Abstract
The introduction of exotic plant species to the sand dunes of New Zealand has displaced native dune species. To examine our hypotheses that: (1) exotic species have functional traits that allow them to outcompete native species; and (2) that traits of exotic species vary across the dune environment, we measured specific leaf area, leaf dry matter content, leaf area and plant height in five common fore-dune taxa of New Brighton, Christchurch. The native species Spinifex sericeus, Ficinia spiralis and Poa billardierei had traits indicative of a more conservative growth strategy compared with the exotic tussock grass Ammophila arenaria and the exotic succulent Carpobrotus spp., suggesting that natives would be less competitive. In exotics, leaf size was larger at greater distances from the high tide mark, as was plant height in A. arenaria. These differences in traits across the dune environment point to phenotypic plasticity across short, but sharp, environmental gradients.
Introduction
The sand dune environment of New Zealand has been highly modified since European settlement in the 19th century, resulting in a displacement of native dune species, a loss of vegetation cover and unstable shifting dunes (Hesp Citation2000). Exotic species were successfully introduced as dune stabilisers, but because they can displace native species, they caused a further decline in dune areas dominated by native species (Hilton et al. Citation2005). Hence, there is a need to undertake restoration of native sand dune plant communities. To do this successfully, a better understanding of the ecology of native sand dune species and the invasive exotics that threaten them is needed. One approach is to study their plant functional traits, those attributes that impact plant fitness indirectly via their effects on growth, reproduction and survival (Violle et al. Citation2007). However, few studies have examined the functional traits of sand dune species (Sykes & Wilson Citation1988; Feagin & Wu Citation2007; Stanisci et al. Citation2010; Murphy et al. Citation2012) and of these, most have focused on qualitative or ordinal traits, rather than quantitative traits (García-Mora et al. Citation1999; Gallego-Fernandez & Martinez Citation2011).
Coastal sand dune plants must survive stressful environmental conditions (Esler Citation1970). Salinity, wind exposure and burial are highest near the ocean and decrease towards the hind-dunes (Miller & Paul Citation2007; Murphy et al. Citation2012). Nutrients such as nitrogen (N) and phosphorus (P) are generally lowest in the foredunes (Lane et al. Citation2008; Syers & Walker Citation1969). Moisture stress is correlated with the distance to the water table (Gries et al. Citation2003) and hence likely increases towards dune crests. Plant responses to these gradients are associated with traits such as specific leaf area, leaf dry matter content, leaf area and plant height (Lavorel & Garnier Citation2002; Garnier & Navas Citation2012). Hence, these traits can help us understand the resource-use strategies (e.g. nutrient and water use) of sand dune plants. Leaf area is correlated with moisture availability and/or nutrient gradients (Ackerly et al. Citation2001); this commonly results in smaller leaves in habitats with low nutrient or moisture availability (Ackerly & Reich Citation1999; Cornelissen et al. Citation2003). Specific leaf area (SLA) and leaf dry matter content (LDMC) are generally negatively correlated; a low SLA with a high LDMC is related to an efficient conservation of nutrients, and a high SLA with a low LDMC corresponds to a rapid production of biomass (Garnier et al. Citation2001). Within a growth form, plant height is correlated with the competitive vigour of plants and their ability to reproduce (Gaudet & Keddy Citation1995; Westoby Citation1998; Westoby et al. Citation2002; Cornelissen et al. Citation2003; Garnier & Navas Citation2012). Finally, functional traits will vary within species across environmental gradients (Garnier & Navas Citation2012) and such trait plasticity is important in understanding invasion dynamics (Stanisci et al. Citation2010; Davidson et al. Citation2011).
At one dune location, we examined our hypotheses that: (1) the functional plant traits of exotic species enable them to outcompete native species; and (2) traits within exotic species differ across the dune environment, from fore- to hind-dune, in response to the environmental conditions. The aim of this study was to provide information on the resource-use strategy of common sand dune species and the extent of trait plasticity within exotic species, which will aid future restoration efforts by helping to understand invasion processes in this sand dune ecosystem and identifying the management actions that could reduce or reverse these impacts (Funk et al. Citation2008).
