Abstract
The expansion of forests over natural grasslands is observed in many parts of the world. This study aimed to contribute to the investigation into the nursing effects promoted by Araucaria angustifolia during the process of forest expansion over grasslands in southern Brazil. Air temperature and humidity, and soil chemistry were evaluated beneath the canopy of isolated trees of A. angustifolia, and compared with values in adjacent grassland areas and under the canopy of the shrub Baccharis uncinella. Milder summer temperatures and vapour pressure deficits were measured under Araucaria and Baccharis compared with open grassland. There were also ameliorating effects on soil chemistry under individuals of these two species, but these were more pronounced for Araucaria than for Baccharis. The results suggest that the large number of seedlings commonly found beneath the canopy of Araucaria is due not only to perching, but also to nursing, through the amelioration of environmental conditions.
Introduction
The expansion of woody vegetation over natural grasslands is a natural phenomenon observed in many parts of the world, but changes in grazing and fire regimes and global climate changes, such as increasing atmospheric CO2, are accelerating this process (Polley Citation1997; Bond et al. Citation2003; Müller et al. Citation2012). The decrease in fire and grazing practices, for example, is leading to dramatic landscape changes in grasslands (Campos) from southern Brazil, by accelerating the process of forest expansion (Oliveira & Pillar Citation2004; Overbeck et al. Citation2007)
Araucaria angustifolia (Bertol.) Ktze. (Araucariaceae) is a South American conifer that reaches up to 50 m high and 2 m in diameter at breast height. It is the dominant tree species in the upper canopy of the montane forests of the high-altitude plateaus of southern Brazil (known as Araucaria forests). The associated fauna broadly uses its starch-rich seeds, and its high-quality wood was exploited to the limit during the first half of the twentieth century. Araucaria forests often form mosaics with the adjacent grasslands, and also appear as gallery forests along streams and as forest islands on hill tops (Reitz et al. Citation1988).
Palynological evidence, soil carbon isotope studies and dendrochronological analyses have indicated that the montane Araucaria forests have been expanding over the adjacent grasslands in recent millennia (Behling et al. Citation2004; Dümig et al. Citation2008; Silva et al. Citation2009; Silva & Anand Citation2011). Recent studies have not only confirmed such expansion, but have also demonstrated the importance of A. angustifolia as a forest nucleation species in the grassland matrix of the high-altitude plateaus of the southernmost state of Brazil, Rio Grande do Sul (Duarte et al. Citation2006, Citation2007, Citation2010; Dos Santos et al. Citation2011).
Woody vegetation may expand over natural grasslands from the forest edges or ‘in jumps’ (Klein Citation1960; Oliveira & Pillar Citation2004). This second process, called forest nucleation (sensu Yarranton & Morrison Citation1974), can include two mechanisms: perching, plants being used as perches by seed-dispersal birds; and/or nursing effects, amelioration of the microenvironmental conditions. When these two mechanisms act simultaneously, chances of seedling recruitment and establishment beneath nucleation species are high (Flores & Jurado Citation2003; Pausas et al. Citation2006; Duarte et al. Citation2007). Araucaria forest expansion ‘in jumps’ may start by nucleation from rocks, trees and shrubs, but while all these agents may act as perches, only rocks and trees seem to also have a nursing effect (Duarte et al. Citation2006, Citation2010; Carlucci et al. Citation2011).
In a study conducted in the Araucaria forest/grassland mosaics in southern Brazil, a few tree and shrub species were found to grow as isolated individuals and to host forest seedlings under their crowns. The pioneer shrub Baccharis uncinella DC (Asteraceae) was found to be particularly abundant among these individuals, but had a very low density of seedlings (height ≤ 50 cm) growing below them (< 0.05 individuals m−2). By contrast, the fewer isolated trees of A. angustifolia had a much higher density of seedlings under their crowns (c. 0.35 individuals m−2) compared with all other species (Duarte et al. Citation2006). These results led the authors to propose two mechanisms for the success of A. angustifolia in promoting forest seedling recruitment and establishment: greater bird attraction (perching effect) and greater amelioration of abiotic conditions (nursing effect) than other woody species. Although the strong perching effect of this conifer species was later confirmed by a seed rain study (Dos Santos et al. Citation2011), its nursing effects and mechanisms are yet to be explored (Duarte et al. Citation2010).
