, and sterile and fertile lamina lengths that fall well within the range of S. fistulosa. No other specimens were seen that fell within the range of S. australis, and it seems likely that only S. fistulosa occurs on the Chatham Islands.
Outside New Zealand, S. australis occurs in southern Chile, the Falkland Islands, probably Tasmania and possibly southeast Australia. Its occurrence in New Zealand at increasingly higher altitudes northwards to Great Barrier Island is consistent with this distribution. By contrast, S. fistulosa occurs in Peninsular Malaysia, Borneo, New Guinea, southern Australia, New Caledonia, Fiji, the Society Islands and the Marquesas Islands. Its occurrence in the northern North Island and northwest Nelson is consistent with this distribution. The ranges of the two species in southeast Australia and Tasmania require further investigation.
The type of S. fistulosa is from Tasmania and an online image has been examined. It fits the range of variation observed in the species in New Zealand. The type of S. australis is from the Falkland Islands, and it, too, matches the range of variation seen in montane populations of this species in New Zealand. Lectotypes for these names, and two basionyms in Lygodium, are selected below.
Lectotypification
Lectotypes are designated here for the following basionyms:
Schizaea australis Gaudich., Ann. Sci. Nat. (Paris) 5: 98 (1825)
LECTOTYPE (chosen here): Iles Malouines [Falkland Islands], C. Gaudichaud s.n., P 00523210, http://dsiphoto.mnhn.fr/sonnerat/LAPI/scanH/H20080227/P00523210.jpg
NOTES: Schizaea australis was described by Gaudichaud-Beaupré (Citation1825) in a paper entitled ‘Rapport sur la Flore des îles Malouines’. No other collection data is provided, but presumably the plant was collected by Gaudichaud when the French expedition of 1817–1820 in the Uranie and Physicienne, commanded by Louis de Freycinet, was stranded in the Falkland Islands. All Gaudichaud's collections are in P (Stafleu & Cowan Citation1976), although duplicates are now in several other European herbaria.
Collections of S. australis from Iles Malouines are in P (00523210) and B (20 0142845) but, since Gaudichaud's main herbarium is at P, the specimen there is the best choice of lectotype. It consists of two plants with fronds c. 60 mm in length and with fertile portions 4–5 mm long. They are at the lower end of the size range for the species in New Zealand (Brownsey & Perrie Citation2012b), but their size and general appearance match specimens from the Auckland Islands and Campbell Island.
Schizaea fistulosa Labill., Nov. Holl. Pl. 2: 103, t. 250, f. 3 (1807)
LECTOTYPE (chosen here): Nova Hollandia et Terra Diemen [Australia and Tasmania], Labillardière s.n., FI 219617 – 2 plants at left of sheet (image seen).
NOTES: Labillardière (Citation1807) stated that he collected his material of S. fistulosa ‘in capite Van-Diemen’, identified by Stafleu (Citation1966) as Storm Bay, Tasmania. There are collections by Labillardière labelled ‘Van-Diemen’ in FI (219617) and P (00523213). Labillardière's main herbarium is at FI and the material there has the best claim to be selected as lectotype. It consists of two plants with fronds up to 190 mm long, and fertile portions up to 18 mm long. These dimensions are well within the range of variation seen in New Zealand material of S. fistulosa. The length of the fertile portion is slightly longer than even the longest known New Zealand specimens of S. australis, suggesting that Labillardière's collection does not belong to that species.
Lygodium articulatum A.Rich., Essai Fl. Nouv.-Zél. 96, t. 15 (1832)
LECTOTYPE (chosen here): Baie des Iles, Nouvelle Zélande [Bay of Islands, Northland, New Zealand], Astrolabe, Herb. A. Richard in Herb. E. Drake, P00523227! (photo WELT E475/12), http://dsiphoto.mnhn.fr/sonnerat/LAPI/scanH/H20080228/P00523227.jpg
NOTES: Lygodium articulatum was described by Richard (Citation1832) from material collected in New Zealand during the voyage of the Astrolabe from 1826–1829, commanded by Dumont D'Urville. Richard was not on the expedition, but worked on material that was brought back to P. Two specimens from Richard's herbarium are extant (P 00523227 and P 00523228). The specimen labelled “Baie des Iles”, which is larger and has more complete collection data, is selected here as the lectotype.
Lygodium gracilescens Colenso, Trans & Proc. New Zealand Inst. 28: 620 (Citation1896)= Lygodium articulatum A.Rich., Essai Fl. Nouv.-Zél. 96, t. 15 (1832)
LECTOTYPE (chosen here): no locality, Colenso s.n., WELT P003360! http://collections.tepapa.govt.nz/objectdetails.aspx?oid=274853
NOTES: Lygodium gracilescens was described by Colenso (Citation1896) from specimens he collected in the ‘country between lower Waikato (N.), head of Thames, and Kaipara; 1843–4’. There are specimens in K and WELT labelled ‘Lygodium gracilescens Col.’ in Colenso's own hand, but neither specimen has any additional collection data. Both specimens are scrappy, comprising just a few fertile and sterile pinnae. The specimen in WELT is slightly more complete, with a length of the rachis present, and is selected here as lectotype. Neither specimen differs in any way from material of L. articulatum which is the only species currently recognized in New Zealand.