Methods
The sand dunes of New Brighton, Christchurch (43°33′S; 172°47′E) are spread along the southern coast of Pegasus Bay. The mean annual temperature of Christchurch is 12.2 °C and the total annual precipitation is 618.2 mm (NIWA Citation2010). Dominant plants include Spinifex sericeus R.Br., Ficinia spiralis A.Rich. and Poa billardierei (Spreng.) St.-Yves, the exotic tussock grass Ammophila arenaria L. and the exotic succulent Carpobrotus spp. N.E.Br. When conducting our fieldwork it was not possible to distinguish between the two Carpobrotus spp. that occur in the study area, due to the lack of flowers. It could be either C. edulis (L.) N.E.Brown or C. chilensis (Molina) N.E.Brown. Ten individual adult plants of each exotic taxon were randomly selected within three habitat zones: foredune, crest and hind-dune. The native species occur only in restoration plantings on the foredune (see Bergin & Kimberley Citation1999; Bergin Citation2008 for details on plantings), so ten individuals of each species were sampled from this habitat. SLA (mm2 mg−1), LDMC (oven dry mass divided by saturated fresh mass; mg g−1), individual leaf area (mm2) and plant height (m) were measured following the standardised methods described by Cornelissen et al. (Citation2003). Only a slice of the epidermis and some parenchyma of the leaves of Carpobrotus spp. were used to measure its SLA and LDMC, as recommended by Cornelissen et al. (Citation2003) and Vendramini et al. (Citation2002).
Three environmental parameters were measured in the field at each sample plant location: distance to hide tide mark (HTM in m), elevation (m above sea level, using differential GPS) and the percentage cover of other species within 1 m2 around each individual. The distance to HTM provides a proxy for the salinity gradient in the sand dunes (Miller & Paul Citation2007). Elevation is associated with the distance to the water table and is therefore a surrogate for moisture stress (Gries et al. Citation2003). The percentage cover of other species provides a measure of interspecific competition.
SLA, LDMC and leaf area were measured on two leaves of each individual plant and averaged to give one statistical observation (Cornelissen et al. Citation2003). Analysis of variance (ANOVA) with post hoc Tukey's honestly significant difference (HSD) tests were used to determine if trait values differed among the species. Principal component analysis (PCA) was used to explore relationships among traits. Multiple regression was used to examine the relationship between traits and the environmental parameters separately for each of the two exotic species.
Results
There were significant differences between species for all four traits (). The native species had a similar height, but showed some differences in SLA, LDMC and leaf area. The exotic tussock grass A. arenaria was the tallest plant and had similar SLA to P. billardierei and F. spiralis. The shortest plant was Carpobrotus spp., which had similar SLA and leaf area to S. sericeus. The first PCA axis explained 60% of variation and was correlated with plant height and leaf area, whereas the second PCA axis was correlated with SLA and accounted for 25% of variation ().
Table 1 Mean (and standard error of the mean) for specific leaf area (SLA), leaf dry matter content (LDMC), leaf area and plant height for each of five sand dune species.
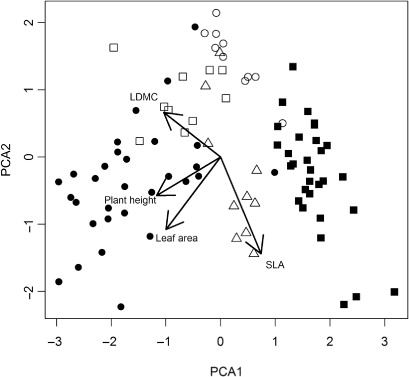
Most plant functional traits varied within exotic species along the environmental gradients of distance to HTM, elevation and per cent cover of other species (), although SLA did not change across the dune gradient for either exotic species. The LDMC of A. arenaria was positively correlated with elevation. Leaf area and height of A. arenaria increased towards the hind-dune, but height was negatively correlated with the cover of other species. Leaf area of Carpobrotus spp. increased towards the hind-dune and its height increased in the presence of other species.
Table 2 Results of the multiple regression, showing the relationships (regression coefficient values and significance) between traits and environmental parameters within exotic species.
Discussion
There have been few quantitative assessments of plant functional traits in coastal sand dune communities worldwide (but see Feagin & Wu Citation2007; Stanisci et al. Citation2010) and in New Zealand coastal dunes (Murphy et al. Citation2012). Here, we show how the key functional traits of SLA, LDMC, leaf area and height vary between native and exotic species and within exotic species across the fore- to hind-dune gradient.
