Abstract
Hawkweed (Hieracium species) invasion in the tussock grasslands of South Island, New Zealand, has been well documented. Distribution of these species ranges from montane to alpine grasslands, from Hawkes Bay to Southland. This study quantifies the distribution of three Hieracium species in a high country tall (snow) tussock grassland along the Upper Cass River. Hieracium pilosella and Hieracium praealtum are widespread throughout the study site, whereas Hieracium lepidulum is rare. Major factors influencing the abundance of H. pilosella and H. praealtum are altitude and dense vegetation cover such as tall tussocks, large herbs and sub-shrubs. Both H. pilosella and H. praealtum are present at high altitude,>1800 m above sea level. Differences in the invasion stages of Hieracium species are evident. Mean abundance of H. pilosella ranged from 0.5% cover at altitudes > 1800 m above sea level, to 60% cover at altitudes < 1200 m above sea level. Hieracium praealtum averaged 0.5% cover at all altitudes.
Introduction
Hawkweeds (Hieracium species: Asteraceae) are environmental weeds with serious impacts on biodiversity and agriculture in New Zealand indigenous grasslands as they displace native species and reduce pasture quality (Espie et al. Citation2001).Footnote Hieracium pilosella (also known as Pilosella officinarum)Footnote1 is an aggressive rosette plant that has been widely recorded invading native vegetation, particularly in the lower-rainfall mountains and basins of the eastern South Island (Treskonova Citation1991; Duncan et al. Citation1997; Meurk et al. Citation2002; Rose et al. Citation2004; Day & Buckley Citation2007; Mark et al. Citation2011). Other Hieracium species have also been documented invading eastern South Island grasslands including Hieracium praealtum (Pilosella piloselloides subsp. praealta) and Hieracium lepidulum (Wiser & Allen Citation2000; Mark & Dickinson Citation2003; Day & Buckley Citation2007, Citation2011; Radford et al. Citation2010; Mark et al. Citation2011). Hieracium species invasion, especially by H. pilosella, has resulted in widespread and dramatic changes in the composition and structure of lower-elevation grasslands to the extent that many areas that were previously dominated by native short tussocks (especially Festuca novae-zelandiae) are now H. pilosella-dominated herbfields (Scott et al. Citation1990; Duncan et al. Citation1997; Rose et al. Citation1998; Norton et al. Citation2006). The reason for the invasion of Hieracium species is a matter of some debate, although a series of ecological and environmental factors have been proposed including high stress tolerance, rapid sexual and asexual reproduction, high competitiveness and allelopathy, often accelerated by land management practices (Treskonova Citation1991; Duncan et al. Citation1997; Rose et al. Citation1998; Rose & Frampton Citation1999; Meurk et al. Citation2002; Day & Buckley Citation2007, Citation2009, Citation2011; Diez et al. Citation2009; Mark et al. Citation2011). What is clear is that Hieracium species were present in New Zealand grasslands for many years before they became dominant. For example, H. pilosella was first recorded in New Zealand in 1878 (Murphy Citation1878), H. praealtum was first recorded in 1924 (Allan Citation1924), and H. lepidulum was first recorded in 1946 (Wiser & Allen Citation2000). The various Hieracium species show ecological differences in both their invasion patterns and the systems that they invade, reflecting differences in their basic biology (Makepeace Citation1980, Citation1985a,Citationb; Espie et al. Citation2001). Hieracium pilosella is most abundant in lower-elevation sites that were previously dominated by short tussock grasslands, whereas the more shade-tolerant H. lepidulum appears more abundant in higher-altitude tall tussock (Chionochloa-dominated) grasslands as well as in forests (Wiser et al. Citation1998; Wiser & Allen Citation2000; Spence et al. Citation2011). Hieracium praealtum appears more common in low-rainfall short tussock grasslands but generally occurs at lower abundance than H. pilosella.