Nurse plants are those that ameliorate the microenvironmental conditions beneath their canopies, including reduction in air temperature, increase in water and nutrient availability, shading and protection from grazing and trampling (Franco & Nobel Citation1989; Tewksbury & Lloyd Citation2001; López et al. Citation2007; Gómez-Aparicio et al. Citation2008). This study aims to identify and explore the nursing mechanisms promoted by A. angustifolia, in order to explain its important role as a nucleating species in the process of forest expansion in the highland forests of southern Brazil. In order to accomplish that, we selected a representative forest/grassland mosaic in the southernmost state of Brazil, and compared some key aspects of the microenvironment under the canopy of A. angustifolia with those operating on adjacent grassland areas, as well as with those under the pioneer shrub, B. uncinella, whose nucleating effects were reported to be smaller than those of our target species.
Materials and methods
Study site
The study was conducted in the Pró-Mata Research and Nature Conservation Centre (CPCN-Pró-Mata). The centre has 4500 ha and is located in São Francisco de Paula, Rio Grande do Sul State, Brazil (29°28′52.04″S and 50°10′28.00″W). The regional climate is classified according to the Köppen system as Cfb. The annual mean temperature is 14.5 °C, with negative temperatures occurring from April to November, and high rainfall levels occurring throughout the year, amounting to an annual mean of 2252 mm (Backes Citation1999). Soils are generally acidic, with low base saturation and high levels of exchangeable Al and organic matter (Streck et al. Citation2008). Vegetation is characterised by tall grasslands (Campos), intermingled with Araucaria forests. Cattle grazing and burning practices were stopped in 1993, allowing for increased regeneration of the forest and woody plant establishment in the grassland (Oliveira & Pillar Citation2004).
The study site was located in an area of c. 78 ha of grassland, surrounded by Araucaria forests, where a significant colonisation of forest species in the grassland matrix is observed. Individuals of the woody species A. angustifolia, B. uncinella, Pinus elliottii and Myrceugenia euosma are commonly found in these grassland areas (Duarte et al. Citation2006).
Sampling methods
Isolated trees of A. angustifolia and shrubs of B. uncinella (hereafter called Araucaria and Baccharis) were randomly sampled in the grassland in January 2009. Starting from an arbitrarily defined initial point, we walked towards one predetermined cardinal point (N, S, E or W). The first isolated individual of Araucaria or Baccharis found was sampled. We considered an individual to be isolated if it had no woody neighbour touching its crown, and if the distance separating it from another mature tree or shrub was at least 10 m (at the end, this minimal distance turned out to be 15 m). For each sampled Araucaria and Baccharis, a point was marked in the open field 10 m away from its main stem and in the direction in which the least number of other isolated woody plants could be seen. This sampling procedure was repeated until we marked 15 Araucaria, 15 Baccharis and 15 points in the open, away from Araucaria, and 15 points in the open, away from Baccharis. We completed the sampling procedure with 30 points in the open field (grassland), and 30 woody individuals (a total of 60 sampling units).
The mean ± SEM height and crown area of the selected Araucaria individuals were 6.46 ± 0.34 m and 48.80 ± 5.97 m2, respectively, and, for Baccharis, 2.05 ± 0.07 m and 6.39 ± 0.67 m2, respectively. The two species also differed on the amount of vertical space available below their crows: c. 5 m for Araucaria and < 1 m for Baccharis. These three microenvironments (Araucaria, Baccharis and open field) were organised into three blocks, separated from each other by c. 500 m. Each block included five Araucaria, five Baccharis, five points in the open near Araucaria and five points in the open near Baccharis (20 samples per block).