The range in SLA of plants in our coastal dune was narrow (4–7 mm2 mg−1) and places them at the lower range of SLA values worldwide (<1–300 mm2 mg−1; Pérez-Harguindeguy et al. Citation2013). These low mean SLA values reflect a conservative growth strategy that is characteristic of plants in resource-poor, stressful environments (Wilson et al. Citation1999). However, these values are in stark contrast to some other published SLA values from coastal sand dunes. Feagin & Wu (Citation2007) recorded SLA values of 36–142 in Texan coastal sand dunes, but did not give units, and Italian coastal dune plants were reported to have SLA values of 81–177 mm2 mg−1 (Stanisci et al. Citation2010). The high SLA values for most species in both these studies places them within the higher range of SLA values globally (Pérez-Harguindeguy et al. Citation2013), suggesting a different life history strategy to New Zealand foredune species. Murphy et al. (Citation2012) recorded SLA values of 2.8–10.9 mm2 mg−1 for shrubs and monocotyledons on a coastal dune at Stewart Island, New Zealand. They recorded an SLA of 2.76 mm2 mg−1 for F. spiralis and 4.13 mm2 mg−1 for P. billardierei, very similar to the SLA of these species measured in our study, further confirming that native foredune species in New Zealand exhibit conservative growth strategies.
Species with high SLA and low LDMC values are able to rapidly produce biomass compared to low SLA/high LDMC species (Garnier et al. Citation2001). Carpobrotus spp. best represents the former life history strategy among our study species. This may explain its successful invasion in New Zealand coastal dunes because higher SLA has been linked with successful invasions elsewhere (Grotkopp & Rejmánek Citation2007; Feng et al. Citation2008).
Taller plants are generally considered to be better competitors than shorter plants (Falster & Westoby Citation2003), because height contributes to a competitive advantage (Hacker et al. Citation2012). Ammophila arenaria is the tallest species on the foredunes at New Brighton and is among the tallest herbaceous species on sand dunes throughout New Zealand (Hesp Citation2000; Gadgil Citation2006). This, coupled with its larger leaves, which aid in light capture, gives it a competitive advantage over other foredune species (Violle et al. Citation2009; Garnier & Navas Citation2012). The native species P. billardierei and F. spiralis have traits that reflect a slow-growing, conservative resource use strategy that has left them susceptible to faster growing, more competitive exotic species. The higher SLA of S. sericeus suggests a faster growth rate relative to the other natives. This may reflect a need to quickly elongate stems in response to the frequent sand burial that occurs in the foredunes, which is close to the HTM (Maze & Whalley Citation1992).
Some traits differed within exotic species across the dune environment. Within A. arenaria, leaf area increased with distance to HTM, which seems likely to be due to a decrease in environmental stresses, such as salinity and hence drought stress (Munns Citation2002), from the foredunes to the hind-dunes (Miller & Paul Citation2007). Gadgil (Citation2006) noted that A. arenaria is less tolerant to salinity than native foredune species, especially when younger (Sykes & Wilson Citation1988), but is tolerant to high temperatures, nutrient stress and wind action, and sand deposition stimulates its growth (Hesp Citation2000). In this study, its height decreased in the occurrence of other species. Generally, a plant will not invest in its vegetative height when the expected decrease in reproductive output, due to this investment, outweighs the potential decrease in shaded conditions (Falster & Westoby Citation2003). According to Gadgil (Citation2006), A. arenaria does not compete well with S. sericeus on the foredunes, although A. arenaria is able to displace F. spiralis and other native species on active dune sites (Hilton et al. Citation2005). Carpobrotus spp. seem to respond to competition by growing taller because the height increases with the per cent cover of other species. Carpobrotus spp. are known to compete successfully for surface soil moisture, nutrients and space with other shallowly rooted plants and also affect herbaceous plants and shrub species in sandy areas (D'Antonio Citation1990). In this study, the leaf area of Carpobrotus spp. increased towards the back of the dunes, likely due to the lower environmental stress (Hesp Citation2000). The plasticity in leaf area and plant height across the dune environment in A. arenaria and Carpobrotus spp. helps explain their ability to grow in a variety of environments (Bradshaw Citation1965). Although we did not test for trait variation across the environmental gradient in natives, this was conducted by Murphy et al. (Citation2012), who found a significant increase in SLA, averaged over the seven species observed in their transects, and a decrease in leaf area of P. billardierei, on transects of higher elevation.
Our examination of functional traits indicates that the exotics, Carpobrotus spp. and A. arenaria, are likely to grow faster, and hence are better competitors in the short-term compared with the native foredune plants on New Zealand sand dunes. There is also very little overlap in the traits of natives and exotics studied in this ecosystem (), suggesting that it will not be possible to prevent reinvasion by planting these natives to fill all available niches in the community (Funk et al. Citation2008). Hence, there will likely need to be ongoing management intervention in the form of intensive weeding of exotic species in areas where there has been planting of native sand dune plants to exclude exotic species and ensure the success of such restoration projects.