All the Hieracium species appear to be very effective competitors with many native species. They have high stress tolerance, being able to withstand a wide range of temperatures, and moisture, nutrient and light levels (Makepeace Citation1985a,Citationb; McIntosh et al. Citation1995; Rose & Frampton Citation1999; Chapman et al. Citation2000). They are good colonizers of both bare ground and low-stature vegetation, taking advantage of the altered disturbance regimens provided by modern agricultural practices. Once established they spread easily because they are wind dispersed. All three Hieracium species can reproduce both sexually and asexually by apomixis. Hieracium pilosella and H. praealtum can also reproduce vegetatively by producing daughter plants at the end of stolons. Genetically, Hieracium species easily evolve and hybridize, thus enabling rapid adaptation (Chapman et al. Citation2000, Citation2003; Morgan-Richards et al. Citation2004). Hieracium species are likely to benefit from increased disturbance (e.g. burning and grazing) but do less well with greater management inputs (e.g. over-sowing, fertilizing and irrigation) (Scott et al. Citation1990). Hieracium species may also have allelopathic properties that alter the soil chemical balance, inhibiting surrounding vegetation (McIntosh et al. Citation1995; Scott et al. Citation2001).
In this study we assessed the extent of invasion by Hieracium species in a Canterbury tall tussock grassland. Specifically we wanted to know how widespread different Hieracium species were, what were the main environmental correlates of their distribution, and if there were any differences in the distribution of the different Hieracium species present.
Methods
Study site
The study was undertaken in the upper Cass River, northwest of Lake Tekapo, Canterbury, New Zealand (43°40′S, 170°22′E, Mt Cook Ecological District, ). The underlying geology comprises Triassic greywacke sandstone and argillite of the Torlesse Composite Terrane (Cox & Barrel Citation2007), with Quaternary glaciations resulting in steeply sloping mountain sides with rocky bluffs, waterfalls and extensive screes. Hanging valleys and cirques with small moraine systems are common, while the braided river flows through gravel river flats. Soils are a mix of Brown Soils and raw Soils, depending on the degree of soil development (Hewitt & Whenua Citation1998). The climate of the area is typical of the eastern South Island mountains, with hot dry summers and cold snowy winters. At altitude 1300 m, mean annual temperature is 5.2 °C, mean July minimum air temperature −2.9 °C, mean January maximum air temperature is 16.1 °C (Norton Citation1985). Annual solar radiation is high at 13.8 MJ m−2 day−1 (LENZ Citation2011). Rainfall at the study site is estimated to be about 2000 mm yr−1, much of it falling as winter snow. The study site regularly experiences strong winds, mainly in the form of ‘Norwesters’. The study site has good drainage, is of moderate fertility and has very low annual water deficit (LENZ Citation2011).
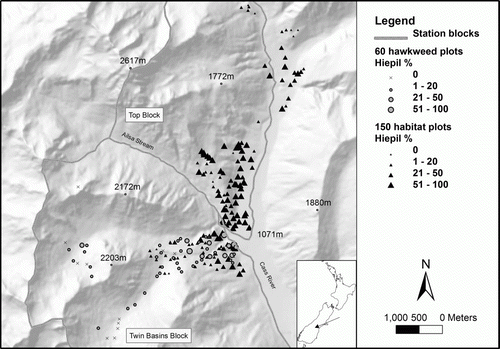
Figure 1 Map of Upper Cass River valley, near Tekapo, depicting the two sets of plots and abundance of Hieracium pilosella.
The study site is located within the summer grazing range of a high country sheep station. Merino ewes are grazed at the site from February to April each year and range widely through the grasslands. Stocking rate is approximately one sheep per hectare over the 2 months that are grazed (equivalent to < 0.2 stock units ha−1 yr−1). A few cattle are also grazed but do not usually venture far above the valley flats. These grasslands are also grazed by tahr and hares, with rabbits on the valley flats; no information is available on the densities of these animals, but pellet abundance would suggest that they are common.