Microclimate and canopy openness
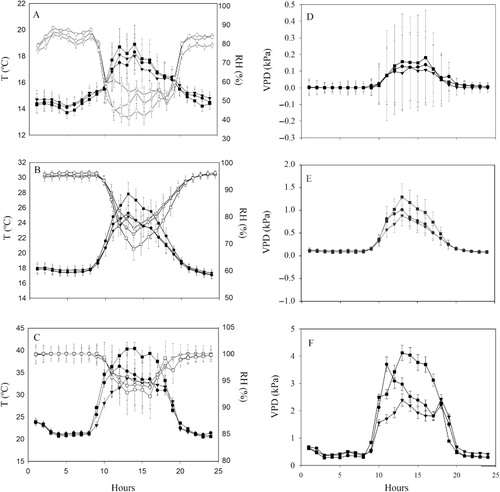
The microclimate (temperature and air humidity) associated with the sampling points was evaluated using 20 data loggers LOGBOX-RHT (NOVUS, Ltda, Porto Alegre, Brazil). Temperature and air humidity were used to calculate the vapour pressure deficit (VPD) of the air. Data loggers were placed 80 cm from the ground, standing above the grassy vegetation in the open sites. Under the canopies, data loggers were placed 70 cm away from the trunk. Data for each block were recorded every hour, for five consecutive days, in the summer of 2009. Measurements were made on 8–12 March 2009, 22–27 February 2009 and 1–5 March 2009, in blocks 1, 2 and 3, respectively. Daily means for mean, minimum and maximum air temperatures, humidity and VPD were based on all hourly measurements taken over five consecutive days. Mean values for each hour of the day were based on all measurements taken at a given hour over the five days. A few winter measurements were also taken in the same year, and because the results followed the same pattern as the summer measurements, only the summer results are shown.
The percentage of open canopy beneath Araucaria and Baccharis was assessed with hemispherical photographs. Pictures were taken with a Nikon Coolpix 8700 camera, coupled with a fisheye lens FC-E9 (Raynox DCR-CF 185° – Pro Fisheye circular) in June 2010, 30 cm above the ground under the shrubs and 1.65 m above the ground under the trees. The images were analysed using the program Gap Light Analyzer 2.0 (Frazer et al. Citation1999).
Soil characteristics
Soil chemistry was analysed in all 60 sampling units in January 2009 (summer) and in August 2010 (winter). Five hundred grams of soil (composed of four subsamples) were taken from the upper 20 cm of soil below the canopies and in the open field, using a shovel. Soil samples were placed in plastic bags and transported to the Analyses Laboratory of the Soil Department of the Federal University of Rio Grande do Sul. Soil variables were determined as follows: pH measured in water solution (1 : 1; v/v), P (mg dm−3) and K (mg dm−3) based on the Mehlich I method; organic matter (OM%) obtained by sulfocromic solution oxidation with external heat, exchangeable Ca (cmolc dm−3), Mg (cmolc dm−3) and Al (cmolc dm−3) extracted with KCl 1 mol L−1; cation-exchange capacity (CEC, cmolc dm−3) at pH 7, and N determined by TKN–Kjeldahl/0.01% method. After extraction, exchangeable cations were determined in an inductively coupled plasma spectrometer (Optima 7300DV ICO-OES, PerkinElmer, Inc., USA). In addition to absolute values, we also report the difference between the value recorded below the canopy of Araucaria or Baccharis and that recorded in the adjacent open field, in order to compare the soil chemical changes induced by the presence of the two woody species.
Soil samples for textural analysis were collected following the same procedures used for chemical analyses, in October 2010. However, only 27 samples were taken (nine Araucaria, nine Baccharis and nine open field, all randomly sampled). Soil moisture was estimated by gravimetric water content. Forty-five samples (15 Araucaria, 15 Baccharis and 15 open field) of 250 g soil (composed of four subsamples) were taken following the same procedures previously described for chemical and textural analyses, in March 2010. Immediately after collection, samples were weighed to obtain the fresh mass (FM) and placed in plastic bags. Back in the lab, they were dried at 60 °C for 24 h to obtain the dry mass (DM). The gravimetric water content (GWC) was then calculated as (FM – DM)/DM.