Acknowledgements
We thank Hannah Lewis and Ruud van Driel for help with fieldwork and Marleen Pierik for advice. Jill Rapson and an anonymous reviewer provided helpful comments on the manuscript.
References
- Ackerly D, Knight C, Weiss S, Barton K, Starmer K. 2001. Leaf size, specific leaf area and microhabitat distribution of chaparral woody plants: contrasting patterns in species and community level analyses. Oecologia 130: 449–457.
- Ackerly D, Reich P 1999. Convergence and correlations among leaf size and function in seed plants: a comparative test using independent contrasts. American Journal of Botany 86: 1272–1281. 10.2307/2656775
- Bergin DO 2008. Establishment of the indigenous sand binder spinifex (Spinifex sericeus) along the sand dunes of Christchurch. Contract report ERL 08/03. Christchurch, Environmental Restoration Ltd.
- Bergin DO, Kimberley MO 1999. Rehabilitation of coastal foredunes in New Zealand using indigenous sand-binding species. Science for Conservation Series no. 122. Wellington, Department of Conservation.
- Bradshaw AD 1965. Evolutionary significance of phenotypic plasticity in plants. Advances in Genetics 13: 115–155.
- Cornelissen J, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich D et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Australian Journal of Botany 51: 335–380. 10.1071/BT02124
- D'Antonio C 1990. Seed production and dispersal in the non-native, invasive succulent Carpobrotus edulis (Aizoaceae) in coastal strand communities of central California. The Journal of Applied Ecology 27: 693–702. 10.2307/2404312
- Davidson AM, Jennions M, Nicotra AB 2011. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecology Letters 14: 419–431. 10.1111/j.1461-0248.2011.01596.x
- Esler AE 1970. Manawatu sand dune vegetation. Proceedings of the New Zealand Ecological Society 17: 41–46.
- Falster D, Westoby M 2003. Plant height and evolutionary games. Trends in Ecology and Evolution 18: 337–343. 10.1016/S0169-5347(03)00061-2
- Feagin RA, Wu B 2007. The spatial patterns of functional groups and successional direction in a coastal dune community. Rangeland Ecology & Management 60: 417–425. 10.2111/1551-5028(2007)60[417:TSPOFG]2.0.CO;2
- Feng Y, Fu G, Zheng Y 2008. Specific leaf area relates to the differences in leaf construction cost, photosynthesis, nitrogen allocation, and use efficiencies between invasive and noninvasive alien congeners. Planta 228: 383–390. 10.1007/s00425-008-0732-2
- Funk JL, Cleland EE, Suding KN, Zavaleta ES 2008. Restoration through reassembly: plant traits and invasion resistance. Trends in Ecology and Evolution 23: 695–703. 10.1016/j.tree.2008.07.013
- Gadgil R 2006. Marram Grass – friend or foe? A review of the use of Ammophila arenaria on New Zealand Sand Dunes. CDVN Bulletin No. 5. Rotorua, New Zealand Forest Research Institute.
- Gallego-Fernandez JB, Martínez, ML 2011. Environmental filtering and plant functional types on Mexican foredunes along the Gulf of Mexico. Ecoscience 18: 52–62. 10.2980/18-1-3376
- García-Mora M, Gallego-Fernández JB, García-Novo F 1999. Plant functional types in coastal foredunes in relation to environmental stress and disturbance. Journal of Vegetation Science 10: 27–34. 10.2307/3237157
- Garnier E, Navas M 2012. A trait-based approach to comparative functional plant ecology: concepts, methods and applications for agroecology. A review. Agronomy for Sustainable Development 32: 365–399. 10.1007/s13593-011-0036-y
- Garnier E, Shipley B, Roumet C, Laurent G 2001. A standardized protocol for the determination of specific leaf area and leaf dry matter content. Functional Ecology 15: 688–695. 10.1046/j.0269-8463.2001.00563.x
- Gaudet CL, Keddy PA 1995. Competitive performance and species distribution in shortline plant communities: a comparative approach. Ecology 76: 280–291. 10.2307/1940649
- Gries D, Zeng F, Foetzki A, Arndt S, Bruelheide H, Thomas F et al. 2003. Growth and water relations of Tamarix ramosissima and Populus euphratica on Taklamakan desert dunes in relation to depth to a permanent water table. Plant, Cell and Environment 26: 725–736. 10.1046/j.1365-3040.2003.01009.x
- Grotkopp E, Rejmánek M 2007. High seedling relative growth rate and specific leaf area are traits of invasive species: phylogenetically independent contrasts of woody angiosperms. American Journal of Botany 94: 526–532. 10.3732/ajb.94.4.526
- Hacker SD, Zarnetske P, Seabloom E, Ruggiero P, Mull J, Gerrity S et al. 2012. Subtle differences in two non-native congeneric beach grasses significantly affect their colonization, spread, and impact. Oikos 121: 138–148. 10.1111/j.1600-0706.2011.18887.x
- Hesp P 2000. Coastal sand dunes: form and function. CDVN Bulletin No. 4. Rotorua, New Zealand Forest Research Institute.