Before sheep farming began, fires swept through the region both in Polynesian times and in early European times and altered the vegetation composition considerably (McGlone Citation2001; McWethy et al. Citation2010). Pre-human vegetation below the tree-line, was probably a mosaic of forest and shrubland including Podocarpus cunninghamii, Hoheria lyallii, Podocarpus nivalis, Brachyglottis cassinioides, Discaria toumatou, Aristotelia fruticosa, Olearia odorata, Dracophyllum spp., small-leaved Coprosma spp. and Hebe spp., with tall tussock grasslands and herbfields above the limit of woody vegetation. When the woody vegetation was burned, tall tussocks would have spread downslope initially, but when more intense grazing occurred, the tall tussocks receded back upslope and were replaced by short tussock grasslands. Tall tussock grasslands comprise chiefly Chionochloa rigida subsp. rigida, but other Chionochloa species are occasionally present (C. pallens subsp. cadens, C. macra, C. rubra subsp. cuprea, C. oreophila and C. crassiuscula). Common inter-tussock species include Celmisia lyalli, Celmisia haastii, Celmisia angustifolia, Poa colensoi, Aciphylla aurea, Dracophyllum spp., Acrothamnus colensoi and many small herbs. Tall tussock grasslands in this area occur from about 1000 to 1600 m above sea level (a.s.l.), where the grasslands give way to alpine herbfields. Short tussock grasslands, defined by the presence of Festuca novae-zelandiae, appear to be replacing the tall tussock grasslands at lower elevations and are now the dominant tussock up to about 1200 m a.s.l. Healthy short tussock grasslands, however, are of limited extent at the study site; degraded short tussock grasslands now cover much of the lower slopes, fans and terraces where the dominant species is Hieracium pilosella. Some open low shrubland occurs on slopes and fans, where Discaria toumatou, Dracophyllum rosmarinifolium, Ozothamnus leptophylla and Brachyglottis cassinioides are the dominant shrubs. In steep gullies, remnant shrubs and small trees such as Podocarpus nivalis, Hoheria lyallii and small-leaved Coprosma species occur.
Field methods
Two sets of vegetation plots were independently established at the study site over the last 5 years. The first set was established in February 2009 specifically for quantifying the distribution of Hieracium species. These 60 plots () were stratified altitudinally (<1200, 1200 to < 1400, 1400 to < 1600, 1600 to < 1800, 1800 to < 2000 m a.s.l.), in proportion to the available area of tussock grassland in different altitudinal bands in the Twin Basins Block south of the confluence of Ailsa Stream and Cass River and were subjectively located to provide a representation of the vegetation present within each altitudinal band. Each plot comprised 10 contiguous 1×1-m quadrats orientated along the contour. Percentage cover of each species was averaged across the 10 quadrats in each plot. The second set of 150 plots comprising 5×5-m quadrats was established as part of a sheep habitat use study in the 2010–11 summer. These plots () were subjectively located in the Twin Basins Block, the Top Block north of Ailsa Stream and on adjacent public conservation land to the east of the Cass River (1080–1600 m a.s.l.). Both sets of plots were GPS marked and could be approximately relocated for future monitoring and have been deposited in the National Vegetation Survey Databank.
For both sets of vegetation plots, percentage cover of each species present was recorded, as well as the cover of bare ground and rock. Average vegetation height in the quadrats was also recorded, as were the environmental variables altitude, aspect and slope. A moisture index was derived from aspect, slope and topography (Duncan et al. Citation1997).