Data analyses
Microclimate and soil parameters were compared in relation to block and microenvironment effects using analysis of variance (ANOVA) with permutation test (Pillar & Orlóci Citation1996). For this, a Euclidean distance matrix between the observations was computed, and the sum of squared distances between groups of observations, defined by two factors (block and microenvironment), was used as test criterion (Qb statistics, see also Pillar Citation2013). The probability of the observed Qb for factor environment being lower than null Qb values was obtained by permuting the observations within blocks and then recomputing Qb for microenvironment. The procedure was repeated 10,000 times, and the number of times the observed Qb value was lower than null Qb values defined that probability. For microclimate and soil parameters, ANOVA with permutation tests included the three microenvironments (Araucaria, Baccharis and open field). Data from each season were analysed separately in the case of soil data. When comparing the differences between each species and the open field for the soil parameters, the ANOVA only included two levels of microenvironment (Araucaria and Baccharis). In all cases, when microenvironment effect was found to be significant, the differences between the levels of the factor microenvironment were compared using orthogonal contrasts with permutation test, in a similar way as the overall effect ANOVA (Pillar & Orlóci Citation1996). The MULTIV v. 2.67 statistical software (V. Pillar, available at http://ecoqua.ecologia.ufrgs.br/ecoqua/MULTIV.html) was used for these analyses.
Results
Microclimate and canopy openness
Mean and maximum daily temperatures were significantly higher in the open field than under Araucaria and Baccharis, but did not differ between these two species (). There were no differences in mean and maximum air humidity among the three microenvironments. Minimum air humidity was higher in Baccharis than in Araucaria and lower in the open field than in Araucaria. Mean and maximum values of VPD were greater in the open field than under the woody individuals. Diurnal variations in these meteorological parameters revealed that maximum temperatures and VPDs at midday were not only higher in the open field, but also higher under Araucaria than under Baccharis (). The percentage of canopy openness was also significantly higher under the former than under the latter species ().
Table 1 Microclimatic characteristics under the canopies of Araucaria and Baccharis and in the open field.
Soil conditions
Textural analyses showed that the soil in the area has higher percentages of silt (c. 50%) and clay (c. 30%) than of sand, with no significant differences among the three microenvironments (data not shown). There were also no differences in gravimetric soil water content (measured in the summer) among them ().
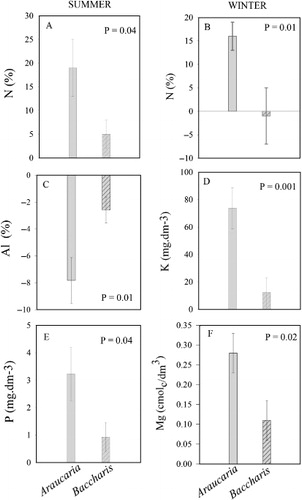
The soil in all three environments and both seasons was characterised by a low pH and high levels of organic matter. Although the CEC did not vary among the environments, the base saturation was greater under the trees and shrubs than in the open field. The Al saturation showed an opposite pattern. Soil concentrations of N, P and K were, in general, greater under Araucaria and Baccharis than in the open field, but these differences were more consistent for the former species. In the particular case of P in the summer and K in the winter, soil concentration under Araucaria was also greater than under Baccharis. The availability of Ca and Mg in the three different soil environments changed markedly from summer to winter. The Ca and Mg availabilities were higher in the open field in the summer, followed by Baccharis and Araucaria. In the winter, the availability of these nutrients reduced substantially in the open field, such that soil under both Araucaria and Baccharis had greater concentrations than the soil in the open ().
Table 2 Chemical characteristics of the soil under Araucaria (n = 15), Baccharis (n = 15), and in the open field (n = 30).
Araucaria had a greater positive effect on the concentrations of N, K, P and Mg (measured by the difference from the open field) than Baccharis (). With respect to Al saturation of CEC (), the reduction effect was also higher for Araucaria than for Baccharis. Deviations of soil parameters not presented in were not significantly different between the two species.