- Hilton M, Duncan M, Jul A 2005. Processes of Ammophila arenaria (Marram grass) invasion and indigenous species displacement, Stewart Island, New Zealand. Journal of Coastal Research 21: 175–185. 10.2112/01041.1
- Lane C, Wright SJ, Roncal J, Maschiniski J 2008. Characterizing environmental gradients and their influence on vegetation zonation in a subtropical coastal sand dune system. Journal of Coastal Research 24: 213–224. 10.2112/07-0853.1
- Lavorel S, Garnier E 2002. Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Functional Ecology 16: 545–556. 10.1046/j.1365-2435.2002.00664.x
- Maze KM, Whalley RDB 1992. Effects of salt spray and sand burial on Spinifex sericeus R. Br. Austral Ecology 17: 9–19. 10.1111/j.1442-9993.1992.tb00775.x
- Miller E, Paul T 2007. Measuring success. Guidelines for the Management of Sand Dune Revegetation Programmes. CDVN Bulletin No. 6. Rotorua, New Zealand Forest Research Institute.
- Munns R 2002. Comparative physiology of salt and water stress. Plant, Cell and Environment 25: 239–250. 10.1046/j.0016-8025.2001.00808.x
- Murphy AL, Silberbauer RB, Streeter RE, Smiley DR, Smith AR, Darling S et al. 2012. An unusual climbing dune, Big Hellfire Pass, Stewart Island, New Zealand: exploration through environment, vegetation and trait patterns. New Zealand Journal of Botany 50: 233–256. 10.1080/0028825X.2012.665807
- NIWA 2010. Climate data and activities. Wellington, National Institute of Water and Atmospheric Research. http://www.niwa.co.nz/education-and-training/schools/resources/climate (accessed 7 December 2013).
- Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P et al. 2013. New handbook for standardised measurement of plant functional traits worldwide. Australian Journal of Botany 61: 167–234. 10.1071/BT12225
- Stanisci A, Acosta ATR, Di Iorio A, Vergalito M 2010. Leaf and root trait variability of alien and native species along Adriatic coastal dunes (Italy). Plant Biosystems 144: 47–52. 10.1080/11263500903454252
- Syers JK, Walker TW 1969. Phosphorus transformations in a chronosequence of soils developed on wind-blown sand in New Zealand. I, Total and organic phosphorus. Journal of Soil Science 20: 57–64. 10.1111/j.1365-2389.1969.tb01554.x
- Sykes MT, Wilson JB 1988. An experimental investigation into the response of some New Zealand sand dune species to salts spray. Annals of Botany 62: 159–166.
- Vendramini F, Díaz S, Gurvich D, Wilson P, Thompson K, Hodgson J 2002. Leaf traits as indicators of resource-use strategy in floras with succulent species. New Phytologist 154: 147–157. 10.1046/j.1469-8137.2002.00357.x
- Violle C, Garnier E, Lecoeur J, Roumet C, Podeur C, Blanchard A et al. 2009. Competition, traits and resource depletion in plant communities. Oecologia 160: 747–755. 10.1007/s00442-009-1333-x
- Violle C, Navas M, Vile D, Kazakou E, Fortunel C, Hummel I et al. 2007. Let the concept of trait be functional! Oikos 116: 882–892.
- Westoby M 1998. A leaf-height-seed (LHS) plant ecology strategy scheme. Plant and Soil 199: 213–227. 10.1023/A:1004327224729
- Westoby M, Falster D, Moles A, Vesk P, Wright I 2002. Plant ecological strategies: some leading dimensions of variation between species. Annual Review of Ecology and Systematics 33: 125–159. 10.1146/annurev.ecolsys.33.010802.150452
- Wilson PJ, Thompson K, Hodgson JG 1999. Specific leaf area and leaf dry matter content as alternative predictors of plant strategies. New Phytologist 143: 155–162. 10.1046/j.1469-8137.1999.00427.x