Data analysis
Correlations between environmental variables and hawkweed abundance were examined first and logistic least squares regressions were performed in R 2.15.0 (R Core Development Team 2012, http://www.r-project.org). To account for zero values, the minimum non-zero value (mnz) was added to all values in each dataset. Each linear model was checked for normality and constant variance. Both response variables, H. pilosella and H. praealtum proportions were transformed to meet these assumptions using log([proportion H. pilosella+mnz]/1 – [proportion H. pilosella+mnz]). Each species was first modelled using the maximal model, which was then reduced to the most parsimonious model based on model fit and best explanation using the Akaike Information Criterion, by removing the explanatory variables with the least influence, one at a time. Correlations between explanatory variables were considered and the most influential variable was kept in the analysis. Analysis was undertaken on the 60-plot and 150-plot datasets separately to avoid differences in cover caused by the different plot sizes. Explanatory variables entered into the model were altitude, slope, tall tussock cover (TT), short tussock cover (ST), bare ground/rock cover (BGR), large herbs cover (LGHRB), shrub cover, sub-shrub cover, vegetation height and moisture index. Aspect was dropped from the model because there was insufficient variation in aspect. Vegetation cover represents the abundance of all the vegetation in the quadrat other than Hieracium species. Regression terms were arranged in order of strength of relationship.
The presence and absence of both hawkweed species were then analysed in R using generalized linear models using the binomial error structure and the logit link function, using the same variables and plots.
Results
Hieracium pilosella was present in 89% of the total 210 plots, whereas H. praealtum was present in 76%. At altitudes < 1800 m a.s.l. H. pilosella was present in 91% of the plots (n=198) but was still found in half of the plots > 1800 m a.s.l. (n=12). Both hawkweed species were present in the highest altitude plot sampled (at 2028 m a.s.l.). Hieracium lepidulum was only recorded in three plots.
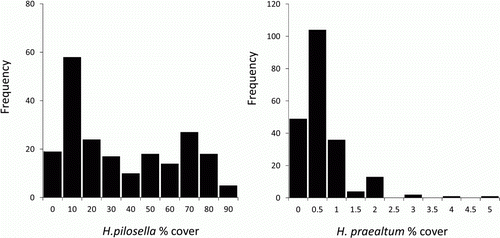
In all plots, H. pilosella cover ranged from 0 to 90% (mean±SD 31.0±29.0, median 22.0). Percentage cover of H. praealtum ranged from 0 to 5% (mean±SD 0.54±0.68, median 0.50). Thirty-four percent of H. pilosella cover values were < 10%, showing a bimodal distribution with one major peak at 1–10% and one minor peak at 61–70% (). Mean H. pilosella cover was 60% at altitudes < 1200 m, 29% at 1200–1400 m. 14% at 1400–1600 m, 5% at 1600–1800 m a.s.l. and 0.5% at > 1800 m. Hieracium praealtum cover was consistently about 0.5% in each altitudinal band.
Figure 2 Histogram of Hieracium pilosella and Hieracium praealtum abundance in both sets of plots, Upper Cass River valley. Bins represent maximum value in % cover.
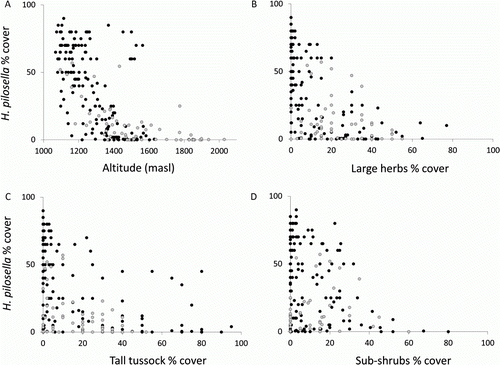
For the 60-plot dataset, the abundance of H. pilosella significantly decreased with increasing altitude, sub-shrub cover and tall tussock cover (). Abundance of H. praealtum changed little in these plots (mean±SD 0.38±0.04%). Presence or absence of H. pilosella showed similar relationships between variables as the abundance data. Slope was not significant in either the abundance or presence/absence models.
Table 1 Results of multiple logistic regression showing coefficient estimates for explanatory variables influencing abundance of log([proportion + mnz]/1 – [proportion + mnz]) Hieracium pilosella using the 60-plot dataset.