Discussion
Changes in microclimate
Mean and maximum temperatures and VPDs during the summer were lower under Araucaria and Baccharis than in the open field, indicating milder and more favourable conditions for seedling establishment under individuals of these species than in the more open grassy environment. Most studies with nurse plants recognise the importance of tree canopies in reducing the temperature and VPD in open areas (Ludwig et al. Citation2004; Gómez-Aparicio et al. Citation2008). Our study was conducted in a montane, cool and very humid environment and, as expected, maximum temperatures and VPDs registered in the study area were much smaller than those reported in other studies, which were mostly conducted in open, but warmer and drier environments (Holl Citation1999; Gómez-Aparicio et al. Citation2005; Munguía-Rosas & Sosa Citation2008). However, we believe that the reported reductions in temperature and VPD promoted by the two woody species are of ecological significance for their nursing effects during the summer. Midday maximum temperature and VPD registered during the summer in the open areas reached c. 40 °C and 4 kPa, respectively. The cooling effect of the canopies amounted to at least 5 °C and caused a reduction of at least 1 kPa in VPD.
Despite striking differences in plant height, crown morphology and canopy openness between the two species, the summer microclimate under their canopies was not very distinct, except for the higher VPDs and maximum temperatures measured under Araucaria during the warmest hours of the day. Because of the much denser and more closed canopy of Baccharis, lower temperatures and VPDs under their crowns than under those of the conifer species were expected. However, the concentration of branches up high in the tall crowns of Araucaria may have allowed for a greater air circulation than in the dense and bushy crowns of Baccharis, attenuating the expected differences.
Shading
Although this study has not directly assessed the availability of light in the different environments, it did show that the canopies of Araucaria impose less shade than the canopies of Baccharis.
Light attenuation might be an important facilitation effect in seedling establishment, by reducing the risks of photoinhibition, which are more likely to occur under suboptimal conditions such as those generated by low temperatures (Long et al. Citation1994). However, less light may also mean less photosynthesis and growth. Thus, it is possible that the lesser shade imposed by the canopy of Araucaria improves the establishment of tree seedlings by limiting the occurrence of photoinhibition, particularly during cold periods, without limiting light availability for seedling growth. However, a better understanding of this possible facilitation mechanism requires measurements of irradiance beneath the canopy of the two species and in the open field, as well as the photosynthetic responses to light of individuals that are recruited into these different environments.
Changes in soil moisture and nutrients
Water content in the soil under the target species did not differ from that under the grassy vegetation. This similarity in water content does not come as a great surprise, considering the large amounts of rainfall in the region (Backes Citation1999). However, the presence of mature individuals of either Araucaria or Baccharis in the grassland resulted in an overall improvement of soil fertility and this effect was more pronounced for the conifer than for the shrub species.
Nurse plants can improve soil chemical conditions by litter deposition, leaching of atmospheric nutrients intercepted by their crowns, mycorrhizal associations and droppings from grazing, perching and hiding animals (Kellman Citation1979; Callaway et al. Citation1991; Gea-Izquierdo et al. Citation2009). The results do not allow us to identify the mechanisms involved in the ameliorating conditions of soil fertility. However, Silva & Anand (Citation2011), working in the same area, also reported a progressive increase in soil fertility (particularly N) and microbial biomass across the vegetation gradient (grasslands < isolated trees < forest patches < forests). By evaluating δ15N in the A. angustifolia leaves, these authors suggested an increasing importance of biological interactions for the N nutrition of plants when moving along this same gradient.
Araucaria angustifolia was found to be highly dependent on mycorrhiza (Moreira-Souza et al. Citation2003; Zandavalli et al. Citation2004). Its presence in the grass matrix might then increase the availability of nutrients in the soil, as a result of this strong biological interaction. Mycorrhizal associations of B. uncinella have not yet been investigated, but Zimmer et al. (Citation2010) reported negative competitive effects (resource competition and mechanical interference) of this species on the colonisation of Podocarpus lambertii in a restoration area. Taking into account the fast growth of this short-lived pioneer shrub species, intense competition for soil resources with the surrounding vegetation is to be expected, and might help explain its less-pronounced amelioration of soil chemical conditions.