Supplementary data
Supplementary file: Collection data and morphological measurements for collections of Schizaea australis, S. fistulosa and their putative hybrids.
Collection data and morphological measurements for collections of <i>Schizaea australis</i>, <i>S. fistulosa</i> and their putative hybrids
Download PDF (64.7 KB)Acknowledgements
This research was supported by Core funding for Crown Research Institutes from the Ministry of Business, Innovation and Employment's Science and Innovation Group.
Notes
Supplementary data available online at www.tandfonline.com/10.1080/0028825X.2012.741066
Supplementary file: Collection data and morphological measurements for collections of Schizaea australis, S. fistulosa and their putative hybrids.
References
- Allan HH 1961 . Flora of New Zealand . Vol. 1 Indigenous Tracheophyta. Psilopsida, Lycopsida, Filicopsida, Gymnospermae, Dicotyledones . Wellington , Government Printer .
- Barrington , DS , Paris , CA and Ranker , TA . 1986 . Systematic inferences from spore and stomate size in the ferns . American Fern Journal , 76 : 149 – 159 . doi: 10.2307/1547723
- Brownlie , G . 1965 . Chromosome numbers in some Pacific Pteridophyta . Pacific Science , 19 : 493 – 497 .
- Brownsey , PJ , Given , DR and Lovis , JD . 1985 . A revised classification of New Zealand pteridophytes with a synonymic checklist of species . New Zealand Journal of Botany , 23 : 431 – 489 . doi: 10.1080/0028825X.1985.10425348
- Brownsey PJ , Perrie LR 2012a . Lygodiaceae . In Breitwieser I , Brownsey PJ , Ford K , Glenny D , Heenan PB , Nelson WA , Wilton A Flora of New Zealand , online edition. http://www.nzflora.info (accessed 20 November 2012) .
- Brownsey PJ , Perrie LR 2012b . Schizaeaceae . In Breitwieser I , Brownsey PJ , Ford K , Glenny D , Heenan PB , Nelson WA , Wilton A Flora of New Zealand , online edition. http://www.nzflora.info (accessed 20 November 2012) .
- Brownsey , PJ and Smith-Dodsworth , JC . 2000 . New Zealand ferns and allied plants , 2nd edition , Auckland : David Bateman .
- Cheeseman , TF . 1925 . Manual of the New Zealand flora , 2nd edition , Wellington : Government Printer .
- Chinnock , RJ . 1998 . Schizaeaceae . Flora of Australia , 48 : 177 – 183 .
- Colenso , W . 1896 . A description of three ferns, believed to be undescribed, discovered more than fifty years ago in the northern district of New Zealand . Transactions and Proceedings of the New Zealand Institute , 28 : 618 – 622 .
- Dawson , MI , Brownsey , PJ and Lovis , JD . 2000 . Index of chromosome numbers of indigenous New Zealand pteridophytes . New Zealand Journal of Botany , 38 : 25 – 46 . doi: 10.1080/0028825X.2000.9512672
- de Lange PJ , Heenan PB , Rolfe JR 2011 . Checklist of vascular plants recorded from Chatham Islands . Wellington , Department of Conservation, Hawke's Bay Conservancy .
- Gaudichaud-Beaupré C 1825 . Rapport sur la Flore des îles Malouines . Annales des Sciences Naturelles (Paris) 5 : 89 – 110 .
- Hooker , JD . 1867 . Handbook of the New Zealand flora , London : Reeve .
- Labillardière JJH de 1807 . Novae Hollandiae Plantarum Specimen , Vol. 2 . Paris , Huzard .
- Lash I 1966 . Studies in Schizaea fistulosa Labill . Unpublished MSc thesis , University of Canterbury , Christchurch .
- Richard A 1832 . Essai d'une flore de la Nouvelle Zélande . In: Dumont d'Urville J, Voyage de découvertes de l'Astrolabe. Botanique. Paris, Tastu .
- Smith , AR , Pryer , KM , Schuettpelz , E , Korall , P , Schneider , H and Wolf , PG . 2006 . A classification for extant ferns . Taxon , 55 : 705 – 731 . doi: 10.2307/25065646
- Stafleu FA 1966 . Labillardière, ‘botaniste-voyageur’ . In Labillardière JJ , Novae Hollandiae plantarum specimen , facsimile edition. Lehre, Cramer .
- Stafleu , FA and Cowan , RS . 1976 . Taxonomic literature, Vol. 1: A–G . Regnum Vegetabile , 94 : 1 – 1136 .
- Thiers B 2012 . Index Herbariorum: a global directory of public herbaria and associated staff . New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/ (accessed 1 July 2012) .