For the 150-plot dataset, abundance of H. pilosella decreased with increasing tall tussock cover, large herb cover, sub-shrubs and altitude (). Abundance of H. praealtumwas low throughout (mean±SD 0.6±0.06%). Presence or absence of H. pilosella was influenced by tall tussock and sub-shrubs only. Slope and moisture showed no influence on hawkweed abundance.
Table 2 Results of multiple logistic regression showing coefficient estimates for explanatory variables influencing abundance of log([proportion + mnz]/1 – [proportion + mnz]) Hieracium pilosella using the 150-plot dataset.
Altitude, tall tussock and sub-shrubs were the most influential variables in both abundance models, while large herbs only appeared in the 150-plot model. Hieracium pilosella is present right up to the highest recorded plot at 2028 m a.s.l. The few plots with a high abundance of H. pilosella at higher altitudes were on broad open ridges. Overall, abundance of H. pilosella decreases with increasing altitude and increasing cover of large herbs, tall tussock and sub-shrubs (A–D, respectively).
Discussion
Both H. pilosella and H. praealtum are widely distributed within the study area, appearing in the majority of plots. Abundance of H. pilosella is highest in the lower-altitude short tussock grasslands, as expected, but is also moderately abundant in the tall tussock grasslands at higher altitude. Although H. praealtum is widespread, it has very low abundance (<2%) in the majority of plots. The high frequency of occurrence of both species throughout the study area is likely to reflect their being in the latter stages of invasion (Radosevich et al. Citation2003). Once a high frequency is reached, it is more informative to record percentage cover (Buckley & Freckleton Citation2010), as both species were present in > 70% of the plots. In our study area, H. lepidulum only appears in three plots and with low cover, so this species is most likely in the early stages of invasion; therefore these plots would be useful for continued monitoring of the spread of H. lepidulum.
The main environmental correlates with Hieracium species distribution are altitude and surrounding vegetation structure. Altitude is a proxy for temperature, with higher elevation sites experiencing cooler average temperatures and shorter growing seasons. The relationship between H. pilosella cover and altitude is strong, appearing in the regression models of both datasets, but the relationship of altitude with H. praealtum is weaker, although still statistically significant. Presumably at higher elevations H. pilosella is less abundant because it is either still expanding its range or it is less competitive because of the prevailing environmental conditions. That both Hieracium species occur in the highest altitude plot suggests that dispersal is not limiting and that temperature is limiting their competitive ability at higher altitudes. Interestingly, on one broad ridge where sheep frequently camp, H. pilosella had much higher abundances than at other comparable plots at a similar altitude, suggesting the factors associated with the camping of sheep (e.g. vegetation disturbance or nutrient transfer) might be facilitating H. pilosella expansion at these sites. A strong relationship between H. pilosella abundance and altitude has been noted in other studies (Duncan et al. Citation1997). However, our study is the first recording of both H. pilosella and H. praealtum spreading well into high-altitude country at up to 2000 m a.s.l. One other Hieracium species has been recorded at high altitudes; Alan Mark recorded H. lepidulum on Treble Cone, Wanaka, at 2000 m a.s.l. (Hunter Citation1992).
The abundance of H. pilosella was low or this species was absent from plots that had abundant tall tussock grasses or herbs, or a good continuous cover of dense shrubs and sub-shrubs at higher altitudes (> 1400 m). This relationship is understandable as these taller species limit the amount of light available for the low-growing hawkweed species. Tall tussock grasslands may have resisted invasion through greater canopy cover, higher rainfall, lower fertility and fewer human impacts such as burning and grazing (Treskonova Citation1991; Rose & Frampton Citation1999) than short tussock grasslands. Rose & Frampton (Citation1999) also found that the abundance of H. pilosella was influenced by vegetation composition and structure, with most H. pilosella seedlings growing among low vegetation, litter and bryophytes, rather than in bare ground. In Otago, H. pilosella has barely invaded where the Chionochloa tussocks are denser (Mark & Dickinson Citation2003; Day & Buckley Citation2009; Mark et al. Citation2011). Large areas of tall tussock grasslands at the study site are quite open, which is where hawkweed species have spread.