One could argue that, in the process of grassland colonisation by Araucaria trees, these would be preferentially established in those spots that are richer than average to begin with, such that we could not be certain that isolated trees cause changes in soil properties or if their distribution is simply a reflection of pre-existing heterogeneity. In a previous study conducted in the same area, Garbin et al. (Citation2006) analysed and compared soil patchiness for inorganic nitrogen in mature Araucaria forest and in an adjacent grassland area. Despite patchiness, there was no overall relationship between the position of mature trees and young individuals of A. angustifolia and patch location in the forest, indicating that the successful establishment of new trees may not depend on whether they find a richer spot of soil or not. These findings further support our suggestion that this conifer species ameliorate soil conditions.
Conclusions
Our results have shown that, under the canopies of Araucaria and Baccharis, seedlings and saplings experience lower summer temperatures and VPDs, as well as overall greater soil fertility, than when growing in the open field. However, when comparing these two pioneer species, Araucaria individuals were found to impose less shade and to promote greater amelioration of soil chemical conditions under their canopies than Baccharis individuals.
Based on these results, we propose that A. angustifolia does act as an effective nucleating species, not only by acting as a perch for seed dispersers (Duarte et al. Citation2006; Dos Santos et al. Citation2011), but by also by nursing the seedlings afterwards. The nursing mechanisms do seem to include the attenuation of extreme temperatures and VPDs during the summer, the imposition of moderate shading and an increase in the availability of soil nutrients. Although individuals of B. uncinella also create a more suitable microclimate under their canopies during the summer, they offer a more closed canopy than individuals of this species, and the reduced canopy openness, associated with the dense and low crown of this shrub, might impose light and space limitations to the colonising seedlings. Also, their ameliorating effects of the soil conditions are less pronounced than those of A. angustifolia.
Although we have made a significant contribution to identifying nursing effects and mechanisms of A. angustifolia in the process of forest expansion over grasslands, future studies that compare the ability of selected forest species to survive and grow under the canopy of this and other species are needed. Also, a more in-depth investigation of changes in irradiance and soil chemical and biological conditions promoted by the presence of A. angustifolia will greatly add to our knowledge of the nursing mechanisms of this conifer species.
Acknowledgements
This study is part of the doctoral thesis of the first author, developed in the Post-Graduate Program in Botany of the Federal University of Rio Grande do Sul. We thank the Inter-American Institute for Global Change Research (IAI), Fernanda Alabarce, Paula Fagundes and Luciano da Silva Figueiredo for field assistance, and the Coordination for Improvement of Higher Education Personnel (CAPES/Brazil) and the Brazilian Council for Scientific and Technological Development (CNPq/Brazil) for fellowships awarded to the first and second author, respectively.
References
- Backes A 1999. Condicionamento climático e distribuição geográfica de Araucaria angustifolia (Bertol.) Kuntze no Brasil. Pesquisas (Série Botânica) 49: 31–51.
- Behling H, Pillar VD, Orlóci L, Bauermann SG 2004. Late Quaternary Araucaria forest, grassland (Campos), fire and climate dynamics, studied by high-resolution pollen, charcoal and multivariate analysis of the Cambará do Sul core in southern Brazil. Palaeogeography, Palaeoclimatology, Palaeoecology 203: 277–297. 10.1016/S0031-0182(03)00687-4
- Bond WJ, Midgley GF, Woodward FI 2003. The importance of low atmospheric CO2 and fire in promoting the spread of grasslands and savannas. Global Change Biology 9: 973–982. 10.1046/j.1365-2486.2003.00577.x
- Callaway RM, Nadkarni NM, Mahall BE 1991. Facilitation and interference of Quercus Douglasii on understory productivity in central California. Ecology 72: 1484–1499. 10.2307/1941122
- Carlucci MB, Teixeira FZ, Brum FT, Duarte LDS 2011. Edge expansion of Araucaria forest over southern Brazilian grasslands relies on nurse plant effect. Community Ecology 12: 196–201. 10.1556/ComEc.12.2011.2.7
- Dos Santos MMG, Oliveira JM, Müller SC, Pillar VD 2011. Chuva de sementes de espécies lenhosas florestais em mosaicos de floresta com Araucária e campos no sul do Brasil. Acta Botanica Brasilica 25: 160–167.