There was little correlation between H. pilosella abundance and the moisture index, indicating that at the scale of our study area, either the differences in soil moisture were minor or the species has adapted to grow in a range of soil moisture conditions. Although Duncan et al. (Citation1997) found moisture index to be a good predictor of H. pilosella abundance at lower altitudes, we did not; their study did, however, span a much greater geographical range.
The results of this study highlight that hawkweed species, and especially H. pilosella, have invaded tall tussock grassland at altitudes> 1000 m a.s.l. with the highest record at 2028 m, although their abundance decreases with increasing altitude. These are the first records of H. pilosella and H. praealtum that we are aware of at these altitudes. As no data are available on temporal patterns in hawkweed abundance at these altitudes it is not possible to determine if the high abundances of hawkweeds that are apparent at lower elevations will also occur at higher altitudes in the future. While much of the study area is subjected to summer grazing by Merino ewes, it is unclear if grazing has facilitated the presence of hawkweed species because hawkweeds were present in plots in parts of the study area that have never had Merino ewe grazing. The upper Cass River tall tussock grasslands are relatively open compared with grasslands under higher rainfall further west and this, together with ongoing grazing by tahr and hares, may make these grasslands vulnerable to hawkweed invasion irrespective of Merino ewe grazing, especially given the high propagule pressure from lower altitudes where hawkweeds are very abundant. The P-values from all these results should be treated with caution because of spatial autocorrelation between plots (Dormann Citation2007; Beale et al. Citation2010).
Supplementary file
Supplementary file: Correlations between variables for each dataset are given first, followed by the results of the Hieracium pilosella presence/absence data analysis using a generalized linear model with a binomial error structure and logit link.
Correlations between variables for each dataset are given first, followed by the results of the H. pilosella presence/absence data analysis using a glm with a binomial error structure and logit link
Download MS Word (65.5 KB)Acknowledgements
We profusely thank the farmers, Will and Emily Murray, for allowing us access to their property. For field assistance, we thank Jo Stillwell and Trevor Blogg. Thanks go to Luis Apiolaza for statistical help. Funding for this project came from the New Zealand Merino Company, School of Forestry at the University of Canterbury and the NZ Federation of Graduate Women. We are most grateful to Hannah Buckley for valuable comments on this script. Much appreciation also goes to the two anonymous referees and associate editor for their valuable comments.
Notes
Supplementary data available online at www.tandfonline.com/10.1080/0028825X.2012.753096
Supplementary file: Correlations between variables for each dataset are given first, followed by the results of the Hieracium pilosella presence/absence data analysis using a generalized linear model with a binomial error structure and logit link.
1Plant names follow nzflora.landcareresearch.co.nz database (2012).
References
- Allan , HH . 1924 . Notes on the occurence of certain exotic plants in New Zealand . New Zealand Journal of Agriculture , 29 : 311 – 314 .
- Beale , CM , Lennon , JJ , Yearsley , JM , Brewer , MJ and Elston , DA . 2010 . Regression analysis of spatial data . Ecology Letters , 13 : 246 – 264 .
- Buckley , HL and Freckleton , RP . 2010 . Understanding the role of species dynamics in abundance–occupancy relationships . Journal of Ecology , 98 : 645 – 658 .
- Chapman , HM , Parh , D and Oraguzie , N . 2000 . Genetic structure and colonizing success of a clonal, weedy species, Pilosella officinarum (Asteraceae) . Heredity , 84 : 401 – 409 .
- Chapman , HM , Robson , B and Pearson , ML . 2003 . Population genetic structure of a colonising, triploid weed, Hieracium lepidulum . Heredity , 92 : 182 – 188 .
- Cox S , Barrel DJA 2007 . Geology of the Aoraki area, Institute of Geological and Nuclear Sciences 1: 250 000 Geological Map . Lower Hutt , , New Zealand (GNS Science) 71 .
- Day N , Buckley HL 2007 . Two decades of vegetation change across tussock grasslands of New Zealand's South Island . Report for Bio-Protection and Ecology Division, Lincoln University .
- Day NJ , Buckley HL 2009 . Colonisation and spread of Hieracium spp in the South Island high country over 25 years . Report prepared for Land Information New Zealand .
- Day , NJ and Buckley , HL . 2011 . Invasion patterns across multiple scales by Hieracium species over 25 years in tussock grasslands of New Zealand's South Island . Austral Ecology , 36 : 559 – 570 .
- Diez , JM , Buckley , HL , Case , BS , Harsch , MA , Sciligo , AR , Wangen , SR and Duncan , RP . 2009 . Interacting effects of management and environmental variability at multiple scales on invasive species distributions . Journal of Applied Ecology , 46 : 1210 – 1218 .
- Dormann , CF . 2007 . Effects of incorporating spatial autocorrelation into the analysis of species distribution data . Global Ecology and Biogeography , 16 : 129 – 138 .
- Duncan , RP , Colhoun , KM and Foran , BD . 1997 . The distribution and abundance of Hieracium species (hawkweeds) in the dry grasslands of Canterbury and Otago . New Zealand Journal of Ecology , 21 ( 1 ) : 51 – 62 .
- Espie PR AgResearch Limited., New Zealand. Ministry for the Environment., New Zealand Fertiliser Manufacturers’ Research Association . 2001 . Hieracium in New Zealand: ecology and management . Mosgiel, N.Z., AgResearch . vi , 66 p.
- Hewitt AE , Whenua M 1998 . New Zealand soil classification . Lincoln , , New Zealand , Manaaki Whenua Press .
- Hunter GG 1992 . The distribution of hawkweeds (Hieracium spp.) in the South Island, indicating problem status . Review, Journal of the New Zealand Mountain Lands Institute ( 48 ): 21 – 31 .
- LENZ 2011 . Land Environments of New Zealand Level 4 Polygons . http://koordinates.com/layer/1101-land-environments-new-zealand-lenz-level-4-polygons (accessed 01 January 2012) .
- Makepeace W 1980 . Ecological studies of Hieracium pilosella and H. praealtum . Unpublished thesis, University of Canterbury , Christchurch . 198 p.
- Makepeace , W . 1985a . Some establishment characteristics of mouse-ear and king devil hawkweeds . New Zealand Journal of Botany , 23 : 91 – 100 .
- Makepeace , W . 1985b . Growth, reproduction, and production biology of mouse-ear and king devil hawkweed . New Zealand Journal of Botany , 23 : 65 – 78 .
- Mark , AF and Dickinson , KJM . 2003 . Temporal responses over 30 years to removal of grazing from a mid-altitude snow tussock grassland reserve, Lammerlaw Ecological Region, New Zealand . New Zealand Journal of Botany , 41 : 655 – 668 .
- Mark , AF , Wilson , JB and Scott , C . 2011 . Long-term retirement of New Zealand snow tussock rangeland: effects on canopy structure, hawkweed (Hieracium spp.) invasion and plant diversity . New Zealand Journal of Botany , 49 : 243 – 262 .
- McGlone , MS . 2001 . The origin of the indigenous grasslands of southeastern South Island in relation to pre-human woody ecosystems . New Zealand Journal of Ecology , 25 : 1 – 15 .
- McIntosh , PD , Loeseke , M and Bechler , K . 1995 . Soil changes under mouse-ear hawkweed (Hieracium pilosella) . New Zealand Journal of Ecology , 19 : 29 – 34 .