- Duarte LDS, Carlucci MB, Hartz SM, Pillar VD 2007. Plant dispersal strategies and the colonization of Araucaria forest patches in a grassland-forest mosaic. Journal of Vegetation Science 18: 847–858. 10.1111/j.1654-1103.2007.tb02601.x
- Duarte LDS, Dos-Santos MMG, Hartz SM, Pillar VD 2006. Role of nurse plants in Araucaria Forest expansion over grassland in south Brazil. Austral Ecology 31: 520–528. 10.1111/j.1442-9993.2006.01602.x
- Duarte LDS, Hofmann GS, Dos Santos MMG, Hartz SM, Pillar VD 2010. Testing for the influence of niche and neutral factors on sapling community assembly beneath isolated woody plants in grasslands. Journal of Vegetation Science 21: 462–471. 10.1111/j.1654-1103.2009.01153.x
- Dümig A, Schad P, Rumpel C, Dignac MF, Kögel-Knabner I 2008. Araucaria forest expansion on grassland in the southern Brazilian highlands as revealed by 14C and δ13C studies. Geoderma 145: 143–157.
- Flores J, Jurado E 2003. Are nurse-protégé interactions more common among plants from arid environments? Journal of Vegetation Science 14: 911–916. 10.1111/j.1654-1103.2003.tb02225.x
- Franco AC, Nobel PS 1989. Effect of nurse plants on the microhabitat and growth of cacti. Journal of Ecology 77: 870–886. 10.2307/2260991
- Frazer GW, Canham CD, Lertzman KP 1999. Gap light analyzer (GLA): imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs, user manual and program documentation, version 2.0. Millbrook, NY, Institute of Ecosystem Studies.
- Garbin ML, Zandavalli, RB, Dillenburg, LR 2006. Soil patches of inorganic nitrogen in subtropical Brazilian plant communities with Araucaria angustifolia. Plant and Soil 286: 323–337. 10.1007/s11104-006-9046-y
- Gea-Izquierdo G, Monteiro G, Cañellas I 2009. Changes in limiting resources determine spatio-temporal variability in tree–grass interactions. Agroforestry Systems 76: 375–387. 10.1007/s10457-009-9211-4
- Gómez-Aparicio L, Gómez JM, Zamora R, Boettinger JL 2005. Canopy vs. soil effects of shrubs facilitating tree seedlings in Mediterranean montane ecosystems. Journal of Vegetation Science 16: 191–198.
- Gómez-Aparicio L, Zamora R, Castro J, Hódar JA 2008. Facilitation of tree saplings nurse plants: microhabitat amelioration or protection against herbivores? Journal of Vegetation Science 19: 161–172.
- Holl KD 1999. Factors limiting tropical rain forest regeneration in abandoned pasture: seed rain, seed germination, microclimate, and soil. Biotropica 31: 229–242. 10.1111/j.1744-7429.1999.tb00135.x
- Kellman M 1979. Soil enrichment by neotropical savanna trees. Journal of Ecology 67: 565–577. 10.2307/2259112
- Klein RM 1960. O aspecto dinâmico do pinheiro brasileiro. Sellowia 12: 17–44.
- Long SP, Humphries S, Falkowski, PG 1994. Photoinhibition of photosynthesis in nature. Annual Review of Plant Physiology and Plant Molecular Biology 45: 633–662. 10.1146/annurev.pp.45.060194.003221
- López RP, Valdivia S, Sanjinés N, Quintana D 2007. The role of nurse plants in the establishment of shrubs seedlings in the semi-arid subtropical Andes. Oecologia 152: 779–790.