- McWethy , DB , Whitlock , C , Wilmshurst , JM , McGlone , MS , Fromont , M , Li , X , Dieffenbacher-Krall , A , Hobbs , WO , Fritz , SC and Cook , ER . 2010 . Rapid landscape transformation in South Island, New Zealand, following initial Polynesian settlement . Proceedings of the National Academy of Sciences USA , 107 : 21343
- Meurk , CD , Walker , S , Gibson , RS and Espie , P . 2002 . Changes in vegetation states in grazed and ungrazed Mackenzie Basin grasslands, New Zealand, 1990–2000 . New Zealand Journal of Ecology , 26 : 95 – 106 .
- Morgan-Richards , M , Trewick , SA , Chapman , HM and Krahulcová , A . 2004 . Interspecific hybridization among Hieracium species in New Zealand: evidence from flow cytometry . Heredity , 93 : 34 – 42 .
- Murphy , M . 1878 . Botany by the wayside . NZ Country Journal Part , 2 : 250 – 257 .
- Norton , DA . 1985 . A multivariate technique for estimating New Zealand temperature normals . Weather and Climate , 5 : 64 – 74 .
- Norton , DA , Espie , PR , Murray , W and Murray , J . 2006 . Influence of pastoral management on plant biodiversity in a depleted short tussock grassland, Mackenzie Basin . New Zealand Journal of Ecology , 30 : 335 – 344 .
- Radford , IANJ , Dickinson , KJM and Lord , JM . 2010 . Does disturbance, competition or resource limitation underlie Hieracium lepidulum invasion in New Zealand? Mechanisms of establishment and persistence, and functional differentiation among invasive and native species . Austral Ecology , 35 : 282 – 293 .
- Radosevich , SR , Stubbs , MM and Ghersa , CM . 2003 . Plant invasions—process and patterns . Weed Science , 51 : 254 – 259 .
- Rose , AB and Frampton , CM . 1999 . Effects of microsite characteristics on Hieracium seedling establishment in tall and short tussock grasslands, Marlborough, New Zealand . New Zealand Journal of Botany , 37 : 107 – 118 .
- Rose , AB , Basher , LR , Wiser , SK , Platt , KH and Lynn , IH . 1998 . Factors predisposing short-tussock grasslands to Hieracium invasion in Marlborough, New Zealand . New Zealand Journal of Ecology , 22 : 121 – 140 .
- Rose , AB , Suisted , PA and Frampton , CM . 2004 . Recovery, invasion, and decline over 37 years in a Marlborough short-tussock grassland, New Zealand . New Zealand Journal of Botany , 42 : 77 – 87 .
- Scott , D , Robertson , JS and Archie , WJ . 1990 . Plant dynamics of New Zealand tussock grassland infested with Hieracium pilosella. I. Effects of seasonal grazing, fertilizer and overdrilling . Journal of Applied Ecology , 27 : 224 – 234 .
- Scott , NA , Saggar , S and McIntosh , PD . 2001 . Biogeochemical impact of Hieracium invasion in New Zealand's grazed tussock grasslands: sustainability implications . Ecological Applications , 11 : 1311 – 1322 .
- Spence , LA , Ross , JV , Wiser , SK , Allen , RB and Coomes , DA . 2011 . Disturbance affects short-term facilitation, but not long-term saturation, of exotic plant invasion in New Zealand forest . Proceedings of the Royal Society B: Biological Sciences , 278 ( 1711 ) : 1457 – 1466 .
- Treskonova , M . 1991 . Changes in the structure of tall tussock grasslands and infestation by species of Hieracium in the Mackenzie country, New Zealand . New Zealand Journal of Ecology , 15 : 65 – 78 .
- Wiser SK , Allen RB 2000 . Hieracium lepidulum invasion of indigenous ecosystems . Wellington , Department of Conservation , Head Office, PO Box 10-420, Wellington, New Zealand .
- Wiser , SK , Allen , RB , Clinton , PW and Platt , KH . 1998 . Community structure and forest invasion by an exotic herb over 23 years . Ecology , 79 : 2071 – 2081 .