- Ludwig F, Kroon H, Berendse F, Prins HHT 2004. The influence of savanna trees on nutrients, water and light availability and the understory vegetation. Plant Ecology 170: 93–105. 10.1023/B:VEGE.0000019023.29636.92
- Moreira-Souza M, Trufem SFB, Gomes-da-Costa SM, Cardoso EJBN 2003. Arbuscular mycorrhizal fungi associated with Araucaria angustifolia (Bert.) O. Ktze. Mycorrhiza 13: 211–215. 10.1007/s00572-003-0221-1
- Müller SC, Overbeck GE, Pfadenhauer J, Pillar VD 2012. Woody species patterns at forest-grassland boundaries in southern Brazil. Flora 207: 586–598.
- Munguía-Rosas MA, Sosa VJ 2008. Nurse plants vs. nurse objects: effects of woody plants and rocky cavities on the recruitment of the Pilosocereus leucocephalus columnar cactus. Annals of Botany 101: 175–185.
- Oliveira JM, Pillar VD 2004. Vegetation dynamics on mosaics of Campos and Araucaria forest between 1974 and 1999 in Southern Brazil. Community Ecology 5: 197–202. 10.1556/ComEc.5.2004.2.8
- Overbeck GE, Müller SC, Fidelis A, Pfadenhauer J, Pillar VD, Blanco CC 2007. Brazil’s neglected biome: the South Brazilian Campos. Perspectives in Plant Ecology, Evolution and Systematics 9: 101–116. 10.1016/j.ppees.2007.07.005
- Pausas JG, Bonet A, Maestre FT, Climent A 2006. The role of the perch effect on the nucleation process in Mediterranean semi-arid oldfields. Acta Oecologica 29: 346–352. 10.1016/j.actao.2005.12.004
- Pillar VD 2013. How accurate and powerful are randomization tests in multivariate analysis of variance? Community Ecology 14: 153–163. 10.1556/ComEc.14.2013.2.5
- Pillar VD, Orlóci L 1996. On randomization testing in vegetation science: multifactor comparisons of relevé groups. Journal of Vegetation Science 7: 585–592. 10.2307/3236308
- Polley HW 1997. Implications of rising atmospheric carbon dioxide concentration for rangelands. Journal of Range Management 50: 561–577.
- Reitz R, Klein RM, Reis A 1988. Projeto Madeira do Rio Grande do Sul. Porto Alegre, Herbário Barbosa Rodrigues. 525 p.
- Silva LCR, Anand M 2011. Mechanisms of Araucaria (Atlantic) Forest Expansion into Southern Brazilian Grassland. Ecosystems 14: 1354–1371. 10.1007/s10021-011-9486-y
- Silva LCR, Anand M, Oliveira JM, Pillar VD 2009. Past century changes in Araucaria angustifolia (Bertol.) Kuntze water use efficiency and growth in forest and grassland ecosystems of southern Brazil: implications for forest expansion. Global Change Biology 15: 2387–2396. 10.1111/j.1365-2486.2009.01859.x
- Streck EV, Kampf N, Dalmolin RSD, Klamt E, Nascimento PC, Schneider P et al. 2008. Solos do Rio Grande do Sul. 2 edition. Porto Alegre, EMATER. 222 p.
- Tewksbury JJ, Lloyd JD 2001. Positive interactions under nurse-plants: spatial scale, stress gradients and benefactor size. Oecologia 127: 425–434. 10.1007/s004420000614
- Yarranton GA, Morrison RG 1974. Spatial dynamics of a primary succession: nucleation. Journal of Ecology 62: 417–428. 10.2307/2258988
- Zandavalli RB, Dillenburg LR, Souza PVD 2004. Growth responses of Araucaria angustifolia (Araucariaceae) to inoculation with mycorrhizal fungus Glomus clarum. Applied Soil Ecology 25: 245–255. 10.1016/j.apsoil.2003.09.009
- Zimmer GO, Paz CP, Ganade G 2010. Effects of different pioneer species on the colonization of Podocarpus lambertii in a restoration area. Neotropical Biology and Conservation 5: 160–166. 10.4013/nbc.2010.53.04
